Lancet 2012; 380: 1241–49 Published Online August 26, 2012 http://dx.doi.org/10.1016/ S0140-6736(12)61384-1
See Comment page 1208
See Articles page 1231 Department of Neurology and Stroke Center
(Prof J L Saver MD) and Department of Radiology and Stroke Center (R JahanMD), University of California, Los Angeles, CA, USA; Department of Neurosurgery, Millard Fillmore Hospital, Buff alo NY, USA (Prof E I LevyMD); Department of Neurology, University of Pittsburgh, PA, USA (T G JovinMD); Department of Radiology, Erlanger Hospital, Chattanooga, TN, USA (B BaxterMD); Department of Neurology, Emory University, Atlanta, GA, USA
(R G NogueiraMD); Department of Neurology, Oregon Health Science University, Portland, OR, USA (Prof W ClarkMD); Department of Radiology, Riverside Methodist Hospital, Columbus, OH, USA (R BudzikMD); and Department of Neurology, Medical College of Wisconsin, Milwaukee, WI, USA (Prof O O ZaidatMD)
Correspondence to: Prof Jeff rey L Saver, UCLA Stroke Center, 710 Westwood Plaza, Los Angeles, CA 90095, USA jsaver@ucla.edu
Solitaire fl ow restoration device versus the Merci Retriever in
patients with acute ischaemic stroke (SWIFT): a randomised,
parallel-group, non-inferior ity trial
Jeff rey L Saver, Reza Jahan, Elad I Levy, Tudor G Jovin, Blaise Baxter, Raul G Nogueira, Wayne Clark, Ronald Budzik, Osama O Zaidat, for the SWIFT Trialists
Summary
Background The Solitaire Flow Restoration Device is a novel, self-expanding stent retriever designed to yield rapid
fl ow restoration in acute cerebral ischaemia. We compared the effi cacy and safety of Solitaire with the standard,
predicate mechanical thrombectomy device, the Merci Retrieval System.
Methods In this randomised, parallel-group, non-inferiority trial, we enrolled patients from 18 sites (17 in the USA and one in France). Patients were eligible for inclusion if they had acute ischaemic stroke with moderate to severe neurological defi cits and were treatable by thrombectomy within 8 h of stroke symptom onset. We used a
computer-generated randomisation sequence to randomly allocatepatients to receive thrombectomy treatment with either
Solitaire or Merci (1:1; block sizes of four and stratifi ed by centre and stroke severity). The primary endpoint was Thrombolysis In Myocardial Ischemia (TIMI) scale 2 or 3 fl ow in all treatable vessels without symptomatic intracranial haemorrhage, after up to three passes of the assigned device, as assessed by an independent core laboratory, which
was masked to study assignment. Primary analysis was done by intention to treat. A prespecifi ed effi cacy stopping
rule triggered an early halt to the trial. The study is registered with ClinicalTrials.gov, number NCT 01054560.
Results Between February, 2010, and February, 2011, we randomly allocated 58 patients to the Solitaire group and
55 patients to the Merci group. The primary effi cacy outcome was achieved more often in the Solitaire group than it
was in the Merci group (61% vs 24%; diff erence 37% [95% CI 19–53], odds ratio [OR] 4·87 [95% CI 2·14–11·10];
pnon-inferiority<0·0001, psuperiority=0·0001). More patients had good 3-month neurological outcome with Solitaire than with
Merci (58% vs 33%; diff erence 25% [6–43], OR 2·78 [1·25–6·22]; p
non-inferiority=0·0001, psuperiority=0·02). 90-day mortality
was lower in the Solitaire group than it was in the Merci group (17 vs 38; diff erence –21% [–39 to –3], OR 0·34
[0·14–0·81]; pnon-inferiority=0·0001, psuperiority=0·02).
Interpretation The Solitaire Flow Restoration Device achieved substantially better angiographic, safety, and clinical outcomes than did the Merci Retrieval System. The Solitaire device might be a future treatment of choice for endovascular recanalisation in acute ischaemic stroke.
Funding Covidien/ev3.
Introduction
Occlusion of proximal intracranial arteries is a common cause of acute ischaemic stroke and causes much disability and mortality.1–3 Treatments for ischaemic stroke that are approved by guidelines or regulatory authorities include systematic supportive care in a stroke unit or stroke centre, pre vention of clot
propa-gation with aspirin, and achievement of reper fusion
through use of intravenous recombinant tissue plas-minogen activator (rt-PA) until 4·5 h after onset, intra-arterial fi brinolysis until 6 h after onset, and mechanical thrombectomy until 8 h after onset.4,5 Available
intra-venous and endovascular reper fusion techniques are
restricted in the extent to which they achieve
recan-alisation and avoid intracranial haemor rhage, so the
development of new devices with diff erent approaches to thrombus removal is desirable.6–11
The Solitaire Flow Restoration device (Covidien/ev3,
Dublin, Ireland) is a self-expanding stent retriever
regulatory authorities in the USA for use in acute ischaemic stroke, in 2004.
We describe the results of the Solitaire With the Intention For Thrombectomy (SWIFT) trial, a multi center, pros-pective, randomised, blinded endpoint assess ment trial comparing the effi cacy and safety of neuro thrombectomy with Solitaire versus the contem porary regulatory standard, the Merci Retriever.
Methods
Study design and participants
SWIFT was a multicenter, randomised, prospective, parallel-group trial with blinded primary endpoint ascertainment. Details of the study design are available elsewhere.19 Briefl y, patients were eligible if they had acute ischaemic stroke with moderate to severe neuro-logical defi cits, harboured angiographically confi rmed occlusions of proximal cerebral arteries, and were treatable by thrombectomy within 8 h of stroke symptom onset. Key inclusion criteria included age (22–85 years), National Institutes of Health Stroke Scale (NIHSS) score (≥8 and ≤30), and ineligibility for or failure to respond to intravenous rt-PA. Key exclusion criteria included uncontrolled hypertension, serious sensitivity to radio-graphic contrast agents, and CT or MRI evidence of intracranial haemorrhage or major ischaemic infarction (acute ischaemic change in more than a third of the middle cerebral artery territory or more than 100 mL of tissue in other territories). The study was approved by the appropriate national regulatory bodies and by the ethics committee at each centre. All patients or their legally authorised representatives provided signed, informed consent.
Randomisation and masking
To assure neurointerventionalist familiarity with the Solitaire device, physicians were trained in the use of the device on a bench vascular model before any procedures were done, and participating sites that had no experience with the Solitaire device were required to use the device on two roll-in patients before randomisation of patients. Roll-in patients received the Solitaire device as the only initial neurothrombectomy intervention and were analysed separately from the patients who were randomly allocated to treatment.
In the main, randomised phase of the trial, study patients were randomly allocated in a one-to-one ratio to receive either Solitaire or Merci as the initial neurothrombectomy intervention. The randomisation sequence was computer-generated and stratifi ed by site and presenting stroke severity (NIHSS ≤17 vs >17), with blocks sizes of four. Allocation concealment was implemented by use of sequentially numbered, opaque, sealed envelopes.
An independent central core imaging laboratory, unaware of the study group assignments, assessed fi nal revascularisation grades on outcome angiograms and haemorrhagic transformation on outcome CT and MR
examinations. A central, independent clinical events committee, whose members were unaware of the study group assignments, categorised all adverse events by severity and relatedness to the study device and to the interventional procedure.
Procedures
Once a patient was assigned to either Solitaire or Merci, the neurointerventionalist selected the proper study device size per device-specifi c instructions for use. A minimum of one deployment of the assigned study device was required to be achieved within 8 h of symptom onset. The neurointerventionalist attempted recanal-isation using the assigned device type, continuing until successful recanalisation was achieved or until three passes of the study group device through any vessel had been done. The primary endpoint outcome angiogram was then done. Intravenous sedation or general anaesthesia was allowed to be used, if deemed appropriate by the attending neurointerventionalist, to assure the comfort and safety of patients.
After the primary endpoint outcome angiogram was done, rescue treatment was permitted in patients in whom adequate recanalisation had not been achieved. All cases requiring rescue treatment were regarded as device treatment failures. Permitted rescue treatment interventions were as follows: a regulatory-agency-cleared neurovascular thrombectomy device diff erent than the initially assigned study device, intra-arterial fi brinolysis according to US guidelines,4 or both. If any rescue treatment had been done, an additional, fi nal, diagnostic angiogram (after all procedures had been done) was obtained.
Follow-up assessment was done at 24 h, 7–10 days (or discharge if earlier), 30 days, and 90 days. The 24-h assessment included a repeat NIHSS score and repeat brain CT or MRI, allowing ascertainment of the symptomatic intracranial haemorrhage component of the composite primary endpoint. The later assessments included the NIHSS, the Barthel Index assessing instrumental activities of daily living, and the modifi ed Rankin Scale (mRS) assessing global disability.
The primary effi cacy endpoint of the study was
subarachnoid haemorrhage, or intraventricular haemor-rhage associated with a worsening of the NIHSS score by four or more within 24 h.
Secondary effi cacy outcomes included the following:
time to achieve initial recanalisation, defi ned as the time from baseline guide catheter run to visualisation of TIMI 2 or 3 fl ow in all treatable vessels; good neurological outcome at 90 days, defi ned as an mRS of 2 or less, or equal to the prestroke mRS if the prestroke mRS was greater than 2, or NIHSS score improvement of 10 points or more; and neurological condition at 90 days, including NIHSS, Barthel Index, and mRS. The primary safety endpoint was the incidence of device-related and procedure-related serious adverse events. Additional safety endpoints included mortality and symptomatic intracranial haemorrhage.
Statistical analysis
We designed the trial to show that the proportion of patients with successful recanalisation with no symp-tomatic haemorrhage would be non-inferior in the Solitaire group compared with the control group, the Merci group, using a clinically relevant non-inferiority margin that was defi ned before starting the trial to be 10%. Superiority analysis of the primary endpoint was undertaken only if non-inferiority was achieved. Effi cacy endpoints were assessed in the intention-to-treat population, which included all patients who consented and were randomly assigned to treatment. We did the primary effi cacy analysis using a one-sided test under Blackwelder’s method of testing non-inferiority at the 0·025 level of signifi cance.20 We did the nested superiority analysis using a two-sided test at the 0·05 level of
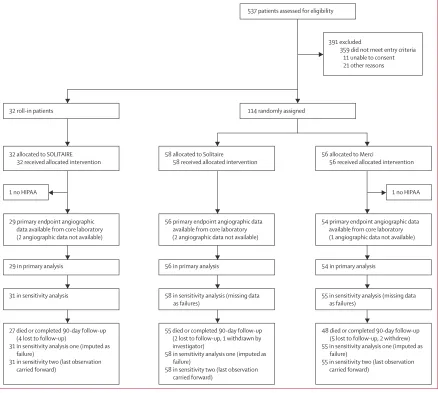
Figure 1: Trial profi le
Two patients signed study consents but not Health Insurance Portability and Accountability Act (HIPAA) consents, including one roll-in patient and one patient randomly allocated to treatment. These patients were excluded from all analyses. Angiographic outcome images forwarded to the core laboratory were insuffi cient to allow Thrombolysis In Myocardial Ischemia outcome assessment in two roll-in and three randomised patients. These patients were censored in the primary analysis. In sensitivity analyses, they were analysed as failures in analysis one, and according to site readings in analysis two.
537 patients assessed for eligibility
391 excluded
359 did not meet entry criteria 11 unable to consent 21 other reasons
114 randomly assigned 32 roll-in patients
32 allocated to SOLITAIRE 32 received allocated intervention
1 no HIPAA
58 allocated to Solitaire
58 received allocated intervention
56 allocated to Merci
56 received allocated intervention
29 primary endpoint angiographic data available from core laboratory (2 angiographic data not available)
56 primary endpoint angiographic data available from core laboratory (2 angiographic data not available)
54 primary endpoint angiographic data available from core laboratory (1 angiographic data not available)
29 in primary analysis 56 in primary analysis 54 in primary analysis
31 in sensitivity analysis 58 in sensitivity analysis (missing data as failures)
55 in sensitivity analysis (missing data as failures)
27 died or completed 90-day follow-up (4 lost to follow-up)
31 in sensitivity analysis one (imputed as failure)
31 in sensitivity two (last observation carried forward)
55 died or completed 90-day follow-up (2 lost to follow-up, 1 withdrawn by investigator)
58 in sensitivity analysis one (imputed as failure)
58 in sensitivity two (last observation carried forward)
48died or completed 90-day follow-up (5 lost to follow-up, 2 withdrew) 55 in sensitivity analysis one (imputed as
failure)
55 in sensitivity two (last observation carried forward)
signifi cance. We did a sensitivity analysis to assess the eff ect of any between-group diff erences in baseline features on the primary effi cacy endpoint, using univariate logistic regression of variables with a p value of 0·1 or less reduced to a fi nal model by backward elimination, with variables used as randomisation stratifi cation factors required to be included. For secondary effi cacy endpoint analyses, statistical tests of non-inferiority were one-sided
and all other tests were two-sided. Resulting p values are shown without multiplicity adjustment.
We calculated the sample size by assuming that the true proportions of patients with successful recanal isation without symptomatic intracranial haemorrhage would be 0·54 in the Merci group and 0·65 in the Solitaire group. A total of 174 assessable patients for this endpoint would give 80% power and a fi nal sample size of 200 was selected to ensure the recruitment of at least 174 assessable patients.20 We planned one formal interim effi cacy analysis according to the method of alpha-spending of Lan-DeMets using a Pocock-type alpha-spending function.
The trial is registered with ClinicalTrials.gov, number NCT 01054560.
Role of the funding source
An academic principal investigator (JLS), academic lead interventional investigator (RJ), and academic steering committee supervised trial design and operations. A publications committee (principal investigator, lead interventional investigator, steering committee, and academic principal investigators the sites that enrolled most patients interpreted the results and wrote the report. The sponsor of the study was responsible for site management, data management, and safety reporting. Key statistical analyses, including the primary endpoint analysis, were validated by an independent external statistician (J Schafer, MS, Integra Group, Brooklyn Park, MN, USA). The corresponding author had full access to all the data in the study and had fi nal responsibility for the decision to submit for publication.
Results
Between February, 2010, and February, 2011, 18 sites (17 in the USA and one in France) consented and enrolled 144 patients, including 31 roll-in patients who received Solitaire and 113 patients who were randomly allocated to one of the two treatment groups (58 to Solitaire and 55 to Merci; fi gure 1). After a safety review in February, 2011, the data and safety monitoring board recommended that enrolment be placed on hold and the
interim effi cacy analysis be undertaken earlier than
initially planned. In September, 2011, after the interim effi cacy analysis was done and pre-specifi ed criteria on which to stop the trial were met, the data safety and monitoring board recom mended halting the trial, and the trial steering committee concurred.
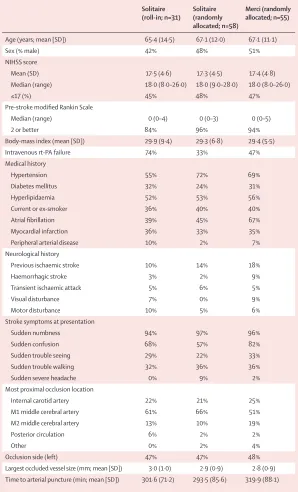
Baseline demographic and clinical characteristics of the two randomised treatment groups were much the same across 35 variables, including age and presenting stroke severity (NIHSS), except that there were more patients with atrial fi brillation, history of previous visual disturbance, and presentation with confusion in the Merci group (before adjustment for multiple com-parisons; table 1).
The mean number of passes with the assigned study device was lower in the Solitaire group than it was in the
Solitaire (roll-in; n=31)
Solitaire (randomly allocated; n=58)
Merci (randomly allocated; n=55)
Age (years; mean [SD]) 65·4 (14·5) 67·1 (12·0) 67·1 (11·1)
Sex (% male) 42% 48% 51%
NIHSS score
Mean (SD) 17·5 (4·6) 17·3 (4·5) 17·4 (4·8)
Median (range) 18·0 (8·0–26·0) 18·0 (9·0–28·0) 18·0 (8·0–26·0)
≤17 (%) 45% 48% 47%
Pre-stroke modifi ed Rankin Scale
Median (range) 0 (0–4) 0 (0–3) 0 (0–5)
2 or better 84% 96% 94%
Body-mass index (mean [SD]) 29·9 (9·4) 29·3 (6·8) 29·4 (5·5)
Intravenous rt-PA failure 74% 33% 47%
Medical history
Hypertension 55% 72% 69%
Diabetes mellitus 32% 24% 31%
Hyperlipidaemia 52% 53% 56%
Current or ex-smoker 36% 40% 40%
Atrial fi brillation 39% 45% 67%
Myocardial infarction 36% 33% 35%
Peripheral arterial disease 10% 2% 7%
Neurological history
Previous ischaemic stroke 10% 14% 18%
Haemorrhagic stroke 3% 2% 9%
Transient ischaemic attack 5% 6% 5%
Visual disturbance 7% 0% 9%
Motor disturbance 10% 5% 6%
Stroke symptoms at presentation
Sudden numbness 94% 97% 96%
Sudden confusion 68% 57% 82%
Sudden trouble seeing 29% 22% 33%
Sudden trouble walking 32% 36% 36%
Sudden severe headache 0% 9% 2%
Most proximal occlusion location
Internal carotid artery 22% 21% 25%
M1 middle cerebral artery 61% 66% 51%
M2 middle cerebral artery 13% 10% 19%
Posterior circulation 6% 2% 2%
Other 0% 2% 4%
Occlusion side (left) 47% 47% 48%
Largest occluded vessel size (mm; mean [SD]) 3·0 (1·0) 2·9 (0·9) 2·8 (0·9)
Time to arterial puncture (min; mean [SD]) 301·6 (71·2) 293·5 (85·6) 319·9 (88·1)
NIHSS=National Institutes of Health Stroke Scale. rt-PA=recombinant tissue plasminogen activator.
Merci group (1·7 [SD 0·9] vs 2·2 [0·9]; p=0·003). Rescue treatment was needed less often in the Solitaire group than it was in the Merci group (table 2). In the randomised Solitaire group, rescue treatment included mechanical devices in nine patients, intra-arterial fi brinolytics in one patient, and both in two patients; in the randomised Merci group, rescue treatment included mechanical devices in 17 patients, intra-arterial fi brinolytics in one patient, and both in six patients.
The primary effi cacy endpoint, successful
recanal-isation with the assigned study device without use of rescue treatment and with no symptomatic intracranial haemorrhage, was achieved more often in the Solitaire group than it was in the Merci group (absolute diff erence of 36·6% [95% CI 18·5–53·4]; table 2). In the sensitivity analysis that adjusted for stratifi cation factors (site and NIHSS) and all additional independent predictors (smoking status) of the primary endpoint, treatment with
Solitaire remained asso ciated with achievement of
successful recanalisation without rescue device use or symptomatic intracranial haemorrhage (odds ratio [OR] 7·52 [2·83–20·02]; pnon-inferiority<0·0001; psuperiority=0·0001). Similarly, sensitivity analyses, treating the two patients in the Solitaire group and the patient in the Merci group who did not have core-laboratory analysable angiograms as treatment failures or per-site investigator ratings did not alter the superiority fi ndings (appendix).
The angiographic effi cacy component of the combined
primary endpoint, successful recanalisation with the assigned study device, as assessed by the core laboratory, was achieved more often in the Solitaire group than it was
in the Merci group (table 2). Site neurointer ventionalists assessed achievement of this endpoint as occurring more often than did the core laboratory in both treatment groups, but still with a substantial advantage for Solitaire compared with Merci (table 2). Median time from initial placement of guide catheter to achievement of recanal-isation or end of procedure was shorter in the Solitaire group (36 min [IQR 18–65]; n=47) than it was in the Merci group (52 min [31–73]; n=46; superiority p=0·038). After use of rescue treatments, a recanalisation advantage remained for patients with initial treatment with Solitaire versus Merci (table 2; rescue treatments used are shown in the appendix).
Good neurological outcome at 90 days was seen more often in the Solitaire group than it was in the Merci group (diff erence 24·9% [5·5–42·9]; table 2). In an analysis adjusting for stratifi cation factors (site and NIHSS) and all additional independent predictors (atrial fi bril lation) of good neurological outcome, treatment
Solitaire (roll-in; n=31)
Solitaire (randomly allocated; n=58)
Merci (randomly allocated; n=55)
Odds ratio (95% CI) for comparison between randomised groups
Non-inferiority p value for comparison between randomised groups
Superiority p value for randomised comparison
Successful recanalisation without symptomatic intracranial haemorrhage (primary endpoint)
55% (16/29) 61% (34/56) 24% (13/54) 4·87 (2·14–11·10) <0·0001 0·0001
Angiographic effi cacy endpoints
Successful recanalisation with study device (assessed by core laboratory)
63% (17/27) 69% (37/54) 30% (16/53) 5·03 (2·22–13·66) <0·0001 0·0001
Successful recanalisation with study device (assessed at study site)
77% (24/31) 83% (45/54) 48% (26/54) 5·38 (2·21–13·15) <0·0001 0·0002
Use of rescue treatment* 23% (7/31) 21% (12/58) 44% (24/55) 0·34 (0·15–0·77) <0·0001 0·015
Successful recanalisation at end of procedure (assessed at study site)
94% (29/31) 89% (48/54) 67% (37/55) 3·89 (1·41–10·78) <0·0001 0·010
Clinical effi cacy endpoints at 90 days
Good neurological outcome† 63% (17/27) 58% (32/55) 33% (16/48) 2·78 (1·25–6·22) 0·0001 0·017
Independent‡ 44% (12/27) 36% (20/55) 29% (14/48) 1·39 (0·61–3·18) 0·0312 0·53
Global disability (modifi ed Rankin Scale)† 3 (1–4) 3 (1–4) 4 (2–6) ·· ·· 0·035
Activities of daily living (Barthel Index)†§ 80 (10–100) 70 (15–100) 22·5 (0–100) ·· ·· 0·054
Neurological defi cit (NIHSS)†¶ 3·0 (1·0–15·0) 4·5 (1·0–12·5) 30·0 (2·0–42·0) ·· ·· 0·007
Data are % (n/N) or median (IQR). NIHSS=National Institutes of Health Stroke Scale. *Specifi c rescue treatments are shown in the appendix. †Prespecifi ed secondary endpoints. ‡Posthoc endpoint. §Fatal outcomes scored as 0. ¶Fatal outcome scored as 42.
Table 2: Effi cacy endpoints
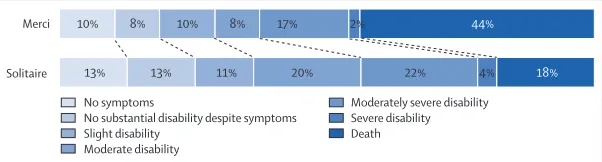
Figure 2: Distribution of modifi ed Rankin Scale scores of disability at 3 months
Percentages for modifi ed Rankin Scale 6 in this fi gure diff er from the mortality rates in table 3 because the denominator for the fi gure population is all patients with an assessable scores at day 90 and the denominator for the table population is all patients randomly allocated to treatment.
10% Merci
Solitaire
No symptoms
No substantial disability despite symptoms Slight disability
Moderate disability
Moderately severe disability Severe disability Death
13% 13% 11% 20% 22% 4% 18%
8% 10% 8% 17% 2% 44%
with Solitaire was still associated with increased good neurological outcomes at 90 days (odds ratio 3·04 [1·23–7·54]; psuperiority=0·02). When analysing the overall distribution of the randomised treatment groups on the modifi ed Rankin scale of global disability, we noted more favourable outcomes in the Solitaire group compared with the Merci group across all scale levels
(Cochran-Mantel-Haenszel test, with adjust ment for
baseline NIHSS, age, and time to start of treatment, p=0·035; fi gure 2).
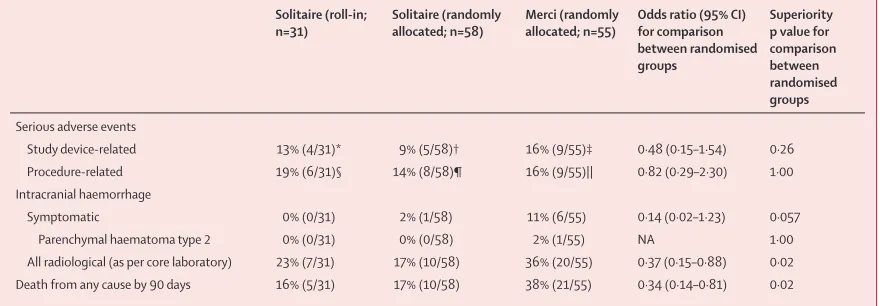
The frequency of total device-related and procedure-related serious adverse events (serious adverse events) did not diff er between the two randomised groups (table 3; appendix). Of all patients randomly allocated to treatment, symptomatic intracranial haemorrhage occurred non-signifi cantly less often in patients in the Solitaire group than it did in patients in the Merci group (diff erence –9·2% [95% CI –27·0 to 9·6]; table 3). Any
intracranial haemorrhage, symptomatic or asymp
to-matic, occurred less often in the Solitaire group than in the Merci group (table 3; appendix).
Death by 90 days occurred less often in the patients in the Solitaire group than it did in those in the Merci group (table 3; appendix). In time-to-event analysis with Kaplan-Meier methods (appendix), we noted a consistent
diver gence in the occurrence of death with time,
beginning at day 4 and increasing through day 50, with the log-rank test favouring Solitaire (p=0·014) with hazard ratio 0·40 (0·19–0·86). In analysis adjusting for all stratifi cation factors and all independent predictors of fatal outcome (age and time to treatment), treatment with Solitaire remained associated with reduced death by 90 days (OR 0·29 [0·11–0·79]; psuperiority=0·015).
Discussion
Our study suggests that, compared with the Merci Retriever, neurothrombectomy with the Solitaire Flow Restor ation device is associated with increased achieve-ment of successful cerebral recanalisation without
symptomatic intracranial haemorrhage, improved fi nal disability outcomes, and reduced mortality.
The combined primary effi cacy endpoint in our trial, successful recanalisation with no symptomatic haemor-rhage, was selected at the request of regulatory authorities and is novel in neurovascular reperfusion trials. Previous trials have used achievement of recanal-isation as the sole component of the primary effi cacy
endpoint.8–10 Our primary endpoint measure regards
good outcomes as only those in which both recanal-isation was technically achieved and symptomatic haemorrhagic transformation did not subsequently develop. This type of combination measure has been termed a metric of unqualifi ed success.21 Measures of unqualifi ed success assess treatment effi cacy adjusted for treatment-induced adverse eff ects in one metric. The between-group diff erences in this trial were driven by both components, but with a larger contribution from the substantially greater recanalisation effi cacy of Solitaire versus Merci. The Solitaire stent retriever, with its multiple points of contact and engagement with the target thrombus, was able to extract clots substantially more often than could the Merci coil retriever.
This trial is the fi rst acute ischaemic stroke trial to undertake a direct, randomised comparison of one endovascular recanalisation technique with another. Previous studies that have led to regulatory clearance of devices for neurovascular thrombectomy have been single group studies with only historical controls.8–10 Compared with previous neuro thrombectomy trials, we took an especially rigorous approach to the assessment of achievement of angio graphic reperfusion. The TIMI scale, developed for the myocardial circulation, cannot be applied in the more complex cervicocephalic arterial tree without the creation of additional operational rules. At least seven diff erent operationalised versions of the TIMI scale have been used in stroke trials.22 Of these, we used the most stringent, requiring revascularisation to be achieved in all treatable vessels, not just the most
Solitaire (roll-in; n=31)
Solitaire (randomly allocated; n=58)
Merci (randomly allocated; n=55)
Odds ratio (95% CI) for comparison between randomised groups
Superiority p value for comparison between randomised groups
Serious adverse events
Study device-related 13% (4/31)* 9% (5/58)† 16% (9/55)‡ 0·48 (0·15–1·54) 0·26
Procedure-related 19% (6/31)§ 14% (8/58)¶ 16% (9/55)|| 0·82 (0·29–2·30) 1·00
Intracranial haemorrhage
Symptomatic 0% (0/31) 2% (1/58) 11% (6/55) 0·14 (0·02–1·23) 0·057
Parenchymal haematoma type 2 0% (0/31) 0% (0/58) 2% (1/55) NA 1·00
All radiological (as per core laboratory) 23% (7/31) 17% (10/58) 36% (20/55) 0·37 (0·15–0·88) 0·02
Death from any cause by 90 days 16% (5/31) 17% (10/58) 38% (21/55) 0·34 (0·14–0·81) 0·02
Data are % (n/N) unless otherwise stated. NA=not applicable. *Five events. †Seven events. ‡13 events. §9 events. ¶17 events. ||12 events.
proximal target vessel. Furthermore, we used angio-graphic readings by a masked, central core laboratory, rather than unmasked site investigators. This method-ological rigor probably accounts for the lower reported rates of partial or better recanalisation in our primary analysis for both devices compared with previous studies.8–10 For Solitaire, the successful recanalisation rate as determined by the core laboratory of 69% is less than the partial recanalisation rates of 88–92% reported in open series,13–17 but the site-determined rate of 83% is not dissimilar from previous reports.13–17 Similarly, for Merci, the successful recanalisation rate as determined by the core laboratory of 30% is lower than the partial recanalisation rates of 48–55% seen in previous multicentre series,8,9 but the site-determined rate of 48% is in the same range.
The results of our trial provide supportive evidence for
a benefi t of endovascular reperfusion on clinical
outcomes in patients with acute ischaemic stroke. As far as we are aware, this trial is the fi rst randomised trial to fi nd a benefi cial eff ect of increased achievement of endovascular reperfusion on mortality. It is also only the second randomised trial (after the PROACT 2 trial in
1999)7 to indicate a benefi cial eff ect of increased
achievement of endovascular reperfusion on fi nal global disability outcomes. The clinical outcome fi ndings in our trial are consistent with a meta-analysis23 of clinical trials and large series that showed cerebral reperfusion to be associated with both reduced mortality and improved global disability outcomes.23
The magnitude of clinical benefi t signals seen in our trial were larger for converting fatal outcomes to mediate levels of disability than for converting inter-mediate and worse outcomes to independence. This pattern is not unexpected in a trial enrolling patients with severe neurological defi cits late after onset, because some enrolled patients will already have accumulated substantial irreversible injury before achieve ment of reper fusion. In these patients, recanalisation might not improve fi nal outcome (futile reperfusion). Nonetheless, the most common outcome alteration we recorded, the achievement of moderate disability (mRS level 3) rather than death, is clearly a treatment eff ect recognised as clinically worth-while by both patients and health providers.24,25 More refi ned methods of selection of patients, such as imaging the extent of the infarct core with perfusion CT or diff usion MRI, might enable better prognostication of attainable outcomes with neuro thrombectomy treatment.26 In any active comparator clinical trial, consideration should be given to whether diff erences in outcomes are due to a benefi cial eff ect of the novel treatment or to an adverse eff ect of standard treatment. Evidence from indirect comparisons indicates that, in patients who are ineligible for rt-PA or have rt-PA failure, the Merci Retriever improves clinical outcomes compared with
supportive medical care (panel).27 Accordingly, the
between-group diff er ences in fi nal outcome among such
patients in our trial, suggest not an adverse eff ect of Merci but rather an additional benefi cial eff ect of Solitaire above and beyond that already provided by Merci. Similarly, in patients just beginning rt-PA treatment, the preliminary IMS 3 trial results suggest that fi rst-generation endo-vascular interventions, in cluding the Merci Retriever, give no additional benefi tover rt-PA alone.28,29 Con sequently, the between-group diff erence in fi nal clinical outcome in our trial suggests a benefi t of Solitaire mechanical
throm bectomy compared with rt-PA alone. However,
defi nitive demonstration of benefi t of mech anical thromb-ectomy with Solitaire can only come from the undertaking of trials to directly compare mechanical thrombectomy
using Solitaire with intravenous throm bolysis and
supportive medical care alone.
We believe the results of our trial are highly gen-eralisable to all centres that do endovascular reperfusion for acute ischaemic stroke. The participating sites included both academic and community tertiary stroke centres. With regards to operator experience, the trial was conservatively biased against the Solitaire device, because sites were all suitably experienced in Merci procedures but 17 of 18 centres had not used the Solitaire device before the brief roll-in phase of the study.
There are several potential reasons for the greater recanalisation effi cacy and lesser haemorrhage trans-formation and subarachnoid haemorrhage with the Solitaire device than with the Merci device. For
Panel: Research in context
Systematic review
We searched for trials published in Medline between Jan 1, 1950, and June 30, 2012, the Cochrane Central Register of Controlled Trials between Jan 1, 1993, and June 30, 2012, the Internet Stroke Trials Center Registry (up to June, 2012), and reference lists in review articles. The Medline search strategy used the terms “thrombectomy”, “retrieval”, “neurothrombectomy”, “recanalization”, or “endovascular” crossed with “ischemic stroke”, “cerebral infarction,” or “cerebral ischemia”. We restricted the search to clinical trials and publications in English. We identifi ed no previous fully reported randomised trials comparing any neurothrombectomy treatment with either supportive care or another neurothrombectomy treatment.27 For patients with M1 or M2 middle cerebral artery occlusion, an indirect comparison suggested that, of patients who were ineligible for rt-PA or had rt-PA failure, the Merci Retriever (used in the control group of our study), improved clinical outcomes compared with supportive medical care. The clinical outcome fi ndings in our trial are consistent with a meta-analysis23 of clinical trials and large series that has shown that cerebral reperfusion is associated with both reduced mortality and improved global disability outcomes.
Interpretation
Covidien/ev3, Genervon, Lundbeck, and Mitsubishi for which the UC Regents received payments based on the clinical trial contracts for the number of participants enrolled. JLS and RS are employees of the University of California, which holds a patent on retriever devices for stroke. The University of California, Regents receive funding for RJ’s services as a scientifi c consultant regarding trial design and conduct from Covidien/ev3 and Chestnut Medical. EIL serves as a scientifi c consultant for Covidien/ev3, Codman and Shurtleff Inc, and TheraSyn Sensors Inc; and receives fees for carotid stent training from Covidien/ ev3, and Abbott Vascular. TGJ has served as a scientifi c consultant to Covidien/ev3, CoAxia, Concentric Medical, and Micrus. RGN has served as a scientifi c consultant to Covidien/ev3, CoAxia, and Concentric Medical. WC and RB have served as scientifi c consultants to Covidien/ ev3. OOZ serves as a scientifi c consultant to Talecris Biotherapeutics, Stryker, Codman, and MicroVention.
References
1 Derex L, Nighoghossian N, Hermier M, Adeleine P, Froment JC,
Trouillas P. Early detection of cerebral arterial occlusion on magnetic resonance angiography: predictive value of the baseline NIHSS score and impact on neurological outcome. Cerebrovasc Dis
2002; 13: 225–29.
2 Sims JR, Rordorf G, Smith EE, et al. Arterial occlusion revealed by CT angiography predicts NIH stroke score and acute outcomes after IV tPA treatment. AJNR Am J Neuroradiol 2005; 26: 246–51. 3 Smith WS, Tsao JW, Billings ME, et al. Prognostic signifi cance of
angiographically confi rmed large vessel intracranial occlusion in patients presenting with acute brain ischemia. Neurocrit Care 2006;
4: 14–17.
4 Adams HP Jr, del Zoppo G, Alberts MJ, et al. Guidelines for the early management of adults with ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups: the American Academy of Neurology affi rms the value of this guideline as an educational tool for neurologists. Stroke 2007; 38: 1655–711.
5 European Stroke Organization Guideline. Update—January 2009.
http://www.eso-stroke.org/pdf/ESO%20Guidelines_update_ Jan_2009.pdf (accessed Aug 14, 2012).
6 Mendonça N, Rodriguez-Luna D, Rubiera M, et al. Predictors of tissue-type plasminogen activator nonresponders according to location of vessel occlusion. Stroke 2012; 43: 417–21.
7 Furlan A, Higashida R, Wechsler L, et al. Intra-arterial prourokinase for acute ischemic stroke. The PROACT II study: a randomized controlled trial. Prolyse in Acute Cerebral Thromboembolism.
JAMA 1999; 282: 2003–11.
8 Smith WS, Sung G, Starkman S, et al. Safety and effi cacy of mechanical embolectomy in acute ischemic stroke: results of the Merci trial. Stroke 2005; 36: 1432–38.
9 Smith WS, Sung G, Saver J, et al. Mechanical thrombectomy for
acute ischemic stroke: fi nal results of the Multi Merci Trial. Stroke
2008; 39: 1205–12.
10 The penumbra pivotal stroke trial: safety and eff ectiveness of a new generation of mechanical devices for clot removal in intracranial large vessel occlusive disease. Stroke 2009; 40: 2761–68.
11 Saver JL. Improving reperfusion therapy for acute ischaemic stroke.
J Thromb Haemost 2011; 9 (suppl 1): 333–43.
12 Jahan R. Solitaire fl ow-restoration device for treatment of acute ischemic stroke: safety and recanalization effi cacy study in a swine vessel occlusion model. AJNR Am J Neuroradiol 2010; 31: 1938–43. 13 Castano C, Dorado L, Guerrero C, et al. Mechanical thrombectomy
with the Solitaire AB device in large artery occlusions of the anterior circulation: a pilot study. Stroke 2010; 41: 1836–40.
14 Miteff F, Faulder KC, Goh AC, Steinfort BS, Sue C, Harrington TJ. Mechanical thrombectomy with a self-expanding retrievable intracranial stent (Solitaire AB): experience in 26 patients with acute cerebral artery occlusion. AJNR Am J Neuroradiol 2011;
32: 1078–81.
15 Mpotsaris A, Bussmeyer M, Loehr C, Oelerich M, Buchner H, Weber W. Mechanical thrombectomy in severe acute stroke: preliminary results of the Solitaire stent.
J Neurol Neurosurg Psychiatry 2012; 83: 117–18.
revascularisation effi ciency, the Solitaire device pushes the clot against the vessel wall and traps it within multiple strut crossings, allowing substantial retrieval traction. It also retains structural integrity during the pull. The Merci retriever’s curved loops can slide through rather than engage target thrombus and the coil can unwind and lose structural integrity during the pull.30 With regards to intracranial haemorrhage, the Solitaire device exerts less outward radial pressure on the vessel than the Merci Retriever, limiting injury to vascular integrity. Also, by displacing the clot toward one part of the wall at the time of deployment, the Solitaire device disrupts circumferential thrombus adherence to the vessel wall before the application of withdrawal pressure. In a preclinical model, Solitaire use was associated with substantially less extensive endothelial and internal elastic lamina injury than was Merci use.31
Our trial has limitations. The design was unavoidably open-label, but potential for bias was mitigated by the use of a masked core imaging laboratory and a masked adjudication committee. Imbalances in a few baseline covariates occurred in the study groups; however, none of the salient results of the trial varied after multivariate adjustment for these diff erences. The trial compared the Solitaire stent retriever with the Merci coil retriever, which the US Food and Drug Administration has
endorsed as the appropriate com parator device for
mechanical thrombectomy trials. Comparisons were not made with suction thrombectomy devices, like the Penumbra System (Penumbra Inc, Alameda, CA, USA), or with other, emerging stent retrievers. Further randomised trials will be needed to delineate Solitaire’s reperfusion and clinical effi cacy and safety relative to these alternative devices. The early halt of the SWIFT trial resulted in a smaller than planned number of study participants, limiting the precision of treatment eff ect estimates. However, the magnitude of the diff erences was large, and the triggering of the interim stopping rule and the substantial mortality imbalance clearly precluded continuation of the study. On the basis of these results, when endovascular recanalisation is done in patients with acute ischaemic stroke, initial treatment with Solitaire might be a future treatment of choice.
Contributors
JLS, RJ, EIL, TGJ, BB, and RGN participated in the design of the study. All authors participated in the interpretation of the data. JLS wrote the report, which was revised after review by all coauthors, all of whom provided critical review of the paper. The statistical analysis was done by the sponsor and independently verifi ed by Jill Schafer. A list of all SWIFT triallists is given in the appendix.
Confl icts of interest
16 Roth C, Papanagiotou P, Behnke S, et al. Stent-assisted mechanical recanalization for treatment of acute intracerebral artery occlusions.
Stroke 2010; 41: 2559–67.
17 Machi P, Costalat V, Lobotesis K, et al. Solitaire FR thrombectomy system: immediate results in 56 consecutive acute ischemic stroke patients. J Neurointerv Surg 2012; 4: 62–66.
18 Noorian AR, Gupta R, Nogueira RG. Acute stroke: techniques and results with the Merci retriever. Tech Vasc Interv Radiol 2012;
15: 47–52.
19 Saver J, Jahan R, Levy E, et al. Solitaire with the intention for thrombectomy (SWIFT) trial: design of a randomized, controlled, multicenter study comparing the Solitaire Flow Restoration Device with the Merci Retriever in acute ischemic stroke. Int J Stroke
(in press).
20 Blackwelder WC. “Proving the null hypothesis” in clinical trials.
Control Clin Trials 1982; 3: 345–53.
21 Mancini GB, Schulzer M. Reporting risks and benefi ts of therapy by use of the concepts of unqualifi ed success and unmitigated failure: applications to highly cited trials in cardiovascular medicine.
Circulation 1999; 99: 377–83.
22 Soares BP, Chien JD, Wintermark M. MR and CT monitoring of recanalization, reperfusion, and penumbra salvage: everything that recanalizes does not necessarily reperfuse! Stroke 2009; 40: S24–27. 23 Rha JH, Saver JL. The impact of recanalization on ischemic stroke
outcome: a meta-analysis. Stroke 2007; 38: 967–73.
24 Rivero-Arias O, Ouellet M, Gray A, Wolstenholme J, Rothwell PM, Luengo-Fernandez R. Mapping the modifi ed Rankin scale (mRS) measurement into the generic EuroQol (EQ-5D) health outcome.
Med Decis Making 2010; 30: 341–54.
25 Hong KS, Saver JL. Quantifying the value of stroke disability outcomes: WHO global burden of disease project disability weights for each level of the modifi ed Rankin Scale. Stroke 2009;
40: 3828–33.
26 Mechanical retrieval and recanalization of stroke clots using embolectomy (MR RESCUE). 2011. http://clinicaltrials.gov/ct2/ show/NCT00389467?term=mr+rescue&rank=1 (accessed June 12, 2011).
27 Josephson SA, Saver JL, Smith WS. Comparison of mechanical embolectomy and intraarterial thrombolysis in acute ischemic stroke within the middle cerebral artery: Merci and Multi Merci compared to PROACT II. Neurocrit Care 2009; 10: 43–49. 28 NINDS statement: Interventional Management of Stroke III trial.
http://www.ninds.nih.gov/disorders/clinical_trials/NCT00359424. htm (accessed June 3, 2012).
29 Khatri P, Hill MD, Palesch YY, et al. Methodology of the interventional management of stroke III trial. Int J Stroke 2008;
3: 130–37.
30 Chueh JY, Wakhloo AK, Gounis MJ. Eff ectiveness of mechanical endovascular thrombectomy in a model system of cerebrovascular occlusion. AJNR Am J Neuroradiol 2012; published online May 3. DOI:10.3174/ajnr.A3103.
31 Mehra M, Hendricks GH, O’Callaghan JM, Wakhloo AK, Gounis MJ. Preclinical in-vivo safety evaluation of thrombectomy devices in the canine cerebrovasculature (abstract). Stroke 2012;