Genes encoding critical steps in the synthesis of osmoprotectant compounds are now being expressed in transgenic plants. These plants generally accumulate low levels of osmoprotectants and have increased stress tolerance. The next priority is therefore to engineer greater osmoprotectant synthesis without detriment to the rest of metabolism. This will require manipulation of multiple genes, guided by thorough analysis of metabolite fluxes and pool sizes.
Addresses
*Department of Horticultural Sciences, University of Florida, Gainesville, FL 32611-0690, USA
†Department of Horticulture, Purdue University, West Lafayette,
IN 47907-1165, USA
‡e-mail: [email protected]
Current Opinion in Plant Biology1999, 2:128–134 http://biomednet.com/elecref/1369526600200128 © Elsevier Science Ltd ISSN 1369-5266
Abbreviations
GlyBet glycine betaine
MCA metabolic control analysis
Introduction
Improving crop resistance to osmotic stresses is a long-standing goal of agricultural biotechnology [1,2•]. Drought, salinity and freeze-induced dehydration consti-tute direct osmotic stresses; chilling and hypoxia can indirectly cause osmotic stress via effects on water uptake and loss. Soil salinity alone affects some 340 mil-lion hectares of cultivated land [2•]. To withstand osmotic stresses, certain plants have evolved a high capacity to synthesize and accumulate non-toxic solutes (osmoprotectants or compatible solutes), predominantly in the cytoplasm, as part of an overall mechanism to
raise osmotic pressure and thereby maintain both turgor and the driving gradient for water uptake [3]. Many microorganisms also produce osmoprotectants [4]. Figure 1 shows the structures of some common osmo-protectant compounds; they fall into three groups — amino acids (e.g. proline), onium compounds (e.g. glycine betaine, dimethylsulfoniopropionate), and polyols/sugars (e.g. mannitol, D-ononitol, trehalose). Being non-toxic, osmoprotectants can accumulate to osmotically significant levels without disrupting metabo-lism; some of them can also protect enzymes and membranes against damage from high salt concentra-tions [4] and others (especially polyols) protect against reactive oxygen species [5].
The accumulation of osmoprotectants has been a target for plant genetic engineering for more than 15 years [6], and work is now in progress on all the compounds in Figure 1. In several cases, introduction of a single foreign gene into a transgenic plant has led to modest accumulation of an osmoprotectant and, apparently in consequence, a small increase in stress tolerance. The physiological and agricul-tural implications of these experiments have been thoroughly reviewed [2•,5,7–9,10•]. We will focus here on the metabolic implications of the genetic engineering work, and on what needs to be done to drive more flux towards osmoprotectant synthesis, with emphasis on glycine betaine, polyols and trehalose.
Overview of progress in engineering
osmoprotectant synthesis
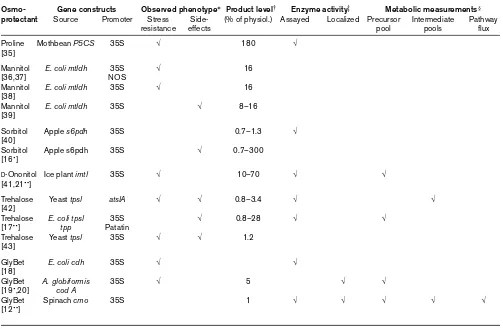
Table 1 catalogs the osmoprotectant work published to date, carried out with tobacco, Arabidopsis, rice and potato. The table illustrates several important points. First, many of the transgenes are of a non-plant origin, which reflects
Metabolic engineering of plants for osmotic stress resistance
Michael L Nuccio
*
, David Rhodes
†
, Scott D McNeil
*
and
Andrew D Hanson
*
‡
Figure 1
N+ COO
-Glycine betaine
S+
COO
-3-dimethylsulfoniopropionate
OH OH
OH O
CH2OH O
O OH OH HO
H2COH
Trehalose
N+ COO
-Proline
CH2OH
CH2OH OH
OH OH OH
Mannitol
CH2OH
CH2OH OH OH OH
OH
Sorbitol OH
CH3O OH
OH
OH OH
D-ononitol
Current Opinion in Plant Biology
the lack of investment in plant biochemistry. The plant gene pool should not be overlooked, as plants have evolved unique genes to synthesize osmoprotectants [11], and plant genes can possess particularly useful characteris-tics. For example, in transgenic tobacco, choline monooxygenase (CMO) is stabilized by salt-stress via a post-transcriptional mechanism, which leads to higher CMO activity when it is most needed [12••]. Second, most of the transgenes have been expressed from a constitutive promoter. Tissue-specific and inducible promoters eventu-ally will be necessary, and could be developed from genes known to be induced when and where high osmoprotec-tant synthesis is required [13,14]. Third, most of the plants described express a single transgene and so represent only the first step in the engineering process, which is by nature iterative [1]. Finally, very few of the transgenic plants pro-duced to date have been thoroughly analyzed at the metabolic level. This is critical because most of them accu-mulate only small amounts of the desired osmoprotectant,
and further progress requires that the metabolic constraints be identified.
What are these constraints likely to be? First, certain meta-bolic networks can be rigid in that they have evolved to maintain metabolite flux distributions that are optimal for growth, and oppose any flux redistribution following expression of a transgene [15]. Second, the engineered pathway may divert flux away from primary metabolism and so create undesirable side effects [16•]. Finally, a for-eign metabolite may be degraded, limiting its accumulation [17••]. We will now use published results to illustrate these points.
Glycine betaine synthesis
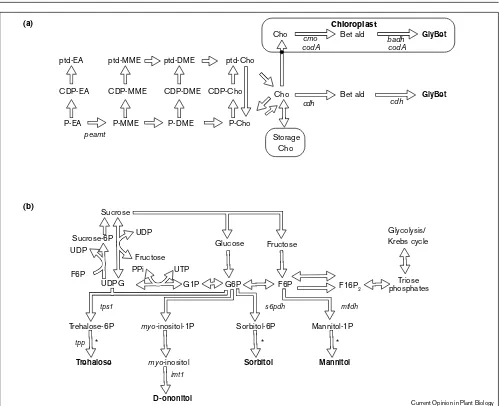
Glycine betaine (GlyBet) is synthesized in the chloroplast in two steps from choline (Figure 2a) and can accumulate to high levels (>20 mM on a tissue water basis, Table 1) in plants such as spinach and sugar beet under osmotic stress.
Table 1
Experiments designed to metabolically engineer osmoprotectant biosynthesis
Osmo- Gene constructs Observed phenotype* Product level† Enzyme activity‡ Metabolic measurements§ protectant Source Promoter Stress Side- (% of physiol.) Assayed Localized Precursor Intermediate Pathway
resistance effects pool pools flux
Proline Mothbean P5CS 35S √ 180 √
[35]
Mannitol E. coli mtldh 35S √ 16
[36,37] NOS
Mannitol E. coli mtldh 35S √ 16
[38]
Mannitol E. coli mtldh 35S √ 8–16
[39]
Sorbitol Apple s6pdh 35S 0.7–1.3 √
[40]
Sorbitol Apple s6pdh 35S √ 0.7–300
[16•]
D-Ononitol Ice plant imtl 35S √ 10–70 √ √
[41,21••]
Trehalose Yeast tpsl atslA √ √ 0.8–3.4 √ √
[42]
Trehalose E. coli tpsl 35S √ 0.8–28 √ √
[17••] tpp Patatin
Trehalose Yeast tpsl 35S √ √ 1.2
[43]
GlyBet E. coli cdh 35S √ √
[18]
GlyBet A. globiformis 35S √ 5 √ √
[19•,20] cod A
GlyBet Spinach cmo 35S 1 √ √ √ √ √
[12••]
*A tick under ‘stress resistance’ indicates that the transgenic plant displayed more resistance than controls. A tick under side-effects indicates that transgenic plants had a growth defect. †This column
reports the level of osmoprotectant synthesis in osmotically-stressed transgenic plants as a percentage of the level found in a representative plant that naturally accumulates the osmoprotectant in similar conditions. These levels (given in mM in tissue water) are:
proline 36mM [35]; polyols 50mM [44]; trehalose 50mM [17••]; GlyBet 24mM [45]. ‡Indicates that the enzyme activity of the
transgene product was assayed and localized to a subcellular compartment. §Indicates measurements were made of the
Several groups have reported engineering GlyBet synthe-sis by introducing choline-oxidizing genes from E. coli[18],
Arthrobacterspp. [19•,20], and spinach [12••]. GlyBet levels in the transgenic plants represented just a few percent of those found in plants that naturally accumulate it. Two groups have shown that supplying choline in axenic cul-tured plants enhanced GlyBet synthesis, indicating a constraint in choline synthesis ([12••], Huang et al. abstract in Plant Physiol 1997, 114S:120). Detailed analysis of choline metabolism in tobacco demonstrates that it is embedded in a rigid network in which choline is directed almost exclusively to phosphatidyl-choline synthesis, mak-ing it difficult to divert choline to GlyBet [12••]. Also, in
tobacco de novocholine synthesis is probably constrained at the step catalyzed by phosphoethanolamine-N -methyl-transferase [12••]. In this context, a comparison of the demand for choline moieties between a plant that lacks GlyBet and a plant that synthesizes it is instructive. Figure 3 makes clear that GlyBet synthesis represents a huge demand on choline synthesis, requiring more than 90% of the choline moieties. This suggests that the choline synthesis pathway is far more active in GlyBet accumula-tors. Increasing the choline supply in tobacco will require additional engineering steps. One possibility is to increase choline synthesis Via up-regulation of phospho-ethanolamine-N-methyltransferase activity.
Figure 2
F6P UDP
UDP
Fructose
F6P F16P2
G6P G1P
UDPG
Trehalose-6P
Trehalose
Sorbitol-6P
Sorbitol
Mannitol-1P
Mannitol myo-inositol-1P
myo-inositol
D-ononitol
* *
*
mtldh s6pdh
Triose phosphates Sucrose
Fructose Glucose
tps1
imt1 tpp
UTP PPi
Glycolysis/ Krebs cycle Cho
Storage
Cho Bet ald GlyBet
badh cmo
Chloroplast
P-EA ptd-EA
P-MME ptd-MME
P-DME ptd-DME
P-Cho ptd-Cho
Cho
CDP-EA CDP-MME CDP-DME CDP-Cho Bet ald GlyBet
cdh cdh
codA codA
peamt
Sucrose-6P
Current Opinion in Plant Biology (a)
(b)
Metabolic pathways associated with GlyBet, polyol and trehalose synthesis. (a)GlyBet synthesis (modified from [12••]) and (b)polyol
and trehalose synthesis. The osmoprotectants are given in bold. Abbreviations are CDP-, cytidyldiphospho-; EA, ethanolamine; MME, monomethylEA; DME, dimethylEA; Bet ald, betaine aldehyde; cmo, choline monooxygenase; nsdh, endogenous non-specific aldehyde
Polyol synthesis
In some plants polyol synthesis is upregulated in response to osmotic stress, leading to significant polyol accumula-tion (up to 50 mM on a tissue water basis, Table 1). Polyols are derived from sugar phosphates by reduction and dephosphorylation (Figure 2b). Mannitol, sorbitol and D-ononitol synthesis have been introduced into transgenic plants. Two reports are of particular interest from a meta-bolic standpoint; one illustrates a competitive or antagonistic effect between transgene activity and host-plant metabolism, and the other a synergistic effect. First, analysis of sorbitol levels in transgenic tobacco showed that high sorbitol accumulation was associated with growth defects and necrosis. This was attributed to myo-inositol depletion, although sorbitol toxicity and cytosolic Pi deple-tion due to sorbitol-6-P build-up were not ruled out [16•]. Second, in tobacco, the myo-inositol pool expands as the plants are salt- or drought-stressed, and in plants engi-neered to synthesize D-ononitol from myo-inositol, the increased precursor supply makes D-ononitol production stress inducible [21••].
Trehalose synthesis
The non-reducing disaccharide trehalose is believed to enable desiccation resistant organisms to survive dehydra-tion stress [17••]; it is synthesized from glucose-6-phosphate and uridine-diphosphoglucose (Figure 2b). In plants engineered to synthesize trehalose, only very small amounts accumulate (Table 1). In two reports trehalose-6-P-synthase (tps) alone was introduced, leaving dephosphorylation of trehalose-6-P to an endoge-nous non-specific phosphatase activity. In one report both
tpsand trehalose-6-P phosphatase (tpp) were inserted, but even with both genes, a dramatic increase in trehalose did not occur due to its degradation by trehalase [17••]. This was demonstrated by the use of a trehalase inhibitor, which increased trehalose accumulation and identified a con-straint on trehalose synthesis in plants that do not naturally accumulate it [17••].
Metabolic engineering requires a guided,
iterative approach
As noted above, Table 1 highlights the relative lack of attention to metabolic analysis of transgenic plants, in par-ticular in vivo estimates of pathway fluxes and intermediate pool sizes made using isotopic tracer methods. Such data can be incorporated into metabolic models, which are very helpful tools to describe and predict the behavior of the tar-get pathway (Figure 4). Models have a long track record of application to the metabolic engineering of microorganisms [22••], and to metabolic pathway characterization in plants [23]. Model development begins with a metabolic map as shown for GlyBet synthesis (Figure 2a) or polyol synthesis (Figure 2b). The input data consist of intermediate and end-product pool size measurements, and flux-rates calcu-lated from timed in vivo isotope labeling data. The modeling program is used to fit the data to the described pathway, which is done interactively by varying flux rates
and pool sizes (see [23]). Once developed, the model can be tested experimentally to assess its accuracy. The most important feature of models is their potential to predict the impact of a modification (e.g. inserting a transgene to upregulate a step in the pathway) on pathway flux. This can guide the engineering process by simulating the outcome of various experimental strategies. For instance, a model for choline metabolism in tobacco has been developed to sim-ulate strategies for engineering GlyBet synthesis. It can be accessed at the world wide web site (http://www.hort.pur-due.edu/cfpesp/models/models.htm) for constructing interactive metabolic models.
A related, but distinct, approach to understanding and pre-dicting metabolic flux is metabolic control analysis (MCA). MCA is basically concerned with the steady state of a metabolic pathway, in which the enzyme activities and metabolite pool sizes remain constant. MCA shows that control of pathway flux is typically shared among all the enzyme activities in the pathway, in sharp contrast with the traditional concept of a single ‘rate-limiting’ step [24]. Each enzyme’s contribution to control of flux can vary and MCA defines this behavior as it relates to an individual step, or the entire pathway. The analysis can be used in simulation experiments similar to those outlined above. MCA has contributed both to microbial metabolic engi-neering [22••], and to basic understanding of the control of metabolism [25••]. For example, a recent report, using MCA to evaluate transgenic plants, clearly demonstrates that the control of pathway flux is shared among the com-ponent reactions within the pathway and is not limited to an individual step [26••].
Figure 3
Glycine betaine synthesis represents a large demand on choline synthesis. Total demand for choline moieties in a GlyBet accumulator (spinach) and a non-accumulator (tobacco). Abbreviations are Cho, choline; P-Cho, phosphocholine; G-P-Cho, glycerophosphorylcholine; ptd-Cho, phosphatidylcholine; GlyBet, glycine betaine. The data are derived from [12••,45–48] and also from the authors’ unpublished results.
Molecule
GlyBet
0 10
20 Tobacco
Spinach
Cho
P-Cho
G-P-Cho ptd-Cho
µ
mol g
-1 fw
Advancing technology to aid the
engineering process
Two useful tools to help diagnose problems in transgenic plants are DNA micro-array technology and in vivoNMR spectroscopy. Micro-array technology was developed to evaluate the expression of many genes at once. The ana-lytical power of this technology has been demonstrated in yeast [27••], and is being applied to plants [28]. It provides high-resolution gene expression data which can be used to identify (unanticipated) changes in expression that follow the insertion of a transgene. In vivoNMR spectroscopy can be used to detect and quantitate several metabolites at once. In some cases the compartmentation of a metabolite can be distinguished [29••]. Although rarely as sensitive as standard in vivobiochemical or mass spectral methods, in vivo NMR techniques can be used to make otherwise impossible measurements — for instance, high resolution two-dimensional 31P-NMR spectroscopy was recently used to directly measure metabolic flux rates [30•].
Regulon engineering — manipulating entire
pathways using regulatory genes
Expression of regulatory genes that control several steps in a pathway or in related pathways (i.e. a regulon [31••]), may provide an alternative to introducing pathway genes
one at a time. Such an approach was used to manipulate secondary metabolite production in maize cells [32]. The basic principle is to manipulate the expression of a regu-latory gene (e.g. a specific transcription factor), which in turn alters the expression of the entire regulon. Over-expression of CBF1, a transcription factor that controls the expression of cold-responsive genes in Arabidopsis, demonstrated the feasibility of this approach in a plant stress context [31••]. Other distinct transcription factors that may function in a similar way to CBF1have also been described [33]. An Arabidopsisregulatory locus (esk1) that governs genes involved in both the synthesis and break-down of proline has been identified [34•], and is, therefore, an attractive candidate for regulon engineer-ing. It is important to note, however, that ectopic expression of regulatory genes can only modify a plant’s natural capabilities; it cannot confer new ones (e.g. the ability to synthesize a novel osmoprotectant).
Conclusions
Engineering metabolic change is not a trivial endeavor. It will typically require iterative rounds of transformation guided by thorough analysis of each transgenic generation. Interactive metabolic modeling promises to streamline this process by helping predict which step(s) will require alter-ation through additional rounds of engineering. Emerging
Figure 4
technology including DNA micro-arrays and in vivoNMR could also speed and simplify the analysis of genetically engineered plants. Only when a biochemical trait, such as osmoprotectant biosynthesis, is successfully engineered can the physiological consequences of that trait be assessed. Metabolic engineering research will teach us not only how to engineer biochemical change but also much about the metabolic pathways themselves.
Acknowledgements
Work in the authors’ laboratories is supported by the National Science Foundation (IBN 9816075 and IBN 9813999), the United States Department of Agriculture National Research Initiative Competitive Grants Program (95-37100-1596 and 98-35100-6149), the Office of Naval Research (N00014-96-1-0364 and N000149-96-1-0366) and the CV Griffin Sr. Foundation and the Florida Agricultural Experiment Station. Journal series number R-06645.
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest
••of outstanding interest
1. McCue KF, Hanson AD: Drought and salt tolerance: towards understanding and application.Trends Biotech1990, 8:358-362. 2. Jain RK, Selvaraj G: Molecular genetic improvement of salt
• tolerance in plants.Biotechnol Annu Rev1997, 3:245-267. An excellent review of recent progress towards engineering salt-resistance in plants. It covers the physiological aspects of salt-tolerance and includes a discussion of the adaptive strategies found in nature.
3. Rhodes D, Samaras Y: Genetic control of osmoregulation in plants.
In Cellular and Molecular Physiology of Cell Volume Regulation.
Edited by Strange K. Boca Raton: CRC Press; 1994:347-361. 4. Yancey PH: Compatible and counteracting solutes.In Cellular and
Molecular Physiology of Cell Volume Regulation. Edited by
Strange K. Boca Raton: CRC Press; 1994:81-109.
5. Smirnoff N: Plant resistance to environmental stress.Curr Opin
Biotechnol 1998, 9:214-219.
6. LeRudulier D, Strom AR, Dandekar AM, Smith LT, Valentine RC: Molecular biology of osmoregulation.Science1984, 224:1064-1068.
7. Nelson DE, Shen B, Bohnert HJ: Salinity tolerance-mechanisms, models and the metabolic engineering of complex traits.Genet
Engineering1998, 20:153-176.
8. Bohnert HJ, Sheveleva E: Plant stress adaptations — making metabolism move.Curr Opin Plant Biol1998, 1:267-274.
9. Holmberg N, Bülow L: Improving stress tolerance in plants by gene transfer.Trends Plant Sci1998, 3:61-66.
10. Yeo A: Molecular biology of salt tolerance in the context of whole
• plant physiology.J Exp Botany1998, 49:915-929.
A whole-plant perspective on molecular approaches to engineering stress tolerance which points out that osmotic stress resistance encompasses a number of mechanisms, many of which are unknown. It cautions against focusing on a single trait such as osmoprotectant synthesis as a solution to introducing resistance, and favors the identification and exploitation of quan-titative trait loci associated with stress resistance.
11. Hanson AD, Rathinasabapathi B, Rivoal J, Burnet M, Dillon DO, Gage DA: Osmoprotective compounds of the Plumbaginaceae: a natural experiment in metabolic engineering of stress tolerance.
Proc Natl Acad Sci USA1994, 91:306-310.
12. Nuccio ML, Russell BL, Nolte KD, Rathinasabapathi B,
•• Gage DA, Hanson AD: The endogenous choline supply limits glycine betaine synthesis in transgenic tobacco expressing choline monooxygenase.Plant J1998, 16:487-496.
This paper illustrates the utility of metabolic analysis in the engineering process. It demonstrates that the spinach choline monooxygenase enzyme functions in tobacco, and describes experiments that show how the choline supply limits GlyBet synthesis in these plants. It also suggests engineering steps that may enhance GlyBet synthesis in the next generation of trans-genic plants.
13. Nelson DE, Rammesmayer G, Bohnert HJ: Regulation of cell-specific inositol metabolism and transport on plant salinity tolerance.Plant Cell1998, 10:753-764.
14. Russell BL, Rathinasabapathi B, Hanson AD: Osmotic stress induces expression of choline monooxygenase in sugar beet and amaranth.Plant Physiol1998, 116:859-865.
15. Stephanopoulos G, Vallino JJ: Network rigidity and metabolic engineering in metabolite overproduction.Science1991, 252:1675-1681.
16. Sheveleva EV, Marquez S, Chmara W, Zegeer A, Jensen RG,
• Bohnert HJ:Sorbitol-6-phosphate dehydrogenase expression in transgenic tobacco: high amounts of sorbitol lead to necrotic lesions.Plant Physiol1998, 117:831-839.
This report highlights a downside in metabolic engineering. It shows that extensive redirection of metabolic flux to sorbitol depletes the myo-inositol pool, and that this correlates with necrosis and growth defects. Clearly, transgene expression created an imbalance in primary metabolism; this could perhaps be corrected through additional rounds of engineering. 17. Goddijn OJM, Verwoerd TC, Voogd E, Krutwagen RWHH,
•• de Graaf PTHM, Poels J, van Dun K, Ponstein AS, Damm B, Pen J: Inhibition of trehalase activity enhances trehalose accumulation in transgenic plants.Plant Physiol1997, 113:181-190.
The analytical experiments in this paper show that an endogenous trehalase activity is one factor limiting trehalose synthesis in transgenic plants. Specific inhibition of trehalase increased trehalose accumulation. It also reports the expression of trehalose-6-P synthase both alone and with tre-halose-6-P phosphatase (tpp), and that the presence of tpphas a small pos-itive effect on trehalose accumulation.
18. Lilius G, Holmberg N, Bülow L: Enhanced NaCl stress tolerance in transgenic tobacco expressing bacterial choline dehydrogenase.
Biotechnology1996, 14:177-180.
19. Hayashi H, Alia, Mustardy L, Deshnium P, Ida M, Murata N:
• Transformation of Arabidopsis thalianawith the codAgene for choline oxidase; accumulation of glycinebetaine and enhanced tolerance to salt and cold stress.Plant J1997, 12:133-142. This was the first report on the expression of a foreign choline oxidizing gene in chloroplasts. The transformed plants synthesized GlyBet and were slight-ly more tolerant to salt and cold stress.
20. Alia, Hayashi H, Sakamoto A, Murata N: Enhancement of the tolerance of Arabidopsisto high temperatures by genetic engineering of the synthesis of glycine betaine.Plant J1998, 16:155-162.
21. Sheveleva EV, Chmara W, Bohnert HJ, Jensen RG: Increased salt
•• and drought tolerance by D-ononitol production in transgenic
Nicotiana tabacumL.Plant Physiol1997, 115:1211-1219.
This paper reports the production of D-ononitol in transgenic plants. The
myo-inositol pool size increased with stress, which led to greater D-ononitol production and osmotic stress resistance. It illustrates how metabolic engi-neering can lead to insight into endogenous metabolic characteristics. 22. Bailey JE: Mathematical modeling and analysis in biochemical
•• engineering: past accomplishments and future opportunities.
Biotechnol Prog1998, 14:8-20.
A historical discussion of mathematical modeling and its application to meta-bolic problems. It highlights the importance of modeling complex metameta-bolic networks which, in turn, facilitate the engineering process.
23. Kocsis MG, Nolte KD, Rhodes D, Shen T-L, Gage DA, Hanson AD: Dimethylsulfonioproprionate biosynthesis in Spartina alterniflora: evidence that S-methylmethionine and dimethylsulfonio propylamine are intermediates.Plant Physiol1998, 117:273-281. 24. ap Rees T: Prospects of manipulating plant metabolism.Trends
Biotech1995, 13:375-378.
25. Fell DA: Understanding the Control of Metabolism. London:
•• Portland Press; 1997.
This book provides a thorough and highly readable introduction to metabol-ic control analysis (MCA). It bridges the gap between traditional enzymolo-gy and MCA, and demonstrates how MCA overcomes some of the descriptive limitations of traditional enzymology.
26. Thomas S, Mooney PJF, Burrell MM, Fell DA: Finite change analysis
•• of glycolytic intermediates in tuber tissue of lines of transgenic potato (Solanum tuberosum) overexpressing
phosphofructokinase.Biochem J1997, 322:111-117.
27. DeRisi JL, Iyer VR, Brown PO: Exploring the metabolic and genetic
•• control of gene expression on a genomic scale.Science1997, 278:680-686.
DNA micro-array technology enables the expression of many genes to be measured at once. This paper is the first to report bringing this analytical pro-cedure to the genomic scale, in yeast. The experiments clearly demonstrate how alterations in growth, mutations and genetic manipulations can affect the expression of many genes.
28. Ruan Y, Gilmore J, Conner T: Towards Arabidopsisgenome analysis: monitering expression profiles of 1400 genes using cDNA microarrays.Plant J1998, 15:821-833.
29. Aubert S, Curien G, Bligny R, Gout E, Douce R: Transport,
•• compartmentation, and metabolism of homoserine in higher plant cells: carbon-13- and phosphorus-31-nuclear magnetic
resonance studies.Plant Physiol1998, 116:547-557.
A good example of how in vivoNMR spectroscopy can complement classi-cal biochemiclassi-cal analysis. 13C- and 31P-NMR were used to monitor the
uptake and metabolism of homoserine. The experiments demonstrate how metabolite pool size data and enzymatic rate data can be derived from NMR spectra. An experiment to determine the distribution of phosphohomoserine between the cytosol and the vacuole is also described.
30. Roscher A, Emsley L, Raymond P, Roby C: Unidirectional steady
• state rates of central metabolism enzymes measured simultaneously in a living plant tissue.J Biol Chem1998, 273:25053-25061.
This paper describes how in vivoNMR spectroscopy can be used to mea-sure the flux of phosphate from substrates to products. The methods to mon-itor four enzymatic reactions in central metabolism are described and used to show how each reaction responds to changes in external stimuli. 31. Jaglo-Ottosen KR, Gilmour SJ, Zarka DG, Schabenberger O,
•• Thomashow MF: Arabidopsis CBF1overexpression induces COR
genes and enhances freezing tolerance.Science1998, 280:104-106.
This is the first report to demonstrate ‘regulon engineering’ in a plant stress context. CBF1had been shown to interact with a number of cold-reponsive genes during the ‘cold-acclimation’ process, which leads to an increase in freezing tolerance. The experiments show that overexpressing CBF1
induces the acclimation effect and enhances freezing tolerance.
32. Grotewold E, Chamberlin M, Snook M, Siame B, Butler L, Swenson J, Maddock S, St Clair G, Bowen B: Engineering secondary metabolism in maize cells by ectopic expression of transcription factors.Plant Cell1998, 10:721-740.
33. Liu Q, Kasuga M, Sakuma Y, Abe H, Miura S, Yamaguchi-Shinozaki K, Shinozaki K: Two transcription factors, DREB1 and DREB2, with an EREBP/AP2 DNA binding domain separate two cellular signal transduction pathways in drought- and low-temperature-responsive gene expression, respectively, in Arabidopsis. Plant Cell1998, 10:1391-1406.
34. Xin Z, Browse J: eskimo1mutants of Arabidopsisare constitutively
• freezing tolerant.Proc Natl Acad Sci USA1998, 95:7799-7804. This paper describes the identification and characterization of an
Arabidopsismutant that is defective in a key regulatory gene. It is a new
locus, distinct from other cold-acclimation pathways. The data show that, in proline metabolism, this gene can simultaneously upregulate synthesis
genes and downregulate degradative genes, implying that it is near the top of a signaling cascade.
35. Kavi Kishor PB, Hong Z, Miao G-H, Hu C-AA, Verma DPS: Overexpression of D1-pyrroline-5-carboxylate synthetase increases proline production and confers osmotolerance in transgenic plants.Plant Physiol1995, 108:1387-1394. 36. Tarczynski MC, Jensen RG, Bohnert HJ: Expression of a bacterial
mtlDgene in transgenic tobacco leads to production and accumulation of mannitol.Proc Natl Acad Sci USA1992, 89:2600-2604.
37. Tarczynski MC, Jensen RG, Bohnert HJ: Stress protection of transgenic tobacco by production of the osmolyte mannitol.
Science1993, 259:508-510.
38. Thomas JC, Sepahi M, Arendall B, Bohnert HJ: Enhancement of seed germination in high salinity by engineering mannitol expression in Arabidopsis thaliana.Plant Cell Environ1995, 18:801-806.
39. Karakas B, Ozias-Akins P, Stushnoff C, Suefferheld M, Reiger M: Salinity and drought tolerance of mannitol-accumulating transgenic tobacco.Plant Cell Environ1997, 20:609-616. 40. Tao R, Uratsu SL, Dandekar AM: Sorbitol synthesis in transgenic
tobacco with apple cDNA encoding NADP-dependent sorbitol-6-phosphate dehydrogenase. Plant Cell Physiol1995, 36:525-532. 41. Vernon DM, Tarczynski MC, Jensen RG, Bohnert HJ: Cyclitol
production in transgenic tobacco.Plant J1993, 4:199-205. 42. Holmström K-O, Mäntylä E, Wellin B, Mandal A, Palva ET, Tunnela OE,
Londesborough J: Drought tolerance in tobacco.Nature1996, 379:683-684.
43. Romero C, Bellés JM, Vayá JL, Serrano R, Culiáñez-Macià FA: Expression of the yeast trehalose-6-phosphate synthasegene in transgenic tobacco plants: pleiotropic phenotypes include drought tolerance.Planta1997, 201:293-297.
44. Popp M, Smirnoff N:Polyol accumulation and metabolism during water deficit.In Environment and Plant Metabolism: Flexibility and
Acclimation.Edited by Smirnoff N. Oxford: Bios Scientific Publishers
Limited; 1995:199-215.
45. Storey R, Wyn Jones RG: Salt stress and comparative physiology in the Gramineae. II: glycinebetaine and proline accumulation in two salt- and water-stressed barley cultivars.Aust J Plant Physiol
1978, 5:817-829.
46. Hanson AD, May AM, Grumet R, Bode J, Jamieson GC, Rhodes D: Betaine synthesis in chenopods: localization in chloroplasts.
Proc Natl Acad Sci USA1985, 82:3678-3682.
47. Bligny R, Gardestrom P, Roby C, Douce R: 31P NMR studies of
spinach leaves and their chloroplasts.J Biol Chem1990, 265:1319-1326.
48. Roughan PG, Batt RD: The glycerolipid composition of leaves.