Mineral nutrition in White Spruce (
Picea glauca
[Moench] Voss) seeds
and somatic embryos; II. EDX analysis of globoids and Fe-rich
particles
Daryl A. Reida, John N.A. Lotta,*, Stephen M. Attreeb,1, Larry C. Fowkeb
aDepartment of Biology,McMaster Uni6ersity,Hamilton,ON,L8S4K1,Canada bDepartment of Biology,Uni6ersity of Saskatchewan,Saskatoon,SK,S7N5E2,Canada Received 13 July 1998; received in revised form 26 October 1998; accepted 28 October 1998
Abstract
The elemental compositions of globoids and Fe-rich particles were investigated in white spruce (Picea glauca[Moench] Voss) somatic embryos, zygotic embryos and female gametophytes using energy dispersive X-ray analysis. Globoids, phytate deposits in seed protein bodies, were found throughout the female gametophyte of seeds and in the ground meristem and procambium from all regions of both somatic and zygotic embryos. Iron-rich particles, believed to be Fe-associated phytate deposits in seed proplastids, were found throughout the female gametophyte of seeds and in the protoderm, ground meristem, and procambium from all regions of both somatic and zygotic embryos. Globoids in somatic and zygotic embryos ranged from 0.5 – 3.0mm in
diameter, but globoids typically ranged from 1.5 – 2.0 mm in diameter in somatic embryos and from 2.0 – 3.0 mm in diameter in
zygotic embryos. Globoids in female gametophyte tissue ranged from 0.5 – 6.0mm in diameter. All Fe-rich particles studied from
somatic embryos and from seeds ranged from 0.14 – 0.25mm in diameter. Globoids in somatic embryos and seeds contained high
P, moderate K and Mg with occasional traces of Fe and little if any Ca and Zn. Globoids in the zygotic embryo cotyledon procambium tissue also contained moderate levels of Fe and had significantly higher Fe:P ratios, which were not found in any other regions in seeds or in somatic embryos. Iron-rich particles in somatic embryos and seeds contained high P and Fe, moderate K and Mg, and little if any Ca and Zn. Typically, spectra of Fe-rich particles in somatic embryos had P peaks higher than Fe peaks and spectra of Fe-rich particles in seeds had P peaks lower than Fe peaks. Overall, the composition of globoids and Fe-rich particles in somatic embryos and zygotic embryos were very similar with only minimal differences found. © 1999 Elsevier Science Ireland Ltd. All rights reserved.
Keywords: Somatic embryos; Seeds; White spruce; EDX analysis; Globoids; Fe-rich particles
1. Introduction
In this study, energy dispersive X-ray (EDX) analysis was used to investigate the mineral nutri-ent reserves of white spruce seeds and somatic embryos. This paper will focus on those elements
measured on a whole tissue basis in paper I of this two paper series. Energy dispersive X-ray analysis is a convenient tool for studying the elemental compositions of specific spots or larger areas within the sample and allows the simultaneous detection of elements with a high detection sensi-tivity [1,2]. We used EDX analysis to determine if phytate reserves in mature somatic embryos of white spruce have similar element compositions to those found in mature white spruce seeds. This research will provide information on mineral
nu-* Corresponding author. Tel.: +1-905-5259140 ext. 24589; fax:
+1-905-5226066; e-mail: lott@mcmaster.ca.
1Present address: Pacific Regeneration Technologies Inc., Victoria, BC, V8T 2W1, Canada.
trient storage which may be useful to those devel-oping artificial seeds.
Globoids (or globoid crystals) are naturally elec-tron-dense deposits within seed protein bodies and are composed of phytate. Phytate is a mixed cation salt of myo-inositol hexa-kisphosphoric acid or phytic acid [3,4]. Phytate is the typical mineral nutrient storage compound in seeds. Due to their natural electron density and relatively large size, these inclusions are convenient particles for spectral EDX analysis. Energy dispersive X-ray analysis of globoids from different tissues and species have shown the presence of P, Mg and K with occasional storage of Ca, Mn, Na, Zn, Ba, and Fe [5].
Studying mature seeds from eleven Pinus spe-cies, small (50.33 mm in diameter) naturally
elec-tron-dense inclusions were found in
membrane-bound structures resembling proplas-tids and were termed Fe-rich particles based on EDX analysis [6]. These particles contained high P and Fe, moderate levels of K and Mg, and traces of Ca, Mn, and Zn [6]. Based on the similarities of EDX analysis spectra of Fe-rich particles, globoids and precipitated ferric phytate, it was proposed that these particles could represent storage de-posits of Fe-rich phytate [6,7]. The high levels of P also suggested that these deposits were not phyto-ferritin inclusions, which is a common iron storage compound found in chloroplasts [8].
In this study the elemental composition of globoids and Fe-rich particles in various regions of white spruce somatic embryos, zygotic embryos and female gametophytes was investigated using EDX analysis. This paper will concentrate on the levels of P, K, Mg, Ca, Fe, and Zn within these deposits since these nutrients were of focus in part one of this two paper series. We believe this to be the first study of globoid and Fe-rich particle composition in white spruce and the first compari-son of their composition in somatic embryos and seeds of any species.
2. Materials and methods
2.1. Tissue preparation for EDX analysis
White spruce (Picea glauca [Moench] Voss) seeds were collected from the Big River and Christopher Lake regions north of Saskatoon,
Saskatchewan. Mature zygotic embryos were ex-cised from artificially dried seeds using fine-tipped forceps. White spruce somatic embryos were pre-pared and desiccated as previously described [9,10]. To prevent the extraction of water-soluble phytate, while allowing enough tissue expansion that epoxy resin can penetrate the tissue, a low-water-content preparation procedure was used [11]. Tissue samples were initially placed into 80% ethanol overnight. These samples were then placed into 100% ethanol for at least 8 h and again in 100% ethanol overnight. To complete the dehydra-tion, the tissue samples were treated with propy-lene oxide for at least 7 h.
Using a standard hardness Spurr’s epoxy resin, the samples were infiltrated on a tissue rotator using a propylene oxide:Spurr’s epoxy resin series (2:1, 1:2, 0:1, and 0:1) for 24 h in each solution. Following infiltration, each embryo was sectioned into cotyledons, hypocotyl and radicle regions and each region was placed into a separate rubber mold. Additionally, each female gametophyte was divided into cotyledon-associated, hypocotyl-asso-ciated and radicle-assohypocotyl-asso-ciated regions prior to de-hydration and placed into separate molds following infiltration with Spurr’s resin. All molds were filled with Spurr’s resin and allowed to harden in a 70°C oven overnight.
A Reichert OM U2 ultramicrotome (Reichert, Austria) was used to cut thick sections (1 – 1.5 mm)
using dry glass knives. Each section was flattened using eyelashes and cactus spines mounted on wooden sticks and picked up using 100 mesh Formvar-carbon coated copper grids. Drops of 100% ethanol were used to help the sections ad-here to the Formvar.
2.2. EDX analysis of globoids and Fe-rich
particles
For each of five somatic embryos and five zygotic embryos, five spectra of globoids from different cells and of different sizes were collected from both the ground meristem and procambium in each of the cotyledon, hypocotyl and radicle regions. Additionally, for each of these same five embryos, five spectra of Fe-rich particles were collected from each of the protoderm, ground meristem and procambium in each of the cotyle-don, hypocotyl and radicle regions. Five spectra of globoids and five spectra of Fe-rich particles were collected from each of the cotyledon-associated, hypocotyl-associated and radicle-associated re-gions from each of five female gametophytes. Therefore in total, 525 spectra of Fe-rich particles and 375 spectra of globoids were collected.
X-ray counts for all the spectra were collected by integrating the peaks at the selected window widths: Mg, 1153.7 – 1354.3 eV; P, 1905.3 – 2120.7 eV; K, 3193.7 – 3432.3 eV; Ca, 3568.6 – 3813.4 eV; Mn, 5758.5 – 6037.5 eV; Fe, 6259.9 – 6546.1 eV; Cu, 7891.7 – 8200.3 eV; and Zn, 8478.9 – 8795.1 eV [12]. In order to produce the best fit background line for all elements, points were connected at the following eV values: 510, 660, 814, 1453, 1730, 2500, 2800, 3020, 4200, 5400, 8400, 9400, and 11 000 eV. Background subtraction was performed for each element in each spectrum by subtracting the number of counts in the background from the total number of counts in the element. A useful format to express peak values is in the form of peak-to-background (P/B) ratios, which have been defined as the number of counts above the back-ground for a peak divided by the number of background counts [13]. Peak-to-background ra-tios were calculated for each element in each spec-trum by using the total number of counts before and after subtraction [14]. Ratios of elements to P were also calculated since P is the main anion to which the elements are bound in phytate.
Due to peak overlaps, correction factors were used to calculate the actual Ca, Fe, and Zn counts in each spectrum [7]. Correction factors were used prior to background subtraction. The Kb peak of K overlaps the Ka peak of Ca and therefore a correction factor of 8.80% of the total X-ray counts for the K Kapeak was subtracted from the total counts in the Ca window to give the actual counts for Ca. Similarly, the Kb peak of Mn overlaps the Fe Kapeak and therefore a correction factor of 11.60% of the total X-ray counts for Mn
Ka peak was subtracted from the total counts in the Fe window to give a corrected Fe value. Finally, the Kb peak of Cu overlaps the Ka peak of Zn and therefore 2.00% of the net Cu Kacounts was subtracted from the total Zn counts to give a corrected Zn value.
2.3. Statistical analysis
The statistical significance between means was determined by using the MINITAB analysis of variance test. When a difference was found, Tukey’s test was used to determine which of the means were statistically different at P\0.05 [15]. Due to the use of manually predetermined back-ground points and relatively short sampling times, several negative counts were obtained for Ca, Fe and Zn P:B values as well as for the Ca:P, Fe:P, and Zn:P ratios. For each P/B value and ratio of P/B values, all the negative values from all the spectra collected were averaged and any means equal to or less than the absolute value of this mean were considered to be not significantly dif-ferent from the background line and were assigned a value of zero. Peak-to-background values and ratios of P/B values for individual embryos were analysed to see if there were any significant varia-tions from embryo-to-embryo.
The MINITAB two-sample t-test for the differ-ence between two means at P\0.05 was used to compare EDX analysis values for somatic em-bryos to values from zygotic emem-bryos. In this comparison, for each P/B value and ratio of P/B values all the globoids in somatic embryos were pooled together and compared to all the globoids pooled together for zygotic embryos. Each element P/B value and each ratio of an element to P in somatic embryo globoids were compared to the corresponding value in zygotic embryo globoids. This was then repeated for all the EDX analysis values for Fe-rich particles.
3. Results
3.1. General obser6ations
so-Figs. 1 – 4. Scanning transmission electron micrographs of thick sections (1 – 1.5mm) from white spruce somatic embryos,
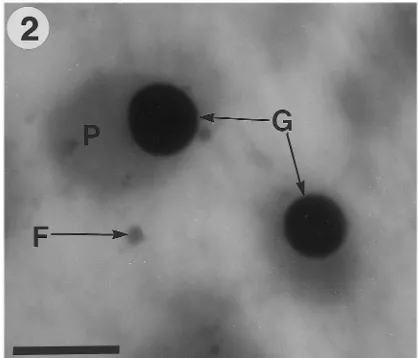
zygotic embryos and female gametophytes. Fig. 1. Naturally electron-dense globoids (G) variable in size and a smaller Fe-rich particle (F) in ground meristem tissue from the radicle of a somatic embryo. Scale bar, 2mm.
Fig. 3. Iron-rich particles (F) and protein bodies (P), which contain globoids (G), are shown in the hypocotyl ground meristem tissue of a zygotic embryo. Scale bar, 2 mm.
diameter (Fig. 4). Globoids in the ground meris-tem of somatic and zygotic embryos tended to be larger than those found in the procambium of the same embryo.
Generally only one globoid was observed per section of a single protein body in somatic and zygotic embryos, but occasionally as many as three were visible. Protein bodies from female gametophyte tissue varied in the number of globoids per section, with some protein bodies containing one globoid and another protein body within the same cell containing four or more globoids. Globoids were most frequent per unit area in female gametophyte tissue and least fre-quent per unit area in somatic embryos.
matic and zygotic embryos. Globoids were not found in the protoderm of either somatic or zygotic embryos. Typically, globoids in somatic embryos were found to be 0.5 – 2.0mm in diameter
(Figs. 1 and 2), but were occasionally observed to be 3.0 mm in diameter. Globoids in zygotic
em-bryos were also found to be between 0.5 and 3.0
mm in diameter (Fig. 3), but typically they were
found to be between 2.0 and 3.0 mm in diameter.
Female gametophyte tissue was found to contain globoids that ranged in size from 0.5 – 6.0 mm in
Fig. 2. Hypocotyl ground meristem tissue of a somatic em-bryo showing a small Fe-rich particle (F) and protein bodies (P), which contain globoids (G). Scale bar, 2mm.
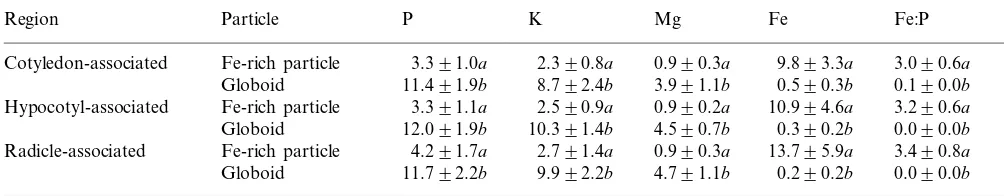
Table 1
Mean peak-to-background ratios and ratios of Fe:P in globoids and Fe-rich particles from resin embedded thick sections for five white spruce female gametophytesa
P K Mg Fe
Region Particle Fe:P
3.391.0a 2.390.8a
Cotyledon-associated Fe-rich particle 0.990.3a 9.893.3a 3.090.6a Globoid 11.491.9b 8.792.4b 3.991.1b 0.590.3b 0.190.0b Hypocotyl-associated Fe-rich particle 3.391.1a 2.590.9a 0.990.2a 10.994.6a 3.290.6a
12.091.9b 10.391.4b 4.590.7b
Globoid 0.390.2b 0.090.0b
Fe-rich particle
Radicle-associated 4.291.7a 2.791.4a 0.990.3a 13.795.9a 3.490.8a Globoid 11.792.2b 9.992.2b 4.791.1b 0.290.2b 0.090.0b
aEach mean (9S.D.) was calculated using 25 values; each value in a single column followed by the same letter is not significantly different at P\0.05.
Iron-rich particles were found in the protoderm, ground meristem and procambium from the cotyledons, hypocotyl and radicle regions of so-matic and zygotic embryos (Figs. 1 – 3). Iron-rich particles were also found throughout the female gametophyte tissue, but they tended to be very difficult to locate. Typically Fe-rich particles were 0.14 – 0.25mm in diameter and were also naturally
electron-dense. Occasionally Fe-rich particles were found in clusters in embryo tissues, but never in female gametophyte tissue.
3.2. EDX analysis of globoids and Fe-rich
particles
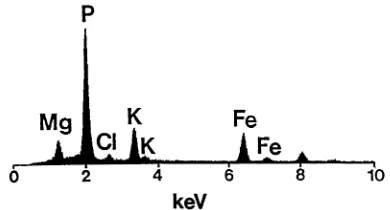
Typical EDX analysis spectra (based on the mean P/B values given in Tables 1 – 3) of globoids from somatic embryos, zygotic embryos and fe-male gametophytes were similar in composition and a representative spectrum is shown in Fig. 5. Typical globoids contained high levels of P, mod-erate levels of Mg and K, occasional traces of Fe and little if any Ca and Zn. One exception to this typical composition was that globoids from the procambium of zygotic embryo cotyledons con-tained moderate levels of Fe and slightly reduced K and Mg (Fig. 6). Spectra of globoids from the same tissue region in somatic embryos showed no significant levels of Fe and more typical K and Mg levels.
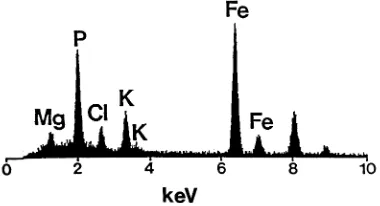
Iron-rich particles in somatic embryos were found to be similar in composition to those found in zygotic embryos and female gametophytes. All Fe-rich particles contained high P and Fe with moderate levels of K and Mg, and little if any Ca and Zn. Iron-rich particles sampled from somatic embryos typically had P peaks higher than Fe peaks (Fig. 7); whereas, Fe-rich particles from
seeds typically had P peaks lower than Fe peaks (Fig. 8). Although Fe-rich particles in seeds and somatic embryos had means that differed in the proportions of P to Fe, particles of similar P and Fe proportions were found in both seeds and somatic embryos. Comparison of a typical globoid spectrum (Fig. 5) with Fe-rich particle spectra (Figs. 7 and 8), show that K and Mg peaks were slightly lower in Fe-rich particles.
Mean P/B values and the ratio of Fe:P for female gametophytes, zygotic embryos, and so-matic embryos are given in Tables 1 – 3, respec-tively. Since little if any Ca or Zn were detected in globoids and Fe-rich particles, these values are not given in these tables. No significant differences were found in P/B values from different female gametophytes or from embryo-to-embryo for ei-ther somatic or zygotic embryos. The majority of the variation was found to be from particle-to-particle.
Comparison of P/B ratios and ratios of Fe:P in globoids and Fe-rich particles tested in various regions of female gametophyte tissue showed no significant variation from region-to-region (Table 1). The P P/B values were between 2.7 and 3.6 times higher in globoids than in Fe-rich particles. Globoids also had between 3.2 and 4.5 times higher K and 4.3 to 5.2 times higher Mg than Fe-rich particles. Iron P/B values were between 19.6 and 68.5 times higher in Fe-rich particles than in globoids. Globoids in the female gametophyte tissue had trace amounts of Fe and therefore very low Fe:P ratios. The Fe:P ratio was 3.0 and higher in Fe-rich particles.
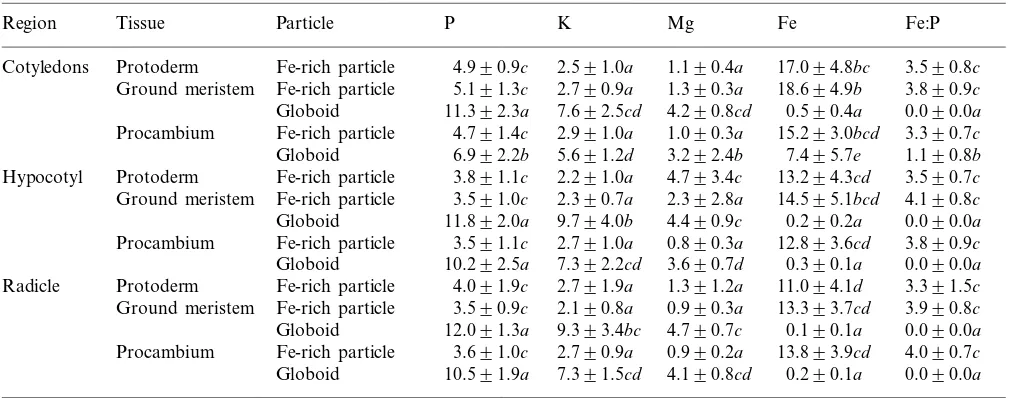
Table 2
Mean peak-to-background ratios and ratios of Fe:P in globoids and Fe-rich particles from resin embedded thick sections for five white spruce zygotic embryosa
Particle P K Mg
Tissue Fe
Region Fe:P
Fe-rich particle
Cotyledons Protoderm 4.990.9c 2.591.0a 1.190.4a 17.094.8bc 3.590.8c Fe-rich particle 5.191.3c 2.790.9a
Ground meristem 1.390.3a 18.694.9b 3.890.9c Globoid 11.392.3a 7.692.5cd 4.290.8cd 0.590.4a 0.090.0a Fe-rich particle 4.791.4c
Procambium 2.991.0a 1.090.3a 15.293.0bcd 3.390.7c Globoid 6.992.2b 5.691.2d 3.292.4b 7.495.7e 1.190.8b Protoderm
Hypocotyl Fe-rich particle 3.891.1c 2.291.0a 4.793.4c 13.294.3cd 3.590.7c Fe-rich particle 3.591.0c 2.390.7a 2.392.8a
Ground meristem 14.595.1bcd 4.190.8c
Globoid 11.892.0a 9.794.0b 4.490.9c 0.290.2a 0.090.0a Procambium Fe-rich particle 3.591.1c 2.791.0a 0.890.3a 12.893.6cd 3.890.9c Globoid 10.292.5a 7.392.2cd 3.690.7d 0.390.1a 0.090.0a Fe-rich particle 4.091.9c 2.791.9a
Protoderm 1.391.2a
Radicle 11.094.1d 3.391.5c
Fe-rich particle 3.590.9c 2.190.8a 0.990.3a
Ground meristem 13.393.7cd 3.990.8c
Globoid 12.091.3a 9.393.4bc 4.790.7c 0.190.1a 0.090.0a Fe-rich particle
Procambium 3.691.0c 2.790.9a 0.990.2a 13.893.9cd 4.090.7c Globoid 10.591.9a 7.391.5cd 4.190.8cd 0.290.1a 0.090.0a
aEach mean (9S.D.) was calculated using 25 values; each value in a single column followed by the same letter is not significantly different at P\0.05.
Fe-rich particles from zygotic embryos had signifi-cantly smaller P/B values for P, K and Mg than globoids. Globoids sampled in the cotyledon pro-cambium tissue in zygotic embryos had signifi-cantly higher Fe values and smaller P, K and Mg values than other globoids within these embryos. Globoids from the cotyledon procambium tissue of zygotic embryos had Fe:P ratios above 1.0. With the exception of this moderate Fe in globoids from the cotyledon procambium tissue of zygotic embryos, the composition of globoids and Fe-rich particles were very consistent from tissue-to-tissue. Table 3 illustrates the P/B ratios and ratios of Fe:P for globoids and Fe-rich particles in somatic embryos. As found with zygotic embryos, the composition of these deposits did not differ greatly from tissue-to-tissue. Globoids had significantly higher P, K and Mg than Fe-rich particles and globoids in the cotyledon procambium of somatic embryos did not have the moderate levels of Fe found in zygotic embryos.
Comparison of globoids and Fe-rich particles in zygotic and somatic embryos using the MINITAB two-samplet-test (Table 4) found that, in general, globoids in zygotic embryos had slightly higher P/B values for P, K, Fe and Fe:P than globoids in somatic embryos. Iron-rich particles from zygotic embryos had slightly higher Fe:P values and sig-nificantly lower P and K values than Fe-rich parti-cles in somatic embryos. Overall, globoid and
Fe-rich particle compositions were very similar in somatic and zygotic embryos.
4. Discussion
The focus of this study was phytate-containing globoids that are found inside protein bodies [16]. The low-water-content preparation procedures used here were designed to retain the phytate in the tissue. It has been shown that K-phytate and Na-phytate are very water-soluble, while phytates containing divalent and trivalent cations tend to be less soluble in water [17,18]. Aqueous fixation and thin sectioning on a water-filled microtome boat can result in major losses of P, Mg and K [19]. Prolonged fixation and washing can cause the complete extraction of globoids [20]. Thicker sec-tions cut on dry knives prevent the shattering of globoids as well as eliminating extraction prob-lems during microtomy. Since globoids are natu-rally electron dense, they can be readily identified in these thicker sections and the addition of elec-tron-dense stains can be omitted.
Table 3
Mean peak-to-background ratios and ratios of Fe:P in globoids and Fe-rich particles from resin embedded thick sections for five white spruce somatic embryosa
Particle P K Mg
Tissue Fe
Region Fe:P
Cotyledons Protoderm Fe-rich particle 4.491.1d 2.690.8e 0.990.3d 12.893.6b 2.990.6bc Fe-rich particle 5.591.6d 3.191.2e
Ground meristem 1.290.4d 14.694.4b 2.690.4c Globoid 10.392.1ab 6.191.4bc 4.291.0b 0.290.2a 0.090.0a Fe-rich particle
Procambium 4.891.4d 2.791.1e 1.190.3d 13.994.9b 2.990.5bc Globoid 10.291.7ab 6.491.9b 4.491.0ab 0.190.1a 0.090.0a Protoderm
Hypocotyl Fe-rich particle 4.891.1d 3.090.8e 1.090.2d 14.093.9b 2.990.5bc Fe-rich particle 4.791.5d 3.691.1de 1.090.3d
Ground meristem 13.694.5b 2.990.4bc
Globoid 11.192.1a 8.892.5a 4.690.7ab 0.290.2a 0.090.0a Procambium Fe-rich particle 4.791.3d 3.090.8e 1.090.4d 14.195.3b 2.990.6bc
Globoid 8.792.5bc 6.391.7bc 3.591.1c 0.190.1a 0.090.0a Fe-rich particle 4.891.3d 3.491.0e
Protoderm 1.090.2d
Radicle 14.794.6b 3.190.5b
Ground meristem Fe-rich particle 5.292.0d 3.491.4e 1.190.4d 16.496.6b 3.190.4b Globoid 10.691.9a 8.392.0a 4.891.0a 0.190.2a 0.090.0a Fe-rich particle 4.992.0d 3.291.5e
Procambium 1.090.4d 14.496.1b 3.090.4b
Globoid 7.492.2c 4.991.9cd 3.290.7c 0.190.2a 0.090.0a
aEach mean (9S.D.) was calculated using 25 values; each value in a single column followed by the same letter is not significantly different at P\0.05.
embryos and female gametophytes had this typical composition except for globoids from the cotyle-don procambium of zygotic embryos, which had Fe peaks similar in height to K. Energy dispersive X-ray analysis of globoids for white spruce, as investigated in this study, showed similar composi-tions to globoids from seeds of various Pinus
species [6]. As found here for white spruce, the protein bodies inPinusalso contained one or more globoids [6]. In zygotic and somatic embryos of white spruce, protein bodies often contained one globoid, but as many as three were observed in a
single section.
It has been proposed, based on studies of an-giosperm seeds, that the size and frequency of globoids was related to the ratio of divalent cations (Mg2+ and Ca2+) to monovalent cations (K+), as measured per g dry weight [21]. Accord-ing to this hypothesis, seed tissues with a high (Mg+Ca):K ratio have larger and more frequent globoids; whereas, a low ratio is correlated to smaller and less frequent globoids. A lower ratio would result in more K-phytate, which is more water-soluble and therefore would be present throughout the proteinaceous matrix rather than in discrete globoids [21,22]. In part I of this two paper series, atomic absorption spectroscopy was used to measure the concentrations of K, Mg and Ca. From these results the (Mg+Ca):K ratios for
Figs. 5 – 8. Energy dispersive X-ray analysis spectra of globoids and Fe-rich particles from thick sections of white spruce zygotic and somatic embryos. The energy lines for each element illustrated are as follows: Mg (Ka=1.2 keV); P
(Ka=2.0 keV); Cl (Ka=2.6 keV); K (Ka=3.3 keV; Kb=3.6
keV); and Fe (Ka=6.4 keV; Kb=7.1 keV). Note the peaks at
8.0 and 8.9 keV are the Cu Kaand Cu Kbpeaks, respectively,
from the copper grids used for holding the sections. Fig. 5. Typical EDX analysis spectrum of a globoid from the hypocotyl ground meristem tissue of a somatic embryo.
Fig. 7. Typical EDX analysis spectrum of an Fe-rich particle from the radicle ground meristem of a somatic embryo show-ing the P peak higher than the Fe peak.
Fig. 8. Typical EDX analysis spectrum of an Fe-rich particle from the radicle ground meristem of a zygotic embryo show-ing the P peak lower than the Fe peak.
white spruce somatic embryos, zygotic embryos and female gametophytes, were 0.38, 0.49 and 0.62, respectively. White spruce somatic embryos, with the smallest (Mg+Ca):K ratio, had smaller globoids and less frequent globoids than in either zygotic embryos or female gametophytes. White spruce female gametophyte tissue, with the highest (Mg+Ca):K ratio, had the largest globoids and the highest frequency of globoids. These results support the theory proposed for angiosperm seeds [21] and this is the first report of this ratio for conifer seeds or somatic embryos. The relationship of this ratio to globoid size and frequency there-fore appears to be valid in both gymnosperm and angiosperm seed tissues.
The second type of storage material studied here was Fe-rich particles. The composition of Fe-rich particles in white spruce seeds and somatic em-bryos are consistent with those reported for Pinus
seeds [6]. Recently, seeds from nine genera in the Pinaceae family were studied for the presence and composition of Fe-rich particles [23]. It was found that Fe-rich particles are a common characteristic of conifers in the family Pinaceae and that they have similar compositions with the exception of
lower Fe:P ratios in Cedrus and Abies [23]. From EDX analysis studies and comparisons to pre-pared phytate and phytoferritin deposits, it was proposed that Fe-rich particles represent stores of Fe-rich phytate [6,7,23]. The findings of this cur-rent study are consistent with that proposal.
The current study has found that phytate stores within white spruce somatic embryos are very similar in location and composition to those found in white spruce zygotic embryos. It was found that the (Mg+Ca):K ratio for whole tissue described for angiosperm seeds holds true for this conifer species. The fact that somatic embryos are pro-duced using prepared media holds the possibility of altering the media composition of Mg, Ca, and K to study their effects on phytate biosynthesis. It may be possible to influence globoid size and frequency by optimizing the ratio of these ele-ments within the media. In addition, since a fully mature white spruce somatic embryo now closely resembles a mature white spruce zygotic embryo, this system of culturing white spruce somatic em-bryos may be very useful in studying the processes of phytate biosynthesis. It can be very difficult to obtain large quantities of conifer embryos at
vari-Table 4
MINITAB two-samplet-test comparison of mean peak-to-background values and ratios of Fe:P in globoids and Fe-rich particles from white spruce somatic and zygotic embryos. For each value, all the globoids in somatic embryos were compared to all the globoids in zygotic embryos and all the Fe-rich particles in somatic embryos were compared to all the Fe-rich particles in zygotic embryosa
Fe Fe:P
K
Particle type Embryo type P Mg
4.191.1a 0.190.2a
Globoid Somatic 9.792.4a 6.892.3a 0.090.0a
0.290.5b 1.493.5b
3.991.1a 7.892.9b
Zygotic 10.592.6b
1.090.3c 14.395.0c
Fe-rich particle Somatic 4.991.5c 3.191.1c 2.990.5c 1.090.5c 14.494.7c
Zygotic 4.191.3d 2.591.1d 3.790.9d
ous stages of development to perform detailed biochemical and molecular studies. Somatic bryos offer a virtually unlimited supply of em-bryos at every stage and these emem-bryos may be useful in discovering some of the key aspects of phytate production, which could then be further explored in zygotic embryos. Somatic embryos offer the potential to learn about aspects of min-eral nutrition that have, for various reasons, been very difficult to study in natural zygotic embryos.
Acknowledgements
This work was supported by Natural Sciences and Engineering Research Council of Canada (NSERC) grants awarded to John N.A. Lott and Larry C. Fowke and an NSERC/industrial grant with Pacific Regeneration Technologies Inc. (Vic-toria, BC) awarded to Larry C. Fowke and Stephen M. Attree. We would like to thank Mar-cia West, Klaus Schultes and Tim Dament for their advice and technical assistance in this study.
References
[1] J.N.A. Lott, E. Spitzer, X-ray analysis studies of ele-ments stored in protein body globoid crystals ofTriticum grains, Plant Physiol. 66 (1980) 494 – 499.
[2] J.A. Chandler, X-ray microanalysis in the electron mi-croscope, in: A.M. Glauert, (Ed.), Practical Methods in Electron Microscopy, Elsevier/North-Holland Biomedi-cal Press, Amsterdam, The Netherlands, 1977, pp. 327 – 518.
[3] D.J. Cosgrove, The chemistry and bio-chemistry of inos-itol polyphosphates, Rev. Pure Appl. Chem. 16 (1966) 209 – 224.
[4] L.F. Johnson, M.E. Tate, Structure of ‘phytic acids’, Can. J. Chem. 47 (1969) 63 – 73.
[5] J.N.A. Lott, Accumulation of seed reserves of phospho-rus and other minerals, in: D.R. Murray (Ed.), Seed Physiology, vol. 1 Development, Academic Press, Aus-tralia, 1984, pp. 139 – 166.
[6] M.M. West, J.N.A. Lott, Studies of mature seeds of elevenPinusspecies differing in seed weight. II. subcellu-lar structure and localization of elements, Can. J. Bot. 71 (1993) 577 – 585.
[7] J. Pittermann, M. West, J.N.A. Lott, Characterization of globoids and iron-rich particles in cotyledons of Pinus banksiana seeds and seedlings, Can. J. For. Res. 26
(1996) 1697 – 1702.
[8] B.B. Hyde, A.J. Hodge, A. Kahn, M.L. Birnstiel, Studies on phytoferritin. I. identification and localization, J. Ultrastruct. Res. 9 (1963) 248 – 258.
[9] S.M. Attree, M.K. Pomeroy, L.C. Fowke, Production of vigorous, desiccation tolerant white spruce (Picea glauca [Moench.] Voss.) synthetic seeds in a bioreactor, Plant Cell Rep. 13 (1994) 601 – 606.
[10] S.M. Attree, M.K. Pomeroy, L.C. Fowke, Development of white spruce (Picea glauca(Moench.) Voss) somatic embryos during culture with abscisic acid and os-moticum, and their tolerance to drying and frozen stor-age, J. Exp. Bot. 46 (1995) 433 – 439.
[11] J.N.A. Lott, D.J. Goodchild, S. Craig, Studies of mineral reserves in pea (Pisum sati6um) cotyledons using low-wa-ter-content procedures, Aust. J. Plant Physiol. 11 (1984) 459 – 469.
[12] P. Beecroft, J.N.A. Lott, Changes in the element compo-sition of globoids fromCucurbita maximaandCucurbita andreana cotyledons during early seedling growth, Can. J. Bot. 74 (1996) 838 – 847.
[13] I. Ockenden, J.N.A. Lott, Beam sensitivity of globoid crystals within seed protein bodies and commercially prepared phytates during X-ray microanalysis, Scanning Microsc. 5 (1991) 767 – 778.
[14] A. Stewart, H. Nield, J.N.A. Lott, An investigation of the mineral content of barley grains and seedlings, Plant Physiol. 86 (1988) 93 – 97.
[15] J.H. Zar, Biostatistical analysis, Prentice-Hall, Engle-wood Cliffs, NJ, 1984.
[16] J.N.A. Lott, Protein bodies in seeds, Nord. J. Bot. 1 (1981) 421 – 432.
[17] E.C. Brown, M.L. Heit, D.E. Ryan, Phytic acid: an analytical investigation, Can. J. Bot. 39 (1961) 1290 – 1297.
[18] D.E.C. Crean, D.R. Haisman, The interaction between phytic acid and divalent cations during the cooking of dried peas, J. Sci. Food Agric. 14 (1963) 824 – 833. [19] H.R. Skilnyk, J.N.A. Lott, Mineral analyses of storage
reserves of Cucurbita maxima and Cucurbita andreana pollen, Can. J. Bot. 70 (1992) 491 – 495.
[20] T. Wada, E. Maeda, An improved method for the reten-tion of globoids in aleurone grains in light microscopy and electron microscopy, Jpn. J. Crop Sci. 48 (1979) 206 – 213.
[21] J.N.A. Lott, P.J. Randall, D.J. Goodchild, S. Craig, Occurrence of globoid crystals in cotyledonary protein bodies ofPisum sati6umas influenced by experimentally induced changes in Mg, Ca and K contents of seeds, Aust. J. Plant Physiol. 12 (1985) 341 – 353.
[22] C.A. Prattley, D.W. Stanley, Protein-phytate interactions in soybeans. I. localization of phytate in protein bodies and globoids, J. Feed Biochem. 6 (1982) 243 – 245. [23] D.A. Reid, H.C. Ducharme, M.M. West, J.N.A. Lott,
Iron-rich particles in embryos of seeds from the family Pinaceae, Protoplasma 202 (1998) 122 – 133.