The pharmacokinetics of cefepime in
The pharmacokinetics of cefepime in
E. coli
E. coli
lipopolysaccharide
lipopolysaccharide
induced febrile buffalo calves
induced febrile buffalo calves
Bharat Joshi, andBharat Joshi, and Suresh Kumar Sharma*Suresh Kumar Sharma*
Department of Veterinary Pharmacology and Toxicology, College of Veterinary Sciences, Guru Angad Dev Department of Veterinary Pharmacology and Toxicology, College of Veterinary Sciences, Guru Angad Dev
Veterinary and Animal Sciences University, Ludhiana, India Veterinary and Animal Sciences University, Ludhiana, India JOSHI, B., S. K. SHARMA
JOSHI, B., S. K. SHARMA: The pharmacokinetics of cefepime in The pharmacokinetics of cefepime in E. coliE. coli lipopolysaccharide induced febrile buffalo calves.
lipopolysaccharide induced febrile buffalo calves. Vet. arhiv 79, 523-530, 2009.
ABSTRACT
The pharmacokinetics of cefepime after its single intravenous administration (10 mg/kg) was The pharmacokinetics of cefepime after its single intravenous administration (10 mg/kg) was investigated in experimentally induced fever in buffalo calves (n = 4). The fever was induced by single/ investigated in experimentally induced fever in buffalo calves (n = 4). The fever was induced by single/ repeated intravenous injection of
repeated intravenous injection of E. coliE. coli lipopolysaccaride (1 lipopolysaccaride (1 μμg/kg). The drug was estimated in plasma samples g/kg). The drug was estimated in plasma samples by microbiological assay using
by microbiological assay using E. coliE. coli (MTCC 739) test organism. The pharmacokinetic behaviour of cefepime (MTCC 739) test organism. The pharmacokinetic behaviour of cefepime
in febrile animals was described by a two compartment open model. At 1 min, the concentration of cefepime in febrile animals was described by a two compartment open model. At 1 min, the concentration of cefepime
in plasma was 40.8 ± 0.98 μg/mL which rapidly declined to 23.0 ± 0.64 μg/mL at 15 min. The drug
in plasma was 40.8 ± 0.98 μg/mL which rapidly declined to 23.0 ± 0.64 μg/mL at 15 min. The drug
was detected up to 24 h. The elimination half-life and volume of distribution were 3.00 ± 0.18 h and was detected up to 24 h. The elimination half-life and volume of distribution were 3.00 ± 0.18 h and 0.42 ± 0.02 L/kg, respectively. The distribution half-life, AUC and total body clearance (Cl
0.42 ± 0.02 L/kg, respectively. The distribution half-life, AUC and total body clearance (ClBB) were ) were
0.08 ± 0.002 h, 101 ± 7.65 μg/mL.h and 98.8 ± 6.06 mL/kg/h, respectively. To maintain a minimum
0.08 ± 0.002 h, 101 ± 7.65 μg/mL.h and 98.8 ± 6.06 mL/kg/h, respectively. To maintain a minimum
therapeutic concentration of 1
therapeutic concentration of 1 μμg/mL, a satisfactory dosage regimen of cefepime in febrile buffalo calves would g/mL, a satisfactory dosage regimen of cefepime in febrile buffalo calves would be 7 mg/kg repeated at 12 h intervals.
be 7 mg/kg repeated at 12 h intervals.
Key words:
Key words: buffalo calf, cefepime, dosage regimen, fever, pharmacokinetics buffalo calf, cefepime, dosage regimen, fever, pharmacokinetics
Introduction Introduction
Cefepime, is a parenteral semisynthetic bactericidal cephalosporin having Cefepime, is a parenteral semisynthetic bactericidal cephalosporin having excellent activity against a broad spectrum of clinically important pathogens, including excellent activity against a broad spectrum of clinically important pathogens, including
Staphylococcus aureus
Staphylococcus aureus and and Pseudomonas aeruginosa Pseudomonas aeruginosa strains resistant to other strains resistant to other
broad spectrum cephalosporins (
broad spectrum cephalosporins (BARBHAIYA et al., 1992BARBHAIYA et al., 1992). The pharmacokinetics of ). The pharmacokinetics of chemotherapeutic agents are markedly altered in disease conditions (
chemotherapeutic agents are markedly altered in disease conditions (LESAR and ZASKE LESAR and ZASKE 1984; HARY et al., 1989; TOTH et al., 1991;
1984; HARY et al., 1989; TOTH et al., 1991; SINGH et al., 1998; CHAUDHARY et al., 1999; SINGH et al., 1998; CHAUDHARY et al., 1999; DARDI et al., 2005; SHARMA et al., 2005
DARDI et al., 2005; SHARMA et al., 2005), hence the dosage regimen obtained in healthy ), hence the dosage regimen obtained in healthy subjects cannot be always extrapolated in clinical cases to treat diseased animals. Fever, subjects cannot be always extrapolated in clinical cases to treat diseased animals. Fever, *Corresponding author:
Dr Suresh Kumar Sharma, Associate Professor, Department of Veterinary Pharmacology and Toxicology, College of Veterinary Dr Suresh Kumar Sharma, Associate Professor, Department of Veterinary Pharmacology and Toxicology, College of Veterinary Sciences, Guru Angad Dev Veterinary and Animal Sciences University, Ludhiana
Sciences, Guru Angad Dev Veterinary and Animal Sciences University, Ludhiana - 141 004 - 141 004, India, India, , Phone: +91 161 241 4032; Phone: +91 161 241 4032; Fax: +91 161 240 0822; E-mail: sureshpau2000@yahoo.com
which is one of the most common manifestations of many infectious diseases, is reported which is one of the most common manifestations of many infectious diseases, is reported to induce a series of biochemical and physiological alterations in cells (
to induce a series of biochemical and physiological alterations in cells (MONSHOUWER MONSHOUWER et al., 1996
et al., 1996). So, a study of the in). So, a study of the inflfl uence of fever on the pharmacokinetics of antibiotics uence of fever on the pharmacokinetics of antibiotics is essential. The purpose of this study was to determine the pharmacokinetic variables is essential. The purpose of this study was to determine the pharmacokinetic variables of cefepime in febrile buffalo calves following intravenous administration. From the of cefepime in febrile buffalo calves following intravenous administration. From the pharmacokinetic data, recommendations were made for the optimal dosage regimen of pharmacokinetic data, recommendations were made for the optimal dosage regimen of cefepime in febrile buffalo calves.
cefepime in febrile buffalo calves. Materials and methods Materials and methods
The experiment was performed on four male buffalo calves, 6-10 months of age and The experiment was performed on four male buffalo calves, 6-10 months of age and weighing an average of 91 kg. The animals were housed in a departmental shed with a weighing an average of 91 kg. The animals were housed in a departmental shed with a concrete fl oor and were provided green fodder and water ad libitum. Each animal was concrete fl oor and were provided green fodder and water ad libitum. Each animal was quarantined for three weeks before the start of the experiment and was determined to be quarantined for three weeks before the start of the experiment and was determined to be healthy by regular clinical examination. Fever was induced by single/repeated intravenous healthy by regular clinical examination. Fever was induced by single/repeated intravenous administration of
administration of E. coliE. coli lipopolysaccaride (LPS) at the dose rate of 1 lipopolysaccaride (LPS) at the dose rate of 1μμg/kg body mass g/kg body mass
((DARDI et al., 2005DARDI et al., 2005). Once fever was induced (1-2 h after LPS injection) cefepime was ). Once fever was induced (1-2 h after LPS injection) cefepime was injected intravenously to these four animals at a dose rate of 10 mg/kg of cefepime, in a injected intravenously to these four animals at a dose rate of 10 mg/kg of cefepime, in a 10% solution with sterilized distilled water. Blood samples (4-5 mL each) were withdrawn 10% solution with sterilized distilled water. Blood samples (4-5 mL each) were withdrawn from the contralateral jugular vein into heparanized glass test tubes before administration from the contralateral jugular vein into heparanized glass test tubes before administration and at 1, 2.5, 5, 7.5, 10, 15, 30, 45 and 60 minutes and 2, 3, 4, 5, 6, 8, 10, 12, 16 and 24 h and at 1, 2.5, 5, 7.5, 10, 15, 30, 45 and 60 minutes and 2, 3, 4, 5, 6, 8, 10, 12, 16 and 24 h after administration of the drug. Plasma was collected after centrifugation at 2000g for 15 after administration of the drug. Plasma was collected after centrifugation at 2000g for 15 minutes at room temperature and kept at -20
minutes at room temperature and kept at -2000 C until analysis, usually the next day. The C until analysis, usually the next day. The
concentration of cefepime in plasma was estimated by employing the microbiological concentration of cefepime in plasma was estimated by employing the microbiological assay technique (
assay technique (ARRET et al., 1971ARRET et al., 1971) using ) using Escherichia coliEscherichia coli (MTCC 739) as the test (MTCC 739) as the test
organism. The bioassay method used in this work could not distinguish between the parent organism. The bioassay method used in this work could not distinguish between the parent compound and its active metabolites. However, it measured the overall microbiological compound and its active metabolites. However, it measured the overall microbiological activity of the drug. The standard curve of cefepime equivalent in buffalo calf plasma was activity of the drug. The standard curve of cefepime equivalent in buffalo calf plasma was linear between 0.25 to 1.25
linear between 0.25 to 1.25 μμg/mL. The drug could be detected up to a minimum limit of g/mL. The drug could be detected up to a minimum limit of 0.1
0.1 μμg/mL. The intra day and inter day coefg/mL. The intra day and inter day coeffifi cient of variance was 4.2 and 9.39 per cent, cient of variance was 4.2 and 9.39 per cent, respectively. The plasma concentration-time data for each buffalo calf were determined respectively. The plasma concentration-time data for each buffalo calf were determined according to the computed least squares regression technique. The kinetic parameters according to the computed least squares regression technique. The kinetic parameters were calculated from the formulae derived for a two- compartment open model (
were calculated from the formulae derived for a two- compartment open model (NOTARI, NOTARI, 1980; GIBALDI and PERRIER, 1982
1980; GIBALDI and PERRIER, 1982). ).
The dosage regimen of cefepime was also determined based on the kinetic data. The The dosage regimen of cefepime was also determined based on the kinetic data. The priming (D) and maintenance (D
priming (D) and maintenance (D11) doses are calculated from the equation:) doses are calculated from the equation: D = C D = Cpp(min )(min )αα.V.V dd e e βτβτ D D11 = C = Cp p (min)(min)αα.V.V dd(e(eβτβτ-1)-1) Where C
Where Cpp(min) is the minimum therapeutic concentration of cefepime, (min) is the minimum therapeutic concentration of cefepime, ττ is the dosage is the dosage interval and other parameters are defi ned in Table 1.
Results Results The effect of
The effect of E. coli E. coli lipopolysaccharide on body temperature was recorded. The lipopolysaccharide on body temperature was recorded. The
normal temperature in the buffalo calves was in the range of 37.1-37.6 ºC. In one animal, normal temperature in the buffalo calves was in the range of 37.1-37.6 ºC. In one animal, there was a significant rise in body temperature within 2 h of administration there was a significant rise in body temperature within 2 h of administration of lipopolysaccharide, while in three animals, the dose of lipopolysaccharide of lipopolysaccharide, while in three animals, the dose of lipopolysaccharide was repeated to obtain an optimum increase in body temperature. This dose of was repeated to obtain an optimum increase in body temperature. This dose of lipopolysaccaride caused fever within two hours and fever persisted for 7-10 hours. At lipopolysaccaride caused fever within two hours and fever persisted for 7-10 hours. At least a 1.1 ºC increase of temperature from the normal temperature was taken as the time least a 1.1 ºC increase of temperature from the normal temperature was taken as the time of cefepime administration.
of cefepime administration.
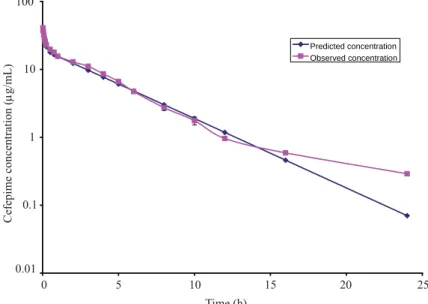
P redicted concentration O bserved concentration
Fig. 1. A semi logarithmic plot of predicted and observed plasma levels of cefepime after a single Fig. 1. A semi logarithmic plot of predicted and observed plasma levels of cefepime after a single intravenous dose of 10 mg/kg body mass in febrile buffalo calves. Values given are mean ± SE of intravenous dose of 10 mg/kg body mass in febrile buffalo calves. Values given are mean ± SE of
four animals. four animals.
The mean plasma concentrations of cefepime as a function of time in the febrile The mean plasma concentrations of cefepime as a function of time in the febrile buffalo calves were plotted on a semilogarithmic scale (Fig. 1).
buffalo calves were plotted on a semilogarithmic scale (Fig. 1). At 1 minute the mean At 1 minute the mean plasma concentration of cefepime was 40.8
plasma concentration of cefepime was 40.8 ± ± 0.98 0.98 μμg/mL, which rapidly declined to g/mL, which rapidly declined to plasma concentration of 23.0
plasma concentration of 23.0 ± ± 0.64 0.64 μμg/mL at 15 minutes. Then levels gradually decreased g/mL at 15 minutes. Then levels gradually decreased to 0.29
to 0.29 ± ± 0.01 0.01 μμg/mL at 24 hours. The elimination half life (tg/mL at 24 hours. The elimination half life (t1/21/2ββ), volume of distribution ), volume of distribution (Vd
(Vd(area)(area)) and total body clearance in febrile animals were 3.00 ) and total body clearance in febrile animals were 3.00 ± ± 0.18 h, 0.42 0.18 h, 0.42 ± ± 0.02 L/kg 0.02 L/kg
and 98.8
and 98.8 ± ± 6.06 mL/kg/h, respectively.6.06 mL/kg/h, respectively. Various pharmacokinetic parameters for cefepime Various pharmacokinetic parameters for cefepime in buffalo calves, in which fever was induced before administration of the drug, are given in buffalo calves, in which fever was induced before administration of the drug, are given in Table 1. in Table 1. 1 0 5 0.01 10 15 20 25 0.1 100 10 Cefepime concentration ( μμ g/ m L ) g/mL) Time (h)
Table1. Pharmacokinetic parameters of cefepime in febrile buffalo calves (n = 4) after a single Table1. Pharmacokinetic parameters of cefepime in febrile buffalo calves (n = 4) after a single
intravenous injection of 10 mg/kg body mass intravenous injection of 10 mg/kg body mass
Parametera Unit Mean ± SE
Cpo μg/mL 44.9 ± 1.49 A μg/mL 25.3 ± 1.70 B μg/mL 19.6 ± 0.84 α h-1 8.35 ± 0.29 β h-1 0.234 ± 0.015 t1/2α h 0.08 ± 0.002 t1/2β h 3.00 ± 0.18 K12 h-1 4.36 ± 0.28 K21 h-1 3.77 ± 0.20 K12/K21 ratio - 1.17 ± 0.11 AUC μg/mL.h 101.0 ± 7.65 Vd(area ) L/kg 0.42 ± 0.02 Vd(SS) L/kg 0.48 ± 0.02 ClB mL/kg/h 98.8 ± 6.06 a
a Kinetic parameters as described by Gibaldi and Perrier (1982). CKinetic parameters as described by Gibaldi and Perrier (1982). C
ppoo = Intercept of the back extrapolated = Intercept of the back extrapolated concentration time curve on the concentration axis; A, B = zero-time plasma drug concentration intercepts of concentration time curve on the concentration axis; A, B = zero-time plasma drug concentration intercepts of regression lines of distribution and elimination phases, respectively;
regression lines of distribution and elimination phases, respectively; α α and and ββ = distribution and elimination rate = distribution and elimination rate constants, respectively; t
constants, respectively; t1/21/2α α = distribution half life; t= distribution half life; t1/21/2ββ = elimination half life; K = elimination half life; K1212, K, K2121 = rate of transfer of drug = rate of transfer of drug from central (blood) to peripheral (tissues) compartment and vice-versa; AUC = total area under plasma drug from central (blood) to peripheral (tissues) compartment and vice-versa; AUC = total area under plasma drug concentration-time curve; V
concentration-time curve; Vd(area)d(area) = apparent volume of distribution, based on area under curve; V = apparent volume of distribution, based on area under curve; Vd(ss) d(ss) = apparent = apparent volume of distribution, based on steady state plasma levels; Cl
volume of distribution, based on steady state plasma levels; ClB B = total plasma clearance.= total plasma clearance.
Discussion Discussion
Evaluation of the results of plasma cefepime levels against time indicated that the Evaluation of the results of plasma cefepime levels against time indicated that the pharmacokinetics of cefepime in febrile buffalo calves, after intravenous administration, pharmacokinetics of cefepime in febrile buffalo calves, after intravenous administration, was best described by the two-compartment open model. The plasma concentration-time was best described by the two-compartment open model. The plasma concentration-time data were adequately described by the equation:
data were adequately described by the equation: C
C pp = Ae = Ae--ααt t + Be+ Be--ββt t
where Cp is cefepime concentration in plasma at time‘t’. A and B are zero time where Cp is cefepime concentration in plasma at time‘t’. A and B are zero time intercepts of the distribution and elimination phase of the plasma concentration time curve. intercepts of the distribution and elimination phase of the plasma concentration time curve. α
α and and ββ are the distribution and elimination rate constants, respectively, and ‘e’ represents are the distribution and elimination rate constants, respectively, and ‘e’ represents the base of the natural logarithm. The disposition of cefepime, following intravenous the base of the natural logarithm. The disposition of cefepime, following intravenous injection, has been reported to follow the 2-compartment open model in calves (
injection, has been reported to follow the 2-compartment open model in calves (ISMAIL, ISMAIL, 2005a; PAWAR and SHARMA, 2008
1998; GARDNER and PAPICH, 2001
1998; GARDNER and PAPICH, 2001) buffalo calves () buffalo calves (JOSHI and SHARMA, 2007JOSHI and SHARMA, 2007) and dogs ) and dogs ((NAKANOMYO et al., 1992NAKANOMYO et al., 1992).).
A comparison of plasma levels of cefepime in febrile animals with healthy animals A comparison of plasma levels of cefepime in febrile animals with healthy animals ((JOSHI and SHARMA, 2007JOSHI and SHARMA, 2007), indicated that the peak plasma levels of cefepime in febrile ), indicated that the peak plasma levels of cefepime in febrile buffalo calves (40.8
buffalo calves (40.8 ± ± 0.98 0.98 μμg/mLg/mL)) was almost identical to healthy buffalo calves (42.7 was almost identical to healthy buffalo calves (42.7 ± ±
1.85
1.85 μμg/mLg/mL ). The high values of the distribution rate constant ). The high values of the distribution rate constant αα (8.35 (8.35 ± ± 0.29 h0.29 h-1-1) indicates ) indicates
that cefepime is rapidly distributed into various body fl uids and tissue compartments. The that cefepime is rapidly distributed into various body fl uids and tissue compartments. The rapid distribution of cefepime is further substantiated by high values of K
rapid distribution of cefepime is further substantiated by high values of K1212/K/K 21 21 (1.17 (1.17 ± ± 0.11). The elimination half-life of cefepime in febrile animals (3.00
0.11). The elimination half-life of cefepime in febrile animals (3.00 ± ± 0.18 h) is similar to 0.18 h) is similar to that reported in healthy animals, but longer than the values reported in other species. The that reported in healthy animals, but longer than the values reported in other species. The elimination half-life of cefepime had been reported as 2.67
elimination half-life of cefepime had been reported as 2.67 ± ± 0.29 h in healthy buffalo 0.29 h in healthy buffalo calves (
calves (JOSHI and SHARMA, 2007JOSHI and SHARMA, 2007), 2.38 h in calves (), 2.38 h in calves (ISMAIL, 2005aISMAIL, 2005a), 1.76 h in ewes ), 1.76 h in ewes ((ISMAIL, 2005bISMAIL, 2005b), 2.1 h in horses (), 2.1 h in horses (GUGLICK et al., 1998GUGLICK et al., 1998), 2 h in humans (), 2 h in humans (KIEFT et al., KIEFT et al., 1993
1993), 1.65 h in foals and 1.09 h in dogs (), 1.65 h in foals and 1.09 h in dogs (GARDNER and PAPICH, 2001GARDNER and PAPICH, 2001). The calculated ). The calculated value of volume of distribution (V
value of volume of distribution (Vd(area)d(area)) in febrile buffalo calves (0.42 ) in febrile buffalo calves (0.42 ± ± 0.02 L/kg) is 0.02 L/kg) is more than the reported value of V
more than the reported value of Vd(area) d(area) (0.225 L/kg) in horses ((0.225 L/kg) in horses (GUGLICK et al., 1998GUGLICK et al., 1998). ). The value of V
The value of Vd(area)d(area) in buffalo calves suggested that cefepime is distributed principally in buffalo calves suggested that cefepime is distributed principally through extracellular fl uid space. Despite low V
through extracellular fl uid space. Despite low Vd(area) d(area) values for cefepime in most species, values for cefepime in most species, its effi cacy against infections located in barrier restricted compartments, such as the CNS, its effi cacy against infections located in barrier restricted compartments, such as the CNS, has been documented (
has been documented (GRASSI and GRASSI, 1993GRASSI and GRASSI, 1993). Apparently, the low degree of plasma ). Apparently, the low degree of plasma protein binding and the probable decrease in integrity of the blood brain barrier caused protein binding and the probable decrease in integrity of the blood brain barrier caused by infl ammation promote the attainment of therapeutic concentrations of cefepime in the by infl ammation promote the attainment of therapeutic concentrations of cefepime in the brain tissue (
brain tissue (GUGLICK et al., 1998GUGLICK et al., 1998). The value of V). The value of Vd(ss)d(ss) in buffalo calves (0.48 in buffalo calves (0.48 ± ± 0.02 L/kg) 0.02 L/kg) also indicated good extravascular distribution of the drug. As with other cephalosporins, also indicated good extravascular distribution of the drug. As with other cephalosporins, the extravascular distribution of cefepime was limited to the extracellular fl uid (
the extravascular distribution of cefepime was limited to the extracellular fl uid (BALANT BALANT et al., 1985
et al., 1985). In spite of its limited distribution into the extracellular ). In spite of its limited distribution into the extracellular flfl uid, as reported in uid, as reported in most species studied, it appears that low protein binding and high bacterial activity are most species studied, it appears that low protein binding and high bacterial activity are considered the major determinants for promotion of its therapeutic effi cacy in all species considered the major determinants for promotion of its therapeutic effi cacy in all species ((KALMAN et al., 1992KALMAN et al., 1992). ).
While comparing the total body clearance in febrile animals with that of healthy While comparing the total body clearance in febrile animals with that of healthy animals (
animals (JOSHI and SHARMA, 2007JOSHI and SHARMA, 2007), it was found that the value of Cl), it was found that the value of ClBB in febrile animals in febrile animals (98.8
(98.8 ± ± 6.06 mL/kg/h) is almost identical to that of healthy animals (86.1 6.06 mL/kg/h) is almost identical to that of healthy animals (86.1 ± ± 3.65 mL/kg/3.65 mL/kg/ h). Endotoxin causes hepatic and renal dysfunction (
h). Endotoxin causes hepatic and renal dysfunction (WILKINSON, 1977WILKINSON, 1977) as well ) as well as hemodynamic depression (
as hemodynamic depression (VAN MIERT, 1973VAN MIERT, 1973). Due to significant alterations in ). Due to significant alterations in hepatic function, the levels of various enzymes, responsible for the metabolism of hepatic function, the levels of various enzymes, responsible for the metabolism of these antimicrobials, is altered, changing the elimination and biotransformation these antimicrobials, is altered, changing the elimination and biotransformation pattern of the drug during fever (
pattern of the drug during fever (SINGH et al., 1997SINGH et al., 1997). Cefepime is not significantly ). Cefepime is not significantly metabolized, but is excreted unchanged, primarily in urine by the glomerular metabolized, but is excreted unchanged, primarily in urine by the glomerular filteration process, and is poorly bound to plasma proteins (
1992
1992), and these may be the reasons why febrile conditions did not affect the ), and these may be the reasons why febrile conditions did not affect the pharmacokinetics of cefepime.
pharmacokinetics of cefepime.
The ultimate objective of the present study was to determine a satisfactory dosage The ultimate objective of the present study was to determine a satisfactory dosage regimen in febrile buffalo calves. It is not axiomatic to be able to compute the dosage regimen in febrile buffalo calves. It is not axiomatic to be able to compute the dosage regimen of cefepime to be used effectively in clinical practice for the treatment of mild regimen of cefepime to be used effectively in clinical practice for the treatment of mild to severe bacterial infections, without having fi rst conducted a detailed pharmacokinetic to severe bacterial infections, without having fi rst conducted a detailed pharmacokinetic study. With a minimum therapeutic plasma concentration of cefepime as 1.0 study. With a minimum therapeutic plasma concentration of cefepime as 1.0 μμg/mLg/mL
((KNUDSEN et al., 1997KNUDSEN et al., 1997) which has been shown to be most effective against the majority ) which has been shown to be most effective against the majority of sensitive Gram-positive and Gram-negative pathogens, the convenient and suitable of sensitive Gram-positive and Gram-negative pathogens, the convenient and suitable dosage regimen of cefepime in the febrile buffalo calves after intravenous administration dosage regimen of cefepime in the febrile buffalo calves after intravenous administration would be 7 mg/kg, repeated at 12h intervals. The dosage schedule of cefepime reported would be 7 mg/kg, repeated at 12h intervals. The dosage schedule of cefepime reported in healthy buffalo calves (
in healthy buffalo calves (JOSHI and SHARMA, 2007JOSHI and SHARMA, 2007) and febrile calves () and febrile calves (PAWAR and PAWAR and SHARMA, 2008
SHARMA, 2008) was 8 mg/kg, repeated at 12h and 8.2 mg/kg repeated at 8h, ) was 8 mg/kg, repeated at 12h and 8.2 mg/kg repeated at 8h, respectively.
respectively. Antibiotic dosing regimens have traditionally been determined by Antibiotic dosing regimens have traditionally been determined by pharmacokinetic parameters only. However, pharmacodynamics play an equal role, pharmacokinetic parameters only. However, pharmacodynamics play an equal role, because pharmacodynamic parameters may be used to design dosing regimens, which because pharmacodynamic parameters may be used to design dosing regimens, which counteract or prevent resistance. Integrating the pharmacokinetic parameters with the counteract or prevent resistance. Integrating the pharmacokinetic parameters with the MIC gives pharmacokinetic/pharmacodynamic (PK/PD) parameters (T>MIC, Cmax/ MIC gives pharmacokinetic/pharmacodynamic (PK/PD) parameters (T>MIC, Cmax/ MIC, AUC/MIC), which quantify the activity of an antibiotic. The pattern of activity MIC, AUC/MIC), which quantify the activity of an antibiotic. The pattern of activity for cephalosporins is time dependent killing and minimal persistent effects. The ideal for cephalosporins is time dependent killing and minimal persistent effects. The ideal dosing regimen for these antibiotics maximizes the duration of exposure. The T>MIC is dosing regimen for these antibiotics maximizes the duration of exposure. The T>MIC is the parameter that best correlates with effi cacy. For these drugs, maximum killing is seen the parameter that best correlates with effi cacy. For these drugs, maximum killing is seen when the time above MIC is at least 70 per cent of the dosing internal (
when the time above MIC is at least 70 per cent of the dosing internal (HYATT HYATT et al., et al., 19951995). ). The T>MIC values (10 h) of cefepime suggested that this agent is clinically effective in The T>MIC values (10 h) of cefepime suggested that this agent is clinically effective in the treatment of various infections manifested with fever.
the treatment of various infections manifested with fever. References
References
ARRET, B., D. P. JOHNSON, A. KRISHBAUM (1971): Outline of details for microbiological ARRET, B., D. P. JOHNSON, A. KRISHBAUM (1971): Outline of details for microbiological
assay of antibiotics. J. Pharma. Sci. 60, 1689-1694. assay of antibiotics. J. Pharma. Sci. 60, 1689-1694.
BALANT, L., P. DAYER, R. AUCKENTHALER (1985): Clinical pharmacokinetics of the third BALANT, L., P. DAYER, R. AUCKENTHALER (1985): Clinical pharmacokinetics of the third
generation cephalosporins. Clin. Pharmacokinet. 10, 101-143. generation cephalosporins. Clin. Pharmacokinet. 10, 101-143.
BARBHAIYA, R. H., C. A. KNUPP, M. PFEFFER, K. A. PITTMAN (1992): Lack of BARBHAIYA, R. H., C. A. KNUPP, M. PFEFFER, K. A. PITTMAN (1992): Lack of
pharmacokinetic interaction between cefepime and amikacin in humans. Antimicrob. Agents pharmacokinetic interaction between cefepime and amikacin in humans. Antimicrob. Agents Chemother. 36, 1382-1386.
Chemother. 36, 1382-1386.
CHAUDHARY, R. K., A. K. SRIVASTAVA, S. RAMPAL (1999): Modifi cation of the CHAUDHARY, R. K., A. K. SRIVASTAVA, S. RAMPAL (1999): Modifi cation of the
pharmacokinetics and dosage of cefuroxime by endotoxin-induced fever in buffalo calves. pharmacokinetics and dosage of cefuroxime by endotoxin-induced fever in buffalo calves. Vet. Res. Commun. 23, 361-368.
Vet. Res. Commun. 23, 361-368.
DARDI, M. S., S. K. SHARMA, A. K. SRIVASTAVA (2005). Pharmacokinetics and dosage DARDI, M. S., S. K. SHARMA, A. K. SRIVASTAVA (2005). Pharmacokinetics and dosage
regimen of ceftriaxone in
regimen of ceftriaxone in E. coli E. coli lipopolysaccharide induced fever in buffalo calves. J. Vet. lipopolysaccharide induced fever in buffalo calves. J. Vet.
Sci. 6, 147-150. Sci. 6, 147-150.
GARDNER, S. Y., M. G. PAPICH (2001): Comparison of cefepime pharmacokinetics in neonatal GARDNER, S. Y., M. G. PAPICH (2001): Comparison of cefepime pharmacokinetics in neonatal
foals and adult dogs. J. Vet. Pharmacol. Therap. 24, 187-192. foals and adult dogs. J. Vet. Pharmacol. Therap. 24, 187-192.
GIBALDI, M., D. PERRIER (1982): Pharmacokinetics. Marcel and Dekker Inc., New York. GIBALDI, M., D. PERRIER (1982): Pharmacokinetics. Marcel and Dekker Inc., New York. GRASSI, G. G., C. GRASSI (1993): Cefepime: overview of activity
GRASSI, G. G., C. GRASSI (1993): Cefepime: overview of activity in vitro in vitro and and in vivo. in vivo. J. J.
Antimicrob. Chemother.
Antimicrob. Chemother. 32, B87-B94.32, B87-B94.
GUGLICK, M. A., C. G. MACALLISTER, C. R. CLARKE, R. POLLET, C. HAGUE, J. M. GUGLICK, M. A., C. G. MACALLISTER, C. R. CLARKE, R. POLLET, C. HAGUE, J. M. CLARKE (1998): Pharmacokinetics of cefepime and comparison with those of ceftiofur in CLARKE (1998): Pharmacokinetics of cefepime and comparison with those of ceftiofur in horses. Am. J. Vet. Res. 59, 458-463.
horses. Am. J. Vet. Res. 59, 458-463.
HARY, L., M. ANDREJAK, S. LELEU, J. ORFILA, J. P. CAPRON (1989): The pharmacokinetics HARY, L., M. ANDREJAK, S. LELEU, J. ORFILA, J. P. CAPRON (1989): The pharmacokinetics of ceftriaxone and cefotaxime in cirrhotic patients with ascites. Euro. J. Clin. Pharmacol. 36, of ceftriaxone and cefotaxime in cirrhotic patients with ascites. Euro. J. Clin. Pharmacol. 36, 613-616.
613-616.
HYATT, J. M., P. S. MCKINNON, G. S. ZIMMER, J. J. SCHENTAG (1995): The importance of HYATT, J. M., P. S. MCKINNON, G. S. ZIMMER, J. J. SCHENTAG (1995): The importance of pharmacokinetic pharmacodynamic surrogate markers to outcome. Clin. Pharmacokinet. 28, pharmacokinetic pharmacodynamic surrogate markers to outcome. Clin. Pharmacokinet. 28, 143-160.
143-160.
ISMAIL, M. M. (2005a): Disposition kinetics, bioavailability and renal clearance of cefepime in ISMAIL, M. M. (2005a): Disposition kinetics, bioavailability and renal clearance of cefepime in
calves. Vet. Res. Commun. 29, 69-79. calves. Vet. Res. Commun. 29, 69-79.
ISMAIL, M. M. (2005b): Pharmacokinetics of cefepime administered by i.v. and i.m. routes to ISMAIL, M. M. (2005b): Pharmacokinetics of cefepime administered by i.v. and i.m. routes to
ewes. J. Vet. Pharmacol. Therap. 28, 499-503. ewes. J. Vet. Pharmacol. Therap. 28, 499-503.
JOSHI, B., S. K. SHARMA (2007): Pharmacokinetic disposition and bioavailability of cefepime in JOSHI, B., S. K. SHARMA (2007): Pharmacokinetic disposition and bioavailability of cefepime in
buffalo calves. J. Vet. Pharmacol. Therap. 30, 500-502. buffalo calves. J. Vet. Pharmacol. Therap. 30, 500-502.
KALMAN, D., S. L. BARRIERE, B. L. JOHNSON (1992): Pharmacokinetics disposition and KALMAN, D., S. L. BARRIERE, B. L. JOHNSON (1992): Pharmacokinetics disposition and bactericidal activities of cefepime, ceftazidime and cefoperazone in serum and blister fl uid. bactericidal activities of cefepime, ceftazidime and cefoperazone in serum and blister fl uid. Antimicrob. Agents Chemother. 36, 453-457.
Antimicrob. Agents Chemother. 36, 453-457.
KIEFT, H., A. I. HOEPELMAN, C. A. KNUPP, D. A. VAN, J. M. BRANGER, B. A. STRUYVEN, KIEFT, H., A. I. HOEPELMAN, C. A. KNUPP, D. A. VAN, J. M. BRANGER, B. A. STRUYVEN, J. L. VERHOEF (1993): Pharmacokinetics of cefepime in patients with sepsis syndrome. J. L. VERHOEF (1993): Pharmacokinetics of cefepime in patients with sepsis syndrome. Antimicrob. Agents Chemother. 32, 117-122.
Antimicrob. Agents Chemother. 32, 117-122.
KNUDSEN, J. D., K. FUURSTED, N. F. MOLLER, F. ESPERSEN (1997): Comparison of the effect KNUDSEN, J. D., K. FUURSTED, N. F. MOLLER, F. ESPERSEN (1997): Comparison of the effect of cefepime with four cephalosporins against pneumoncocci with various susceptibilities to of cefepime with four cephalosporins against pneumoncocci with various susceptibilities to penicillin,
penicillin, in vitro in vitro and in the mouse peritonitis model. J. Antimicrob. Chemother. 40, 679-686. and in the mouse peritonitis model. J. Antimicrob. Chemother. 40, 679-686.
LESAR, T. S., D. E. ZASKE (1984): Modifying dosage regimen in renal and hepatic failure. In: LESAR, T. S., D. E. ZASKE (1984): Modifying dosage regimen in renal and hepatic failure. In: Antimicrobial Therapy. (Ristuccia A. M., B. A. Cunha, Eds). Raven Press. New York. pp. Antimicrobial Therapy. (Ristuccia A. M., B. A. Cunha, Eds). Raven Press. New York. pp. 95-112.
112.
MONSHOUWER, M., R. F. WITKAMP, S. M. NIJMEIJER, L. A. VANLEENGOOD, H. C. MONSHOUWER, M., R. F. WITKAMP, S. M. NIJMEIJER, L. A. VANLEENGOOD, H. C.
VERNOOY, J. H. VERHEIJDEN, A. S. J. P. A. M. VAN MIERT (1996): A VERNOOY, J. H. VERHEIJDEN, A. S. J. P. A. M. VAN MIERT (1996): A lipopolysaccharide-induced acute phase response in the pig is associated with a decrease in hepatic cytochrome induced acute phase response in the pig is associated with a decrease in hepatic cytochrome P450-mediated drug metabolism. J. Vet. Pharmacol. Therap. 19, 382-388.
NAKANOMYO, H., Y. ESUMI, M. TAKAICHI, H. SEKI, S. NINOMIYA, Y. OKAMURA, Y. NAKANOMYO, H., Y. ESUMI, M. TAKAICHI, H. SEKI, S. NINOMIYA, Y. OKAMURA, Y. SUDO (1992): Study on the pharmacokinetics of cefepime (II). Japanese J. Antibiot. 45, SUDO (1992): Study on the pharmacokinetics of cefepime (II). Japanese J. Antibiot. 45, 938-942.
938-942.
NOTARI, R. E. (1980): Principle of pharmacokinetics. In: Biopharmaceutics and Clinical NOTARI, R. E. (1980): Principle of pharmacokinetics. In: Biopharmaceutics and Clinical
Pharmacokinetics. Marcell Dekker Inc. New York. pp 45-106. Pharmacokinetics. Marcell Dekker Inc. New York. pp 45-106. PAWAR, Y. G., S. K. SHARMA (2008): Infl uence of
PAWAR, Y. G., S. K. SHARMA (2008): Infl uence of E.coliE.coli. lipopolysaccharide induced fever on . lipopolysaccharide induced fever on
the plasma kinetics of cefepime in cross-bred calves. Vet. Res. Commun. 32, 123-130. the plasma kinetics of cefepime in cross-bred calves. Vet. Res. Commun. 32, 123-130. SHARMA, S. K., A. K. SRIVASTAVA, M. D. DEORE (2005): Pharmacokinetics of cefotaxime in SHARMA, S. K., A. K. SRIVASTAVA, M. D. DEORE (2005): Pharmacokinetics of cefotaxime in
hepatic-dysfunctioned buffalo calves. Vet. Arhiv 75, 339-348. hepatic-dysfunctioned buffalo calves. Vet. Arhiv 75, 339-348.
SINGH, R. P., A. K. SRIVASTAVA, S. K. SHARMA, D. C. NAURIYAL (1997): Disposition SINGH, R. P., A. K. SRIVASTAVA, S. K. SHARMA, D. C. NAURIYAL (1997): Disposition kinetics,urinary excretion and dosage regimen of sulfadimidine in febrile crossbred calves. kinetics,urinary excretion and dosage regimen of sulfadimidine in febrile crossbred calves. Indian J. Anim. Sci. 67, 866-867.
Indian J. Anim. Sci. 67, 866-867.
SINGH, R. P., A. K. SRIVASTAVA, S. K. SHARMA, D. C. NAURIYAL (1998): Infl uence of SINGH, R. P., A. K. SRIVASTAVA, S. K. SHARMA, D. C. NAURIYAL (1998): Infl uence of
Escherichia coli
Escherichia coli endotoxin induced fever on the pharmacokinetics and dosage regimen of endotoxin induced fever on the pharmacokinetics and dosage regimen of oxytetracycline in crossbred calves. Acta Vet. Hung. 46, 95-100.
oxytetracycline in crossbred calves. Acta Vet. Hung. 46, 95-100.
TOTH, A. H., Y. ABDALLAH, R. VENKATARAMANAN, L. TEPERMAN, G. HALSF, M. TOTH, A. H., Y. ABDALLAH, R. VENKATARAMANAN, L. TEPERMAN, G. HALSF, M.
RABINOVITCH, G. J. BURCKART, T. E. STARZ (1991): Pharmacokinetics of ceftriaxone RABINOVITCH, G. J. BURCKART, T. E. STARZ (1991): Pharmacokinetics of ceftriaxone in liver-transplant recipients. J. Clin. Pharmacol. 31, 722-728.
in liver-transplant recipients. J. Clin. Pharmacol. 31, 722-728. VAN MIERT, A. S. J. P. A. M. (1973): Clinical symptoms induced by
VAN MIERT, A. S. J. P. A. M. (1973): Clinical symptoms induced by E. coli E. coli endotoxin in goats. J. endotoxin in goats. J. Vet. Med. A 20, 614-623.
Vet. Med. A 20, 614-623.
WILKINSON, S. P. (1977): Endotoxins and liver dis
WILKINSON, S. P. (1977): Endotoxins and liver diseases. Scand. J. eases. Scand. J. Gastroenterol. 12, 385-386.Gastroenterol. 12, 385-386.
JOSHI, B., S. K. SHARMA
JOSHI, B., S. K. SHARMA: Farmakokinetika cefepima u bivolske teladi s vrućicom izazvanom lipopolisaharidom bakterije E. coli. Vet. arhiv 79, 523-530, 2009.
SAŽETAK
Istražena je farmakokinetika cefepima u bivolske teladi (n = 4) s pokusno izazvanom vrućicom nakon
Istražena je farmakokinetika cefepima u bivolske teladi (n = 4) s pokusno izazvanom vrućicom nakon
njegove jednokratne intravenske primjene (10 mg/kg). Vrućica je bila izazvana jednokratnom ili ponovljenom
njegove jednokratne intravenske primjene (10 mg/kg). Vrućica je bila izazvana jednokratnom ili ponovljenom
intravenskom primjenom lipopolisaharida bakterije
intravenskom primjenom lipopolisaharida bakterije E. coli E. coli (1 (1 μμg/kg). Farmakokinetika lijeka odreg/kg). Farmakokinetika lijeka određđivana je u ivana je u
uzorcima plazme mikrobiološkim postupkom upotrebom soja
uzorcima plazme mikrobiološkim postupkom upotrebom soja E. coliE. coli (MTCC 739). Farmakokineti (MTCC 739). Farmakokinetiččko ponašanje ko ponašanje cefepima u febrilnih životinja opisano je na osnovi dva otvorena modela. U prvoj minuti koncentracija cefepima cefepima u febrilnih životinja opisano je na osnovi dva otvorena modela. U prvoj minuti koncentracija cefepima
u plazmi iznosila je 40,8 ± 0,98 μg/mL, a u 15. se naglo smanjila na 23,0 ± 0,64 μg/mL. Lijek je bio dokazan
u plazmi iznosila je 40,8 ± 0,98 μg/mL, a u 15. se naglo smanjila na 23,0 ± 0,64 μg/mL. Lijek je bio dokazan
do 24 sata nakon davanja. Poluvrijeme njegova izlučivanja iznosilo je 3,00 ± 0,18 h, a količina raspodjele 0,42
do 24 sata nakon davanja. Poluvrijeme njegova izlučivanja iznosilo je 3,00 ± 0,18 h, a količina raspodjele 0,42
± 0,02 L/kg. Poluvrijeme njegove raspodjele iznosilo je 0,08 ± 0,002 h, površine ispod koncentracijske krivulje ± 0,02 L/kg. Poluvrijeme njegove raspodjele iznosilo je 0,08 ± 0,002 h, površine ispod koncentracijske krivulje
u plazmi 101 ± 7.65 μg/mL, a ukupni klirens iz organizma (Cl
u plazmi 101 ± 7.65 μg/mL, a ukupni klirens iz organizma (ClB) B) 98,8 ± 6,06 mL/kg/h. Za održavanje minimalne 98,8 ± 6,06 mL/kg/h. Za održavanje minimalne
terapijske koncentracije od 1 μg/mL cefepim bi u febrilne bivolske teladi trebalo davati u dozi od 7 mg/kg s
terapijske koncentracije od 1 μg/mL cefepim bi u febrilne bivolske teladi trebalo davati u dozi od 7 mg/kg s
ponovljenom primjenom u razmaku od 12 sati. ponovljenom primjenom u razmaku od 12 sati.
Ključne riječi:
Ključne riječi: bivolska telad, cefepim, doziranje, vru bivolska telad, cefepim, doziranje, vruććica, farmakokinetika ica, farmakokinetika
Received: 1 August 2008 Accepted: 2 November 2009