Full Terms & Conditions of access and use can be found at
http://www.tandfonline.com/action/journalInformation?journalCode=lpte20
Download by: [National Cheng Kung University] Date: 29 August 2016, At: 01:10
Polymer-Plastics Technology and Engineering
ISSN: 0360-2559 (Print) 1525-6111 (Online) Journal homepage: http://www.tandfonline.com/loi/lpte20
Properties of Polypropylene
(PP)/Ethylene-Propylene Diene Terpolymer (EPDM)/Natural
Rubber (NR) Vulcanized Blends: The Effect of
N,N-m-Phenylenebismaleimide (HVA-2) Addition
Halimatuddahliana & H. Ismail
To cite this article: Halimatuddahliana & H. Ismail (2008) Properties of Polypropylene (PP)/ Ethylene-Propylene Diene Terpolymer (EPDM)/Natural Rubber (NR) Vulcanized Blends: The Effect of N,N-m-Phenylenebismaleimide (HVA-2) Addition, Polymer-Plastics Technology and Engineering, 48:1, 25-33, DOI: 10.1080/03602550802539155
To link to this article: http://dx.doi.org/10.1080/03602550802539155
Published online: 17 Dec 2008.
Submit your article to this journal
Article views: 66
View related articles
Properties of Polypropylene (PP)/Ethylene-Propylene Diene
Terpolymer (EPDM)/Natural Rubber (NR) Vulcanized Blends:
The Effect of
N
,
N
-
m
-Phenylenebismaleimide (HVA-2) Addition
Halimatuddahliana
1and H. Ismail
21
Department of Chemical Engineering, Universitas Sumatera Utara, Indonesia 2
School of Materials and Mineral Resources Engineering, Universiti Sains Malaysia, Penang, Malaysia
This paper discusses process development, tensile properties, morphology, oil resistance, gel content, and thermal properties of polypropylene (PP)/ethylene-propylene diene terpolymer (EPDM)/ natural rubber (NR) vulcanized blends with the addition of N,N-m-phenylenebismaleimide (HVA-2) as a compatibilizer. Blends were prepared in several blend ratios in a Haake Polydrive with tempera-ture and rotor speed of 180C and 50 rpm, respectively. Results indi-cated that the combination of dicumyl peroxide (Dicup) with HVA-2 shows high torque development and stabilization torque as compared to the blend with Dicup vulcanization alone. In terms of tensile properties, the combination of Dicup with HVA-2 shows higher tensile strength, tensile modulus (M100), elongation at break, oil resistance, and gel content in all blend ratios compared to similar vulcanized blends with Dicup without HVA-2 addition. Scanning electron microscope (SEM) micrographs of the blends support that the cross-linking and compatibilization occur during the process of the vulcanized blend containing HVA-2. In the case of crystallinity of the blends, the addition of HVA-2 in Dicup vulcanized blend revealed a tendency for the percentage of crystallinity (Xc) to decrease. The addition of HVA-2 in Dicup vulcanization also pro-duced blends with good thermal stability dealing with the so-called coagent bridge formation.
Keywords Crystallinity; Dicup; EPDM; HVA-2; Natural rubber; Polypropylene; Thermal stability
INTRODUCTION
There are large combinations of thermoplastics and elastomers that are commercially available. The PP= EPDM blends are the most commonly used types of blends. However, replacement of EPDM with NR in the PP=EPDM blends has been considered due to the reduction of the cost. It has also been observed that the partial replacement of EPDM by NR exhibits lower properties of PP=EPDM blends[1]. Therefore, dynamic
vulcanization has been introduced in the PP=EPDM=NR blend to improve the properties of such blends. Some investigations have been reported regarding the dynamic vulcanization of thermoplastic elastomer, especially on PP=EPDM blends, and most of the investigations focused on the processing and the improvements on mechanical and physical properties of the blends[2–7]. Dynamic vulcani-zation has also been introduced in the PP=EPDM=NR blend using sulfur[8] and dicumyl peroxide (Dicup)[9] to improve the properties of such blends.
The presence of Dicup in the blends is to produce reactive radicals upon decomposition at elevated tempera-ture via exothermic reaction that is beneficial in a rubber compound. It is generally agreed that for the peroxide vul-canization of polyolefin and rubber, cross-links or chain scission may occur simultaneously. According to Ho et al.[10] the polymer free radicals in PP induced by the
peroxide decomposition lead to predominantly scission reaction, whereas in rubber, vulcanized properties of blends are determined by cross-link structures that form[11,12].
Coagents, in the other hand, can be used to compound elastomers, thermoplastics, and thermoplastic=elastomer blends with and without the presence of accelerators or peroxide. Investigatons have been reported regarding the use of coagent, especially HVA-2 and accelerators[13–15]. The addition of HVA-2 for peroxide cure is to eliminate practically all of the problems once considered to be asso-ciated with peroxide cure. According to Dikland et al.[16] and McElwee and Lohr[17], peroxide-coagent system can be visualized in a way similar to that which was postulated for the sulfurization of the rubber molecules by the action of accelerators. After decomposition of peroxide mole-cules, macroradicals are formed and subsequently the coagent incorporation process is expected to initiate by the vulcanization system. The polyfunctional molecules in coagent can also be incorporated into the cross-link
Address correspondence to H. Ismail, School of Materials and Mineral Resources Engineering, Universiti Sains Malaysia, 14300 Nibong Tebal, Penang, Malaysia. E-mail: [email protected]
ISSN: 0360-2559 print/1525-6111 online DOI: 10.1080/03602550802539155
structures during vulcanization, forming a so-called coagent bridge.
Keller[18] has suggested briefly that the process initiates
via thermal decompostition of the peroxide and how the coagent affects the reaction. A generalized scheme is shown as follows:
ð1ÞPeroxide!heat2R Peroxide decomposition
ð2ÞRþP!PþRH H abstraction
ð3Þ2P!PP Cross-link
ð4ÞPþR!PR No cross-link
ð5ÞP!P0þP¼ Scission
The cross-link is completed when the polymer radicals combine as in Reaction (3). If a coagent is present in the compound, Reactions (4) and (5) are repressed because the coagent rapidly combines with P to produce
stable radicals. The polyfunctional nature of the coagent also enhances Reaction (2). In addition, the presence of a reactive olefinic site as in the terpolymer shifts Reac-tion (2) from the polymer backbone to the pendant unsa-turation, thus intensifying Reaction (3) and suppressing Reaction (5).
A previous paper[19] reported that in terms of tensile properties and degree of cross-linking, the presence of HVA-2 in Dicup vulcanization significantly affects the extent of recombination of macroradicals (from chain scis-sion) and improves the cross-lining heat efficiency, which can be attributed to the formation of coagent bridge in PP=EPDM=NR blends. It was concluded that the sup-pression of chain scission and the formation of coagent bridges contributes to an improvement of the cross-linking efficiency. This paper discusses the role of HVA-2 in Dicup vulcanization of PP=EPDM=NR blends and how it can influence the process development, tensile properties, mor-phology, oil resistance, gel content, and thermal properties of PP=EPDM=NR blends.
EXPERIMENTAL
Materials
Polypropylene (PP) homopolymer used in this study was an injection-molding grade, supplied by Titan PP Polymers (M) Sdn Bhd, Johor, Malaysia (TITANPRO 6331 grade), with a melt flow index (MFI) value of 14 gr=10 min at 230C and 2.16 kg. Ethylene-propylene diene terpolymer (EPDM-EPT 3072E), with Mooney Viscosity ML (1þ4) at 100C of 74 was purchased from Luxchem Trading Sdn. Bhd. Natural rubber (NR-HSL) with Mooney vis-cosity ML (1þ4) at 100C of 73 was obtained from Hokson Rubber Trading Sdn. Bhd, Seremban. Dicumyl peroxide (Dicup) was purchased from Aldrich. The coagent used for the blend was N,N-m-phenylenebismaleimide
(HVA-2), a free radical cross-linking agent from DuPont Dow Elastomer.
Preparation and Processing
Studies were conducted on PP=EPDM=NR blends con-sisting of two systems viz. blend with Dicup vulcanization and blend with the combination of Dicup vulcanization and HVA-2, where each blend covered different blend compositions viz. 50=40=10, 50=30=20, 50=20=30, 50=10=40, and 50=0=50. Blends were prepared by melt mix-ing in an internal mixer, Haake Polydrive with Rheomix R600=610, at temperature and rotor speed of 180C and 50 rpm, respectively. Table 1 shows the mixing sequence of components in preparation of the PP=EPDM=NR blends. During blending, thermoplastics (PP) were first loaded into the internal mixer and pre-mixed for 2 min fol-lowed by the rubbers (EPDM and NR). Mixing times were determined based on the recorded torque at stable value confirming the homogeneous mixing of the blends. For the dynamic vulcanization process, Dicup was added at 5 min of mixing and the mixing time was set for 8 min. The corresponding blends with combination of Dicup and HVA-2 had mixing time further increased to 10 min. The samples were then sheeted by passing through a 2 roll-mill and allowed to cool at room temperature.
Tensile Properties
Tensile tests were carried out according to ASTM D412 on an Instron machine; 2-mm thick dumbbell specimens were cut from the molded sheets with a Wallace die cutter.
TABLE 1
Mixing sequence of components in preparation of the PP=EPDM=NR blends
0 PP loading PP loading
2 Rubbers addition Rubbers addition 5 DCP addition
aIrganox 1010 (0.4 phr). b
Based on the rubbers content (EPDM and NR).
cBased on the NR content.
The specimen was tested using a constant rate (50 mm= min) at room temperature of 25C. The results were quoted based on the average value of five specimens tested for each blend system.
Morphology Studies
Morphological evaluations of PP=EPDM=NR surfaces were done using a scanning electron microscope (SEM), model Stereoscan 200 Cambridge. The vulcanized samples were etched with nitric acid for 2 days, washed with water, and then dried. All the samples were mounted on alumi-num stubs and sputter-coated with a thin layer of gold to avoid electrostatic charging during examination. The examinations were done within 24 h of preparation.
Swelling Test
Determination of the swelling index was carried out according to ASTM D471. The specimens with dimensions of 30 mm5 mm2 mm were cut and weighed using an electrical balance. The test pieces were then immersed in oil (IRM 903) for 48 h at room temperature. The samples were then removed from oil, wiped with tissue paper to remove excess liquid from the surface, and weighed. The swelling index of the blends was then calculated as follow:
Swelling index¼W2
W1
100% ð1Þ
WhereW1andW2are weight of specimen before and after
immersion, respectively.
Gel Content
The degree of cross-linking in the rubber was measured after extraction in boiling cyclohexane for 8 h. The samples were dried at 80C for 30 min and subsequently weighed. The percentage of gel content of the blends was then calcu-lated as follows:
% gel content¼Wg
Wo
100% ð2Þ
Where Wg and W0 are sample weights after and before
extraction, respectively.
Differential Scanning Calorimetry (DSC) Study
Thermal analysis measurements of selected blend sys-tems were performed using a DSC-7 Perkin Elmer differen-tial scanning calorimeter. The samples were programmed and heated at 20C=min to about 200C and maintained at this temperature for 10 min to ensure a complete melting of the crystals. The melting temperature (Tm) and the heat of fusion (DHf) were measured during the heating cycle. The parameter percentage of crystallinity (Xc) was calcu-lated by dividing the measured DHf by the DHf of 100%
polypropylene. The DHf for polypropylene as a fully crystalline material is 209 J=g[20].
Thermogravimetric Analysis (TGA)
Thermogravimetric analysis of the blends was carried out using a Perkin Elmer Pyris 6 TGA analyzer at tempera-tures between 30 and 600C at nitrogen airflow of 50 ml=min and a heating rate of 20C=min.
Ageing Test
The ageing test was done according to ASTM D573. Dumbbell specimens were hung in an air oven at the temperature of 100C for 72 h. At the end of the test period, the specimens were removed from the oven, cooled at room temperature, and allowed to rest for at least 16 h before determination of tensile properties. Retention in properties was calculated as
Properties retentionð%Þ ¼A=O100% ð3Þ
Where A is the value after ageing, and O is the original value.
RESULTS AND DISCUSSION
Process Development
The comparison of stabilization torque values between Dicup vulcanized blend with HVA-2 and vulcanized blend without HVA-2 are presented as a function of blend com-positions in Fig. 2. It shows that the Dicup vulcanized blend with HVA-2 exhibits higher stabilization torque compared with the Dicup vulcanized blend only. In this case, the extended cross-linking and inhibition of chain scission by HVA-2 led to an increase in viscosity of the blend and hence the torque. With the addition of HVA-2 in Dicup vulcanization, even though the radicals are formed by peroxide, the coagent is sufficient to bridge and stabilize the PP macroradicals. As the coagent was added, a dramatic increase of melt viscosity was obtained. This implies that macroradicals formed by peroxide are mostly reacted with HVA-2. Furthermore, the stabilization torque increases with the increase of NR content. This indi-cates that the effect of HVA-2 on the Dicup vulcanized blend was more significant than on the NR richer blend.
Tensile Properties
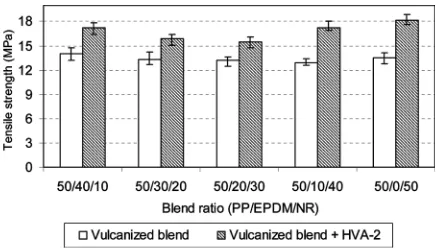
The effect of Dicup vulcanization combined with HVA-2 addition on tensile strength of PP=EPDM=NR blends as
compared with the Dicup vulcanized blend alone is presented in Fig. 3. The tensile strength of PP=EPDM=NR NR blends registers a marked improvement with the com-bination of Dicup and HVA-2. Here, the cross-link becomes more pronounced in the blend with the combi-nation of Dicup and HVA-2 as indicated in Fig. 3. The data demonstrates that the Dicup vulcanized of 50=0=50 PP=EPDM=NR blend gave the highest value in tensile strength, 13.5 MPa to 18.2 MPa, which is about a 35%
increase. This can be attributed to the extensive cross-link formation in the vulcanized blends by the addition of HVA-2. The increase of the cross-linking efficiency in the presence of coagents in the peroxide vulcanization was also reported by Dikland et al.[16]. As mentioned before, the coagent affecting molecular structure of the cross-links where the coagent molecules can be incorporated into the cross-link structure during vulcanization of rubber forming is a so-called coagent bridge. The suggested reaction sequence of cross-linking in rubber phase due to the com-bination of Dicup and HVA-2 is shown in Fig. 4. In addition, the presence of coagent molecules in Dicup vulca-nization have also suppressed unwanted side reactions, such as chain scission of PP[16]. So, the previous obser-vation suggested that the HVA-2 in Dicup vulcanization acted as an effective compatibilizer to increase the adhesion between the phases due to production of a coagent bridge. The increased interfacial adhesion, which facilitates the stress transfer mechanism within the blend, is also expected, and hence the improvement of tensile strength occurred.
The introduction of HVA-2 on the Dicup vulcanization blend has also slightly increased the tensile modulus of the blends, as can be seen in Fig. 5. The Dicup vulcanized (50=0=50) blend containing HVA-2 generated the highest tensile modulus (M100) of 10.3 MPa, increasing 7.3%from
the blend without HVA-2 addition (9.5 MPa). Here again, the formation of extensive cross-linking and compatibiliza-tion improved the stiffness of the blend. This finding was
FIG. 1. The torque development of Dicup vulcanization of PP=
EPDM=NR blends with and without HVA-2 addition.
FIG. 2. The comparison of stabilization torque between Dicup vulcanization with and without HVA-2 addition.
FIG. 3. The effect of Dicup vulcanizationþHVA-2 addition on tensile strength of PP=EPDM=NR blends.
due to the formation of the interfacial cross-linking via formation of a coagent bridge.
Figure 6 shows the comparison of elongation at break of Dicup vulcanized blends with and without HVA-2 addition. It can be seen that the elongation at break of the vulcanized blend containing HVA-2 is higher than that without HVA-2 for each blend composition. This is clearly due to the inherent ductility as a result of the presence of a coagent bridge, which increases the deformability of the
molecules. In addition, the improvement of elongation at break may also be caused by the restriction of the chain scission by the addition of HVA-2. The introduction of HVA-2 in Dicup vulcanization results in the recombination and functionalization of macroradicals competing with the degradation of tertiary alkyl radicals of PP. This indicates that the cross-linking predominates over chain scission, resulting in greater resistance at break. Further, the addition of HVA-2 in Dicup vulcanized blend has
FIG. 4. Reaction sequence of crosslinking in rubber phase by peroxide and HVA-2[11].
FIG. 5. The effect of Dicup vulcanizationþHVA-2 addition on tensile modulus (M100) of PP=EPDM=NR blends.
improved interfacial adhesion between phases due to the low interfacial tension leading to better and finer disper-sion and reduction in the dispersed size as will be discussed in the following section.
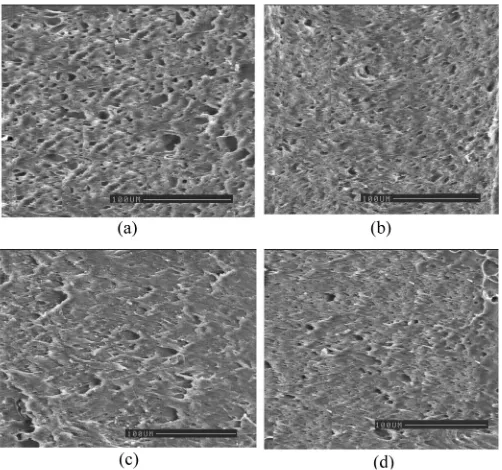
Morphology Study
Figures 7a–d compares the extracted surface morpholo-gies of Dicup vulcanized PP=EPDM=NR blends with and without HVA-2 addition. Through the addition of HVA-2 in vulcanized blends (Figs. 7b and d), the particles are smaller, better dispersed, and more evenly distributed in the PP matrix as compared to Dicup vulcanized blends alone (Figs. 7a and c). It is apparent from the micrographs that the numbers of holes due to the extraction of rubber particles are reduced significantly. This observation indicates that the combination of Dicup vulcanization with HVA-2 has cross-linked the rubber phase and increased interfacial adhesion resulting from the formation of the coagent bridge. After the cross-linking of the rubber particles, the tendency of the rubber particles to dissolve by the solvent decrease.
Although a detailed distribution analysis on the dispersed particles was not carried out in this work, the decrease of the dimension and number of voids could be explained by the production of coagent bridge between PP and rubber in PP=EPDM=NR blends under dynamic cross-linking conditions. The same observation was obtained by Wu[22], who indicated that if the graft
copolymer of plastic and rubber was produced predomi-nantly at an early stage in the dynamic cross-linked pro-cess, it would play a role of compatibilizer to reduce the dimension and number of the particles dramatically. There-fore, this morphology shows evidence for the improvement of the properties of Dicup vulcanized blend with HVA-2.
Swelling Index
The result of oil resistance of Dicup vulcanized blend with and without HVA-2 addition based on the swelling index after oil immersion at room temperature for 48 h is presented in Table 2. It is obvious that the oil resistance increased for Dicup vulcanized blend containing HVA-2 as indicated by the low values of swelling index compared to Dicup vulcanized blend alone. The magnitude of decrease in swelling index of the blend increases with increasing NR content. The higher magnitude changes are observed in the Dicup vulcanized 50=0=50 PP=EPDM=NR blend as shown in Table 2. This indicates that cross-link formation in NR richer content is more pre-dominant. These values represent tight cross-linking of the rubber chains, therefore less oil penetrated into the rubber particles.
Gel Content
The effect of HVA-2 addition on the Dicup vulcaniza-tion on the percentage of gel content of PP=EPDM=NR blend is depicted in Table 3. The amount of gel content determined after Soxhlet extraction of uncross-linked rub-ber phase is in agreement with those determined from swelling index. Gel is formed when a strong interaction between components of the blend exists. It is an indicator of cross-links and compatibilization formed in the blend. Upon addition of HVA-2 in the Dicup vulcanized blend, there is an increase in percentage of gel. This is again associated with the increase of cross-link efficiency due to the formation of a coagent bridge by HVA-2.
FIG. 7. SEM micrographs of extracted surface PP=EPDM=NR blends: (a) Dicup vulcanized 50=30=20, (b) Dicup vulcanized 50=30=
20þHVA-2, (c) Dicup vulcanized 50=0=50, and (d) Dicup vulcanized 50=0=50þHVA-2.
TABLE 2
Comparison of the swelling index between dicup vulcanized PP=EPDM=NR blends with and without
HVA-2 addition
Thermal Analysis
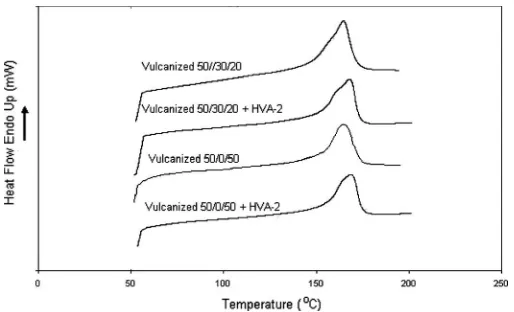
Differential Scanning Calorimetry (DSC)
Figure 8 presents the DSC scan of Dicup vulcanized blends with and without addition of HVA-2 for two dif-ferent blend compositions of PP=EPDM=NR blends. The DSC outputs show characteristics corresponding to the PP phase, where it is mostly governed by crystalline PP alone. This is because rubber is an amorphous poly-mer[23]. However, in the Dicup vulcanized 50=30=20 PP=EPDM=NR blend with HVA-2, a small peak in the shoulder of the endothermic peak is observed, implying that the shape of the melting endothermic was modified by HVA-2 addition. As can be seen, the sharp endo-thermic peak of Dicup vulcanized 50=30=20 PP=EPDM= NR blend without HVA-2 was broadened with HVA-2. This indicates that the chain scission of PP in EPDM molecule was limited by the addition of HVA-2. In addition, the small shoulder in the 50=30=20 PP=EPDM= NR endothermic peak may be related to the formation of a new crystal and change in crystal structure by HVA-2. However, this peak disappeared in the 50=0=50 PP= EPDM=NR blend.
The detailed thermal properties of such blends derived from DSC scan thermograms are tabulated in Table 4.
The PP melting peak (Tm) of Dicup vulcanized blend is shifted toward higher temperatures by the presence of HVA-2, indicating an effective cross-linking of the rubbers. Theoretically, in the thermodynamic sense, the chain scis-sion reactions cause a decrease in the melting temperature by increasing the melt entropy[24]. In this case, melting tem-perature (Tm) can be affected by the effect of scission and cross-linking of PP molecules. Therefore, these results con-firm that the cross-linking was predominant over the chain scission.
The analysis of the DSC traces further reveal that the presence of HVA-2 in Dicup vulcanization has a significant nucleating effect on the blend. The coagent bridge on the PP=rubber interface can improve the phase adhesion, cre-ating additional links between the phases that may contrib-ute to a more effective transmission of stress. However, as can be seen in Table 4, the percentage of crystallinity of the Dicup vulcanized blend decreases with the addition of HVA-2. A cross-linked structure produced under dynamic vulcanization may restrict the spherulitic growth and hence decrease the degree of crystallinity of PP[25]. Here, the reduction of percentage of crystallinity, which leads to the increase of network, was due to the extended cross-linking by HVA-2. Furthermore, the incorporation of the coagent bridge between the phases might cause the restric-tion of the mobility of the PP segment, leading to the lack alignment in the crystal lattice, thus reducing percentage of crystallinity.
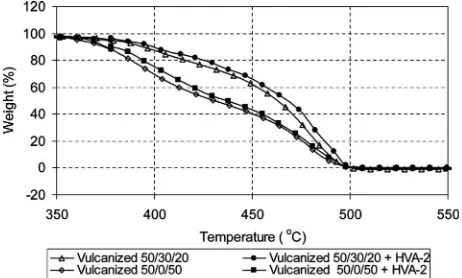
Thermogravimetric Analysis
Thermograms of Dicup vulcanized PP=EPDM=NR blends with and without HVA-2 addition are shown in Fig. 9. It can be observed that a slower degradation of the Dicup vulcanized blend containing HVA-2 over the Dicup vulcanized blend alone took place. Here, the degradation temperatures of the Dicup vulcanized blends containing HVA-2 were shifted to slightly higher TABLE 3
Gel content of selected cicup vulcanized PP=EPDM=NR blend with and without HVA-2 addition
Gel content (%)
FIG. 8. The effect of Dicup vulcanizationþHVA-2 addition on DSC scans of PP=EPDM=NR blends.
TABLE 4
Thermal properties of selected dicup vulcanized PP=EPDM=NR blends containing HVA-2 derived from
DSC scan thermogram as compared without HVA-2
PP=EPDM=NR Dicup vulcanized blendþHVA-2
temperature. This is due to the presence of HVA-2, which formed a coagent bridge during processing that hinders easy transport of thermal energy across the material during heating and subsequently protects the blend from degra-dation. This can slow down the formation of degradation products. The initial decomposition temperature increased with the addition of HVA-2, which is due to the predomi-nant effect of HVA-2 in NR.
The effect of HVA-2 addition on the thermal stability of Dicup vulcanized PP=EPDM=NR blends is depicted in Fig. 10. It is clear that the percentage of weight loss of the Dicup vulcanized blends containing HVA-2 at any tem-perature is lower than those of the blend without HVA-2. This is observed from the curves, where the Dicup vulca-nized blends containing HVA-2 lie above the blends with-out HVA-2 for every percentage of weight loss (20 to 90%). Here, HVA-2 brings out stability in the phase due
to the introduction of cross-links as well as formation of intramolecular cross-linking (coagent bridge)[16]. This could also be understood by SEM analysis, where the mor-phology of the vulcanized blend containing HVA-2 shows
more stable morphology, which is responsible for enhanc-ing the thermal stability of the systems. Therefore, it is observed that when compared with the Dicup vulcanized blend alone, Dicup vulcanization combined with HVA-2 system demonstrated better thermal stability.
Heat Resistance
The percentage of retention of tensile strength and elon-gation at break of blends after hot air ageing for 72 h (100C) is shown in Table 5. The deterioration of the tensile properties of the Dicup vulcanized blend containing HVA-2 after ageing has been monitored. The related results, given in Table 5, demonstrate that the Dicup vulcanized blend containing HVA-2 shows higher retention, which is a hint for good heat resistance compared to Dicup vulca-nized alone. According to the work that has been done by McElwee and Lohr[18], vulcanized blends lead to an increase in the cross-linking density and restriction of degradation and hence in tensile properties. The higher the cross-linking level, the smaller the amount of oxygen that penetrates into the rubber particles, resulting in the increase of the percentage of retention. The cross-linking process protected these polymers from extended oxidation. This effect is more pronounced for richer NR content and indicates further cross-linking in the NR phase. Here, the presence of Dicup combined with HVA-2 reduces the NR degradation during ageing. The HVA-2 is also capable of reacting with the polymer radicals formed by chain scission during blending because the chain scissions are correlated with oxidation processes.
CONCLUSION
The formation of the coagent bridge and the inhibition of chain scission by HVA-2 in Dicup vulcanization lead to an increase in the viscosity of the PP=EPDM=NR blend,
FIG. 9. The effect of HVA-2 addition on Dicup vulcanized blend on the TGA scan of PP=EPDM=NR blends.
FIG. 10. The effect of HVA-2 addition on Dicup vulcanized blend on the thermal stability of PP=EPDM=NR blends.
TABLE 5
Percentage of retention of tensile and elongation at break of cicup vulcanized blends with and without addition
HVA-2
which subsequently increased the stabilization torque of the blend. The incorporation of HVA-2 in vulcanized blends increases tensile properties, oil resistance, and gel content. The SEM micrographs from the surface extraction of the blends also support that the coagent bridge occurred during Dicup vulcanization of PP=EPDM=NR containing HVA-2. Meanwhile, the incorporation of HVA-2 in the Dicup vulcanization blend decreased the percentage of the crystallinity of blend. In addition, the presence of HVA-2 in the blends slows down the degradation and consequently produces a heat stable blend.
REFERENCES
1. Halimatuddahliana; Ismail, H. Polym. Plast. Tech. Eng.2004,43, 357. 2. Coran, A.Y.; Patel, R. Rubb. Chem. Technol.1980,53, 141. 3. Lopez-Manchado, M.A.; Arroyo, M. Rubb. Chem. Technol.2001,74, 211. 4. Sabet, S.A.; Puydak, R.C.; Rader, C.P. Rubb. Chem. Technol.1996,
69, 476.
5. Ellul, M.D. Rubb. Chem. Technol.2003,76, 202.
6. Xiao, H.; Huang, S.; Jiang, T.; Cheng, S. J. Appl. Polym. Sci.2002,83, 315. 7. Sariatpanahi, H.; Nazokdast, H.; Dabir, B.; Sadaghiani, K.;
Hemmati, M. J. Appl. Polym. Sci.2002,86, 3148.
8. Ismail, H.; Halimatuddahliana; Akil, H. Md. J. Solid State Sci. Technol. Lett.2004,11(2), 105.
9. Halimatuddahliana; Ismail, H.; Akil, H. Md. Inter. J. Polym. Mater.
2004,54, 1169.
10. Ho, R.M.; Su, A.C.; Wu, C.H.; Chen, S.I. Polym.1993,34, 3264. 11. Coran, A.Y. In:Encyclopedia of Polymer Science and Engineering, Mark,
H.F.; Bikales, N.M.; Overberger, C.G.; Menges, G.; Kroschwitz, J.I., Eds., 2nd Ed., Canada: John Wiley and Sons, Inc., 1989, 17, 666. 12. Dluzneski, P.R. Rubb. Chem. Technol.2001,74, 451. 13. Inoue, T.J. Appl. Polym. Sci.1994,54, 709.
14. Inoue, T.J. Appl. Polym. Sci.1994,54, 723.
15. Ishikawa, M.; Sugimoto, M.; Inoue, T.J. Appl. Polym. Sci.1996,62, 1495.
16. Dikland, H.G.; Does, V.D.; Bantjes, A. Rubb. Chem. Technol.1992,
66, 196.
17. McElwee, C.B.; Lohr, J.E. Rubber World2001,225, 41. 18. Keller, R.C. Rubb. Chem. Technol.1988,61, 238.
19. Halimatuddahliana; Ismail, H.; Akil, H. Md. Polym. Plast. Tech. Eng.
2005,44, 1217.
20. Greco, R.; Manacarella, C.; Martuscelli, E.; Ragosta, G. Polym.1987,
28, 1929.
21. Dahlan H.M.; Khairul Zaman, M.D.; Ibrahim, A. J. Appl. Polym. Sci.2000,78, 1776.
22. Wu, S. Polym. Eng. Sci.1987,27, 334.
23. Kim, B.C.; Hwang, S.S.; Lim, K.Y.; Yoon, K.J. J. Appl. Polym. Sci.
2000,78, 1267.
24. Stojanovic, Z.; Kacarevic-Popovic, Z.; Galovic, S.; Milicevic, D.; Suljovrujic, E. Polym. Degrad. Stab.2005,87, 279.
![FIG. 4.Reaction sequence of crosslinking in rubber phase by peroxide and HVA-2[11].](https://thumb-ap.123doks.com/thumbv2/123dok/3907861.1860187/6.612.149.462.68.381/fig-reaction-sequence-crosslinking-rubber-phase-peroxide-hva.webp)