1 23
Journal of Food Measurement and
Characterization
ISSN 2193-4126
Volume 9
Number 3
Food Measure (2015) 9:454-462
DOI 10.1007/s11694-015-9253-4
Evaluation of in vitro antioxidant and
brine shrimp lethality activities of different
stem extracts of
Zizyphus rugosa
Lam
Md. Sazzad Hossain, Nizam
1 23
O R I G I N A L P A P E R
Evaluation of in vitro antioxidant and brine shrimp lethality
activities of different stem extracts of
Zizyphus rugosa
Lam
Md. Sazzad Hossain1•Nizam Uddin1•A. F. M. Mahmudul Islam1•
A. H. M. Nazmul Hasan1•Md. Monir Hossain1•Mohammad Raquibul Hasan1•
Md. Farhan Khalik1•Md. Sohel Rana1
Received: 27 May 2014 / Accepted: 26 April 2015 / Published online: 6 May 2015
Springer Science+Business Media New York 2015
Abstract Zizyphus rugosaLam (Z. rugosaLam) (Family: Rhamnaceae), has been regarded as a food and medicinal plant. It is locally known as ‘‘Bon Boroi’’ or as ‘‘Jongli Boroi’’ in Bangladesh. Its stem, roots and fruits are used medicinally for the treatment of carbuncle, syphilis, menorrhea, and ulcer tongue. It is also used for dysentery in Laos, Burma, Thailand, China (Hainan, Yunnan), Sri-Lanka and Vietnam. The present study was designed to investigate antioxidant prop-erties (through in vitro method) as well as brine shrimp lethality and phytochemical group evaluation of stem part of
Z. rugosaLam extracted with different solvents i.e. from non
polar to polar (petroleum ether[ethyl acetate[ethanol[
methanol[water). Phytochemical investigation showed the
presence of alkaloids, flavonoid, glycosides and carbohy-drates which provides evidence on good to moderate antioxidant and good lethality properties of the subjected plant. Ethyl acetate extract of stem was found to contain the highest amount of phenols (97.188±12.816 mg/g gallic
acid equivalent) and flavonoids (15.009±0.385 mg/g
quercetin equivalent). In 2,2-diphenyl,1-picrylhydrazyl (DPPH) free radical scavenging assay, among all the extracts ethanolic stem extract showed the highest scavenging prop-erty (IC5083.550lg/ml) whereas standard drug ascorbic acid
showed (IC5018.348lg/ml). But in nitric oxide (NO)
scav-enging assay maximum scavscav-enging of NO was found with water extract of stem (IC50 5.975lg/ml) comparatively
similar to standard ascorbic acid (IC50 5.934 lg/ml).
Methanolic stem extract was found to contain the greater
reducing power in reducing power capacity assessment (correlation coefficient r =0.99 and P\0.001). In brine
shrimp lethality bioassay (BSLB) among all extracts, ZSM part showed good activity (LC50 40.43 lg/ml) whereas
standard anticancer drug vincristine sulphate showed high toxicity (LC50 2.48lg/ml). The overall findings provide
scientific basis for the use ofZ. rugosaLam stem extracts in traditional medicine in the treatment of aforementioned dis-eases. Hence, the stem may serve as a new potential source of medication.
Keywords Jongli BoroiZizyphus roughIn vitro antioxidantLethalityPhytochemicals
Introduction
Natural products are considered as the prime source of most of the active ingredients of modern medicines. Nat-ural product, a chemical substance produced by a living organism; a term used commonly in reference to chemical substances found in nature that have distinctive pharma-cological effects. When applied to drug discovery in ‘olden times’ before the advent of high-throughput screening and the post-genomic era: more than 80 % of drug substances were natural products or inspired by a natural compound [1]. Natural compounds provide a strategic starting to synthesize new compounds, that have diversified structures and often with multiple stereocenters [2,3]. More than 100 natural-product-derived compounds are currently under-going clinical trials and at least 100 similar projects are in preclinical development. Most are derived from leads from plants and microbial sources [4]. Most of the today’s medicines are obtained directly from the natural source.
& Md. Sazzad Hossain sazzad.phrm@gmail.com
1 Department of Pharmacy, Jahangirnagar University, Savar,
Medicinal plants are various plants that possess therapeutic properties or exert beneficial pharmacological effects on the animal body [5].
Zizyphus rugosaLam (Z. rugosaLam) appears as pulpy
white or pinkish in colour and has a mildly sweet taste. It is the species of plant in the Rhamnaceae family. They are found on the hills in bunches on thorny branches of theZ.
rugosaLam trees. It is found in hills and mountains below
1400 m altitude and its bark and wood are used medicinally for dysentery in Laos, China (Hainan, Yun-nan), India, Burma, Sri Lanka, Thailand and Vietnam. Its leaf size is larger than normal Plum. Leaves are elliptic, often rounded from an oblique or cordate base, 5–13 cm long, densely tawny–villous and paler beneath and Fruits are drupe, globose or pear-shaped, about 2 cm long. Its stem is commonly used for the treatment of diarrhea while the flowers, together with leaves, are used in menorrhagia [6]. Its bark, roots and fruits are also used medicinally for the treatment of carbuncle, syphilis, menorrhea, and ulcer tongue [7]. A new glycoside zizyphoside has been isolated along with the betulicoleanolic, alphitolic and 2-a
-hydroxyrusolic acids; three flavonoids—kaempferol-40 -methylether, luteolin and luteolin-7-O-glucoside have been isolated from the barks of Z. rugosa Lam [8]. The cy-clopeptide alkaloids of the plant show antibacterial as well as antifungal activity [9].
This study aims to investigate antioxidant and brine shrimp lethality activities of five different stem extracts of
Z. rugosaLam using various in vitro assay models.
Methodology
Study design
The present protocol was designed to evaluate the phyto-chemical group, antioxidant and cytotoxic potentiality of stem extract of Z. rugosa Lam extracted with different solvent i.e., from non polar to polar i.e., petroleum ether[ethyl acetate[ethanol[methanol[water.
Collection and identification of plant material
The whole plants ofZ. rugosa Lam were collected from Jahangirnagar University campus, Savar, Dhaka, Bangla-desh in the month of October, 2013 and was identified by Mr. Abdur Rahim, Technical officer, Department of Bot-any, Jahangirnagar University, Savar, Dhaka, Bangladesh (DACB accession No. 38729) where voucher specimen has been deposited for further reference. The specimen samples were kept in the Laboratory of Natural Products Research in the Department of Pharmacy, Jahangirnagar University, Bangladesh.
Chemicals and reagents
DPPH (2,2-diphenyl,1-picrylhydrazyl), ascorbic acid, sodi-um nitroprusside, sodisodi-um phosphate, sulphanilamide, phosphoric acid and naphthylethylenediamine were ob-tained from SD Fine Chem. Ltd. India. Ammonium molyb-date was obtained from Merck, Germany. Ferric chloride and neocaproine were obtained from Sigma Chemical Co. Preparation of plant material and extraction procedure
Stem or branches of the plants were first washed with water to remove adhering dirt and then cut into small pieces and sun-dried for few days and then, sun-dried in a hot air oven (size 1, Gallenkamp) at reduced temperature (not more than 50C). After that stem parts were grinded into coarse powders using high capacity grinding mill which were then stored in air-tight container with necessary markings for identification and kept in cool, dark and dry place for the investigation.
The powdered plant materials were used for extraction by Soxhlet apparatus at elevated temperature (65C) using pet-roleum ether, ethyl acetate, ethanol and methanol con-secutively (500 ml of each solvent). After each extraction the plant material was dried and used again for the next extraction. Extraction was considered to be complete when the plant materials become exhausted of their constituents that were confirmed from cycles of colorless liquid siphoning in the Soxhlet apparatus. After methanol extraction was completed the plant materials were dried and soaked into distilled water (1 L). The plant materials were kept in water for 7 days in sealed container accompanying occasional shaking and string. All five extracts of stem part were filtered individually through fresh cotton bed. The filtrates obtained were dried at tem-perature of 40±2C to have gummy concentrate of the crude extracts. Each extract was kept in suitable container with proper labeling and stored in cold and dry place [10]. Phytochemical test
The freshly prepared crude extract was qualitatively tested for the presence of chemical constituents i.e., carbohy-drates, flavonoids, glucosides, steroids, saponins, tannins and alkaloids. These were identified by characteristic color changes using standard procedures [5].
Antioxidant activity evaluation
Total phenol content determination
Total phenolic contents of the fractions were determined by Folin–Ciocalteu reagent (FCR) [11, 12]. 1.0 ml of each
Evaluation of in vitro antioxidant and brine shrimp lethality activities of different stem… 455
123
plant extract (200lg/ml), standard (gallic acid) of different
concentrations and 5 ml of FCR (diluted 10 fold) reagent solution were taken in marked test tubes and 4 ml of 7.5 % sodium carbonate solution was added. The test tubes were incubated at 20C (30 min for standard solutions and 1 h for extract solution). Absorbance at 765 nm was deter-mined using a UV–Vis spectrophotometer (Shimadzu UV PC-1600) against blank. Total phenol contents of the fractions were expressed as gallic acid equivalents (GAE). Determination of total flavonoid content
Total flavonoid was determined using the aluminum chlo-ride colorimetric method described by Wang and Jiao [13]. 1.0 ml plant extracts (200lg/ml) and standard (Quercetin)
were added to 3 ml of methanol and 200ll of 10 %
alu-minium chloride solution, 200ll of 1 M potassium acetate
solution and 5.6 ml of distilled water were added and then incubated for 30 min at room temperature to complete the reaction. Absorbance of the solution was measured at 415 nm using a spectrophotometer (Shimadzu UV PC-1600) against blank. Total Flavonoid contents of the frac-tions were expressed as Quercetin equivalents (QE). Determination of total antioxidant capacity
Total antioxidant capacity of the plant extract was deter-mined following the method described by ascorbic acid equivalents (AAE) [14]. 300ll of each fraction (200 lg/
ml) and standard (ascorbic acid) in different concentrations were taken in test tubes and 3 ml of reagent solution (0.6 M sulfuric acid, 28 mM sodium phosphate and 4 mM ammonium molybdate) was added. The test tubes were incubated at 95C for 90 min to complete the reaction. Absorbance of the solution was measured at 695 nm using a spectrophotometer (Shimadzu UV PC-1600) against blank after cooling to room temperature. Total antioxidant capacity was expressed as the number of AAE.
DPPH free radical scavenging assay
DPPH free radical scavenging activity of the plant fractions was determined following the method described by Braca et al. [15]. Plant extract (1.0 ml) and standard (ascorbic acid) was added to 2 ml of a 0.004 % methanol solution of DPPH and incubated for 30 min. Absorbance at 517 nm was determined and IC50values were calculated.
Nitric oxide (NO) scavenging capacity assay
Nitric oxide (NO) radical scavenging was estimated on the basis of Griess–Illosvoy reaction [16]. In this investigation, Griess–Illosvoy reagent was modified by using naphthyl
ethylene diamine dihydrochloride (0.1 % w/v) instead of 1-naphthylamine (5 %). 4.0 ml of each fraction and stan-dard (ascorbic acid) was added into 1.0 ml of sodium ni-troprusside (5 mM) solution and incubated for 2 h at 30C to complete reaction. Then 2.0 ml solution was withdrawn from the mixture and mixed with 1.2 ml of Griess reagent. Absorbance of the solution was measured at 550 nm using a spectrophotometer (Shimadzu UV PC-1600) against blank and IC50values were calculated.
Reducing power capacity assessment
Reducing power capacity assessment of the plant extracts was determined following the method described by Oyaizu et al. [17]. 2.0 ml of each plant extracts or standard of different concentration solutions were taken and 2.5 ml of potassium ferricyanide [K3Fe(CN)6], 1 % solution was
added into each of test tubes. The test tubes were incubated for 10 min at 50C and 2.5 ml of trichloro acetic acid, 10 % solution was added. The resultant mixtures were centrifuged at 3000 rpm for 10 min and 2.5 ml supernatant solution was withdrawn from each of the mixtures and mixed with 2.5 ml of distilled water. Then 0.5 ml of ferric chloride (Fe3Cl), 0.1 % solution was added. The
ab-sorbances of the solutions were measured at 700 nm using a spectrophotometer against a typical blank solution. Cupric reducing antioxidant capacity (CUPRAC) Cupric reducing antioxidant capacity of the plant extracts was determined following the method described by Resat et al. [18]. 500ll of each fraction and standard (ascorbic acid) in
different concentrations were taken in test tubes. 1.0 ml of 0.01 M CuCl22H2O solution and 1.0 ml of ammonium
ac-etate buffer (pH 7.0) was added into the test tubes. Then 1.0 ml of 0.0075 M of neocaproine solution and 600ll of distilled
water was added into the test tubes. The total mixture was incubated for 1 h at room temperature then the absorbance of the solution was measured at 450 nm using a spectropho-tometer (Shimadzu UV PC-1600) against blank. The molar absorptivity of the CUPRAC method for each antioxidant was found from the slope of the calibration line concerned. Total alkaloid content determination
Total alkaloid content was determined by slightly modified Fazel et al. [19] method. 4 mg of each plant extract was dissolved in 4 ml 2 N HCl to get the concentration 1 mg/ ml. Then 5 times dilution was done by adding 1 ml of this solution into 4 ml 2 N HCl to get the concentration of stock solution 200 lg/ml. 1 ml stock solution of each plant
extract, 5 ml phosphate buffer and 5 ml BCG (Bromocre-sol green) (Bromocre-solution taken in a separating funnel, shaken
well and 5 ml CHCl3 was added. The funnel was shaken
vigorously and the lower layer of the solution was collected in a labeled test tube. The absorbance of the complex in chloroform was measured at 470 nm. A typical blank so-lution contained the same soso-lution mixture without plant extract or standard. Total alkaloid content is expressed as the number of equivalents of atropine (AE).
Total tannin content determination
The tannins were determined by slightly modified Folin and Ciocalteu method [11]. 0.1 ml of the sample extract (200lg/ml) was added with 7.5 ml of distilled water.
0.5 ml of Folin Phenol reagent (diluted 10 fold) solution was added into the test tubes. 1 ml of 35 % sodium car-bonate solution was added into the test tubes. The volume was adjusted up to 10 ml with distilled water. The mixture was shaken well, kept at room temperature for 30 min. The absorbance was measured at 725 nm. A typical blank was prepared with water instead of the sample. A set of stan-dard solutions of Tannic acid is treated in the same manner as described earlier and read against a blank. The results of tannins are expressed in terms of tannic acid in mg/g TAE of extract.
Brine shrimp lethality bioassay (BSLB)
Lethal activity of the plant extract was determined by Brine shrimp lethality bioassay described by Meyer et al. [20]. Brine shrimp lethality bioassay [20–22] is a rapid and comprehensive bioassay for the bioactive compounds of natural and synthetic origin. Brine shrimp eggs (Artemia
salina leach) are hatched in simulated seawater to get
nauplii. Sample solutions are prepared by dissolving the test materials in pre-calculated amount of DMSO (di-methyl sulphoxide). Ten nauplii are taken in vials taining simulated seawater. The samples of different con-centrations are added and the volume was adjusted up to 5 ml. Survivors were counted after 24 h. Vincristine sul-phate is usually used as the reference cytotoxic drug. The mortality was corrected using Abott’s formula [23] Pt¼ ½ðPo PcÞ=ð100 PcÞ 100;
where, Po=observed mortality and Pc=control
mor-tality. LC50 values of the test samples after 24 h are
ob-tained by regression analysis. Statistical analysis
Values were presented as mean±SD (standard deviation). IC50and LC50 values were obtained with the help of
Mi-crosoft excel 2007. Pearson correlation analysis and one way ANOVA followed by Tukey multiple comparison
were performed to analyze the data set using Graph pad prism version 5.00 (Graph Pad Software Inc., San Diego, CA, USA) and SPSS version 16 (IBM software Inc, USA).
Results
Phytochemical group evaluation test
In the present study, various qualitative tests were done to detect the presence of various phytochemical compounds in the petroleum ether, ethyl acetate, ethanol, methanol and water extracts of the stem or branches part of Z. rugosa
Lam. Preliminary phytochemical screening of the crude extracts of different parts of Z. rugosa Lam showed the presence of different kind of phytochemical groups that can be summarized in Table 1.
Antioxidant activity evaluation
Total phenol content determination
The total phenolic contents of the test fractions were cal-culated using the standard curve of Gallic acid and were expressed as GAE per gram of plant extract. ZSEA was found to contain the highest amount of phenols (97.188±12.816 mg/g) and ZSE showed the lowest
amount (32.500 ±10.607 mg/g). Phenol contents of the
extracts were found to decrease in the following order: ZSEA[ZSPE[ZSM[ZSW[ZSE (Table2).
Total flavonoid content determination
The total flavonoid content was calculated using the standard curve of Quercetin. Flavonoid contents of the test fractions were found as mg/g Quercetin equivalent (QE). ZSEA was found to contain the highest amount of Flavonoid (15.009±0.385 mg/g) and ZSE was found to contain the
lowest amount of flavonoid (0.293±0.003 mg/g). Flavo-noid contents of the extracts were found to decrease in the following order: ZSEA[ZSPE[ZSM[ZSW[ZSE
(Table2).
Total antioxidant capacity assessment
Total antioxidant capacity ofZ. rugosaLam stem extract was evaluated by the phosphomolybdenum method and was ex-pressed as AAE per gram of plant extract. ZSM was found to contain the highest (37.500±3.712 mg/g) and ZSPE was
found to contain the lowest (16.875 ±4.773 mg/g) amount of total antioxidant capacity. Total antioxidant capacities of
Evaluation of in vitro antioxidant and brine shrimp lethality activities of different stem… 457
123
the extracts were found to decrease in the following order: ZSM[ZSW[ZSEA[ZSE[ZSPE (Table2).
DPPH free radical scavenging assay
DPPH scavenging assay has been widely used to evaluate the free radical scavenging capacity of antioxidant. The IC50
values of different extracts ofZ. rugosaLam are summarized in the Table3. ZSE was found to contain the lowest IC50value
(83.550lg/ml) that represents the highest scavenging
prop-erty than the other tested extracts. The IC50value of standard
drug ascorbic acid was 18.348lg/ml. DPPH free radical
scavenging activity of the plant extracts ofZ. rugosaLam was found to decrease in the following order: ZSE[ZSM[
ZSW[ZSEA[ZSPE.
Nitric oxide (NO) scavenging capacity assay
Different extracts of the stem part exhibited dose depen-dent scavenging of nitric oxide. Maximum scavenging of NO was found with ZSW with an IC50value of 5.975lg/
ml; the result was similar to standard ascorbic acid (IC50
value 5.934lg/ml) can be summarized in the Table3. NO
scavenging activity decreased in the following order; ZSW[ZSM[ZSE[ZSPE[ZSEA that are
summa-rized in the Table3.
Reducing power capacity assessment
Reducing power of the tested extracts were assessed using ferric to ferrous reduction activity as determined spec-trophotometrically from the formation of Perl’s Prussian blue color complex [24]. The standard ascorbic acid showed the highest reducing capacity. The stem extracts of
Z. rugosaLam showed reducing capacity. ZSM was found
to contain more reducing power than other extracts (cor-relation coefficient r=0.99 and P\0.001) presented in
Fig.1.
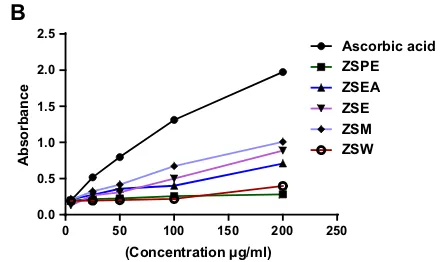
Cupric reducing antioxidant capacity (CUPRAC) Reduction of Cu2? ion to Cu? was found to rise with increasing concentrations of the different extracts. The standard ascorbic acid showed the highest reducing ca-pacity in CUPRAC method. Comparison graph of cupric reducing antioxidant capacity (CUPRAC) between ascor-bic acid and Z. rugosa Lam stem extracts are given in Fig.1.
Total alkaloid content determination
Total Alkaloid content ofZ. rugosaLam was determined by Dragendroff’s method and was expressed as atropine
equivalents (AE) mg/g of plant extract. ZSW was found to contain the highest amount of Alkaloid (38.135±0.003 mg/g) and ZSEA was found to contain the lowest amount of al-kaloid (24.062±0.001 mg/g). Alkaloid contents of five
different extracts did not find significantly (P[0.05).
Alkaloid contents of the extracts were found to decrease in the following order; ZSW[ZSE[ZSPE[ZSM[
ZSEA (Table2).
Table 2 Summarized anti-oxidant results of stem extracts ofZ. rugosaLam
Extract Total phenol
Values are presented as mean±SD (n=3). One way ANOVA followed by Tukey multiple comparisons was performed to analyze the data sets. Values in same column with different superscripts are significantly different from one another (P\0.05)
Table 3 IC50and LC50values of the different extracts in DPPH, NO and Brine shrimp lethality bioassays
Extract DPPH free radical
scavenging assay, IC50(lg/ml)
Nitric oxide scavenging capacity assay, IC50(lg/ml)
Brine shrimp lethality
Ascorbic acid 18,348 5.934 –
Vincristine sulphate – – 2.48
0 50 100 150 200
Fig. 1 aCuprac reducing assessment of five different stem extracts and standard. Values are presented as mean±SEM (n=3). Pearson correlation analysis was performed between different concentrations and absorbance of each stem extract and standard. For ZSPE, correlation coefficient r=0.98 and P\0.01; for ZSEA, r=0.98 andP\0.01; ZSE, correlation coefficient r=0.99 andP\0.0001; for ZSM, correlation coefficient r=0.98 and P\0.01; for ZSW, correlation coefficient r=0.95 and P\0.05; for ascorbic acid, r=0.999 andP\0.001.bReducing power capacity assessment of
five different stem extracts and standard. Values are presented as mean±SEM (n=3). Pearson correlation analysis was performed between different concentrations and absorbance of each stem extract and standard. For ZSPE, correlation coefficient r=0.97 and P\0.01; for ZSEA, r=0.98 and P\0.01; ZSE, correlation coefficient r=0.99 andP\0.001; for ZSM, correlation coefficient r=0.99 andP\0.001; for ZSW, correlation coefficient r=0.93 andP\0.001; for ascorbic acid, r=0.98 andP\0.05
Evaluation of in vitro antioxidant and brine shrimp lethality activities of different stem… 459
123
Total tannin content determination
Total Tannin content ofZ. rugosaLam was determined by slightly modified Folin and Ciocalteu method [11] and was expressed as tannic acid equivalent (TAE) mg/g of plant extract. ZSM was found to contain the highest amount of total tannin that can be expressed as tannic acid equivalent (221.026±0.050 mg/g). Tannin contents of the extracts were found to decrease in the following order; ZSM[ZSEA[ZSPE[ZSW[ZSE (Table2).
Brine shrimp lethality bioassay (BSLB)
In this study methanol extract of stem part (ZSM) showed most toxicity to brine shrimp nauplii, with LC50 value of
40.43lg/ml whereas anticancer drug Vincristine sulphate
showed LC50 value 2.48lg/ml described in the Table3.
The order at which cytotoxic potential of the test samples decreased was as follows: ZSM[ZSPE[ZSW[
ZSEA[ZSE.
Discussion
Several phytochemical study reported that cyclopeptide alkaloids [25,26] and six flavones glycosides [27] and one saponine were [28] isolated from root barks ofZ. rugosa. In continuation of this study, we have extracted this plant with different solvents and a preliminary screening showed that ZSPE, ZSEA and ZSM were rich in flavonoids. It can be assumed that Z. rugosa Lam stem are rich in alkaloids, flavonoids, tannins, carbohydrates, steroids, saponin which may account for their various pharmacological activities.
Antioxidative properties of polyphenols arise from their high reactivity as hydrogen or electron donors which can stabilize and delocalize the unpaired electron (chain-breaking function) and from their potential to chelate metal ions (termination of the Fenton reaction) [29]. Polyphenols have been shown to block LDL oxidation, decrease the formation of atherosclerotic plaques and reduce arterial stiffness, leaving arteries more responsive to endogenous stimuli of vasodilation [30–32]. This ability is believed to be mainly due to their redox properties [33], which play an important role in adsorbing and neutralizing free radicals. The results strongly suggest that phenolics are important components of the tested plant extractsZ. rugosaLam stem extract showed good to moderate amount of phenolic content which is showed in gallic acid equivalent. Flavo-noids play an important role in antioxidant system in plants. The antioxidative properties of flavonoids are due to several different mechanisms, such as scavenging of free radicals, chelation of metal ions, such as iron and copper, and inhibition of enzymes responsible for free radical
generation [34]. Depending on their structure, flavonoids are able to scavenge practically all known ROS. The stem extract ofZ. rugosaLam has been shown to possess mild to moderate amount of flavonoids. The total antioxidant ac-tivity of stem extracts of Z. rugosa Lam was evaluated from their ability to reduce phosphate/Mo(VI) complex to phosphate/Mo(V). According to recent reports, a highly positive relationship between total phenols and antioxidant activity appears to be the trend in many plant species [35].
Z. rugosa Lam stem extracts showed moderate content
which is showed in ascorbic acid equivalent. DPPH radical scavenging is a popular and reliable method for screening the free radical scavenging activity of compounds or an-tioxidant capacity of plant extracts [12,36–38]. In DPPH radical scavenging assays, the stem extracts of Z. rugosa
Lam showed good to moderate scavenging of DPPH radicals in a way similar to that of the reference antioxidant ascorbic acid. NO is generated in biological tissues by specific nitric oxide syntheses (NOSs), which metabolizes arginine to citrulline with the formation of NO via a five electron oxidative reaction [39]. The toxicity and damage caused by NO- and O2
-is multiplied as they react to produce reactive peroxynitrite (ONOO-), which leads to serious toxic reactions with biomolecules as mentioned above [40–42]. Our findings indicate that all of the five plant extracts showed good NO scavenging activity. The reducing power of the extracts of the selected plants might be due to its hydrogen donating ability [43]. The an-tioxidant activity has been reported to be concomitant with the development of reducing power [44]. Among the ex-tracts ZSM exhibited the most reducing power in reducing power capacity assessment. Reducing power is associated with antioxidant activity and may serve as a significant reflection of the antioxidant activity [35]. The standard ascorbic acid showed the highest reducing capacity. The stem extracts ofZ. rugosaLam showed reducing capacity. It also showed to possess moderate amount of Alkaloids which were also identified by phytochemical group evaluation test. Moreover, the extracts also showed to possess good to moderate amount of tannin content. These phytochemicals may strengthen the antioxidant potential of the stem.
The brine shrimp lethality bioassay has been considered as a practical, safe and economic method for determination of bioactivities of synthetic compound as well as plant products [20]. In Brine Shrimp lethality bioassay all of the samples showed considerable lethality. The stem extracts
of Z. rugosaLam has showed good cytotoxic potentiality.
The toxicity of plants is principally contributed by the presence of alkaloids, glycosides, steroids, tannins, phlo-batannins, terpenoids and flavonoids [45–48]. However, phenolics and flavonoids are also known to show cyto-toxicity in Hoechst 33258 fluorescence assay by inhibiting
cellular DNA in a concentration-dependent manner [49]. Different phytochemical group like alkaloids, glycosides, steroids, tannins and flavonoids were found in our obser-vation (Table1). So the observed cytotoxic action may be due to the presence of these phytochemical constituents, although further studies are required to be confirmed.
Conclusion
The results from the experiments confirmed that the stem extract of Z. rugosa Lam in general, possesses good to moderate antioxidant and good brine shrimp lethality prop-erties. Various phytochemical constituents like terpenoids, glycosides and steroids present in the plant, which may be responsible for the observed activities. The good toxicity exerted by the extracts ofZ. rugosaLam in brine shrimp lethality bioassay suggests the presence of bioactive princi-ples in the plant. However, further studies are suggested to be undertaken to understand the underlying mechanism of the observed activities and to isolate, purify and characterize active phytochemical compounds that are responsible for these bioactivities in animal models and to carry out advance research on cancer because the other plants of Rhamnaceae family have significant cytotoxic and inhibitory activity on tumors of various types of cancers. Besides, these extracts can be used as promising source of natural antioxidants which can work as stabilizing agent against oxidative dete-rioration in pharmaceutical applications.
Acknowledgments We are greatly thankful to Laboratory of Nat-ural Products Research (LNPR), Department of Pharmacy, Jahangir-nagar University to provide sufficient laboratory facilities to carry out this research.
References
1. W. Sneader,Drug prototypes and their exploitation(Wiley, UK., 1996)
2. J. Clardy, C. Walsh, Nature432(7019), 829–837 (2004) 3. F.E. Koehn, G.T. Carter, Nat. Rev. Drug Discov.4(3), 206–220
(2005)
4. M.S. Butler, Nat. Prod. Rep.25, 475–516 (2008)
5. Ghani A. Medicinal Plants of Bangladesh. 2ndedn. (The Asiatic Society of Bangladesh. Dhaka, Bangladesh, 2003), pp. 31, 39-40, 418, 500-504, 589-580
6. K.R. Kirtikar, B.D. Basu, E. Blatter, J.F. Cains, K.S. Mhaskar,
Indian medicinal plants, vol. 1 (Lalit Mohan Basu, Allahabad,
India, 1975), p. 594
7. Dinesh Kumar Tyagi,Pharma forestry. Field guide to medicinal
plants (Atlantic Publishers and Distributors, New Delhi, 2005),
p. 147
8. A. Singh, M.B. Pandey, S. Singh, A.K. Singh, J.P. Singh, J. In-dian Chem. Soc.86(2), 177–178 (2009)
9. C.P. Khare, C.P. Khare,Indian medicinal plants: an illustrated
dictionary(Springer Verlag, Berlin, 2007), p. 737
10. R.J.P. Cannel,How to approach the isolation of a natural
pro-duct, in natural products isolation, 1st edn. (Humana Press, New
Jersey, 1998), pp. 1–51
11. Y.S. Velioglu, G. Mazza, L. Gao, B.D. Oomah, J. Agric. Food Chem.46, 4113–4117 (1998)
12. L. Yu, J. Agric. Food Chem.49, 3452–3456 (2001)
13. S.Y. Wang, H. Jiao, J. Agric. Food Chem.48, 5672–5676 (2000) 14. P. Prieto, M. Pineda, M. Aguilar, Anal. Biochem.269, 337–341
(1999)
15. A. Braca, N.D. Tommasi, L.D. Bari, C. Pizza, M. Politi, I. Morelli, J. Nat. Prod.64, 892–895 (2001)
16. R. Govindarajan, S. Rastogi, M. Vijayakumar, A. Shirwaikar, A.K.S. Rawat, S. Mehrotra, P. Palpu, Biol. Pharm. Bull. 26, 1424–1427 (2003)
17. M. Oyaizu, Jpn. J. Nutr.44, 307–315 (1986)
18. A. Resat, G. Kubilay, O. Mustafa, E.K. Saliha, J. Agric. Food Chem.52, 7970–7981 (2004)
19. Fazel Shami, Hamidreza Monsef, Rouhollah Ghamooshi, M. Verdian-Ariza, J. Pharma. Sci.32, 17-20 (2008)
20. B.N. Meyer, N.R. Ferrigni, J.E. Putnam, L.B. Jacobsen, Planta Med.45, 31–34 (1982)
21. X.D. Luo, S.H. Wu, Y.B. Ma, D.G. Wu, Fitoterapia 71(5), 492–496 (2000)
22. J.L. Mclaughlin, J.E. Anderson, L.L. Rogers, Drug Info. J.32, 513–524 (1998)
23. W.S. Abott, J. Am. Mosq. Control Assoc.3(2), 302–303 (1987) 24. A. Yildirim, A. Mavi, M. Oktay, A.A. Kara, J. Agric. Food
Chem.48, 5030–5034 (2000)
25. Y.C. Tripathi, S.K. Maurye, V.P. Singh, V.B. Pandey, Phyto-chemistry28(5), 1563 (1986)
26. V.B. Pandey, Y.C. Tripathi, S. Devi, J.P. Singh, A.H. Shah, Phytochemistry27(6), 1915–1918 (1988)
27. Y.C. Tripathi, S. Devi, V.B. Pandey, A.H. Shah, Fitoterapia 59(2), 158 (1988)
28. V.B. Pandey, Y.C. Tripathi, Fitoterapia64(4), 341–343 (1993) 29. C. Rice-Evans, N. Miller, G. Paganga, Trends Plant Sci. 2,
152–159 (1997)
30. J. Moline, I.F. Bukharovich, M.S. Wolff, Med. Hypoth. 55(4), 306–309 (2000)
31. Y. Arai, S. Wantanabe, M. Kimira, K. Shimoi, Am. Soc. Nutr. Sci.130(9), 2243–2250 (2000)
32. G.G. Duthie, S.J. Duthei, J.A.M. Kyle, Nutr. Res. Rev. 13, 79–106 (2000)
33. W. Zheng, Y.S. Wang, J. Agric. Food Chem. 49, 5165–5170 (2001)
34. O. Benavente-Garcia, J. Castillo, F.R. Marin, A. Ortun˜o, J.A. Del-Rio, J. Agric. Food Chem.45, 4505–4515 (1997)
35. M. Oktay, I. Gulcin, O.I. Kufrevioglu, LWT36, 263–271 (2003) 36. M.E.H. Mazumder, S. Rahman, Pharm. Biol. 46(10), 704–709
(2008)
37. W. Brand-Willams, M.E. Cuvelier, C. Berset, LWT28, 25–30 (1995)
38. J.C. Espin, C. Soler-Rivas, H.J. Wichers, J. Agric. Food Chem. 48, 648–656 (2000)
39. R.J. Ruch et al., Carcinogenesis10, 1003–1008 (1989) 40. R. Radi, J.S. Beckman, K.M. Bush, B.A. Freeman, J. Biol. Chem.
266, 4244–4250 (1991)
41. S. Moncada, R.M. Palmer, E.A. Higgs, Pharmacol. Rev. 43, 109–142 (1991)
42. J.S. Beckman, T.W. Beckman, J. Chen, P.A. Marshall, B.A. Freeman, Proc. Nat. Acad. Sci. USA87, 1620–1623 (1990) 43. K. Shimada, K. Fujukawa, K. Yahara, T. Nakamura, J. Agric,
Food Chem.40, 945–948 (1992)
44. M. Tanaka, C.W. Kuei, Y. Nagashima, T. Taguchi, Nippon Suisan Gakkaishi54, 1409–1414 (1998)
Evaluation of in vitro antioxidant and brine shrimp lethality activities of different stem… 461
123
45. B. Ozcelik, M. Kartal, I. Orhan, Pharm. Biol. 9(4), 396–402 (2011)
46. M.L. Dhar, M.N. Dhar, B.N. Dhawan, Ind. J. Expt. Biol. 11, 43–45 (1973)
47. P. Vijayan, V. Rreethi, S.H. Prashanth, H. Raghu, Biol. Pharm. Bull.24, 528–530 (2004)
48. S. Badami, S.A. Manohara, E.P. Kumar, Phytother. Res. 17, 1001–1004 (2003)
49. C.C. Chang, M.H. Yang, H.M. Wen, J.C.J. Chern, Food Drug Anal.10, 178–182 (2002)