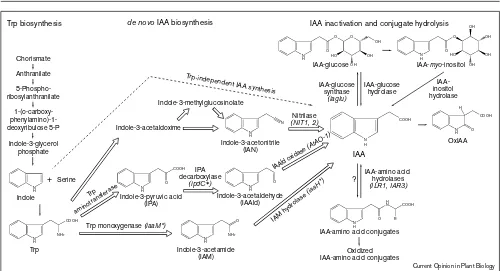
Plants have evolved elaborate systems for regulating cellular levels of indole-3-acetic acid (IAA). The redundancy of this network has complicated the elucidation of IAA metabolism, but molecular genetic studies and precise analytical methods have begun to expose the circuitry. It is now clear that plants synthesize, inactivate and catabolize IAA by multiple pathways, and multiple genes can encode a particular enzyme within a pathway. A number of these genes are now cloned, which greatly facilitates the future dissection of IAA metabolism.
Addresses
*Department of Biochemistry and Molecular Biology, University of Massachusetts, Amherst, MA 01003, USA;
e-mail: [email protected]
†Department of Biochemistry and Cell Biology, Rice University,
Houston, TX 77005, USA; e-mail: [email protected]
Current Opinion in Plant Biology1999, 2:207–213 http://biomednet.com/elecref/1369526600200207 © Elsevier Science Ltd ISSN 1369-5266
Abbreviations
AO aldehyde oxidase
IAA indole-3-acetic acid
IAAld indole-3-acetaldehyde
IAM indole-3-acetamide
IAN indole-3-acetonitrile
IPA indole-3-pyruvic acid
JA jasmonic acid
Introduction
The coordinated control of indole-3-acetic acid (IAA) biosynthesis, inactivation, catabolism and transport is a very dynamic process that responds to developmental and envi-ronmental signals. Biochemical approaches have demonstrated that plants can synthesize IAA from trypto-phan (Trp), and several Trp-dependent pathways have been proposed (Figure 1; reviewed in [1]). Stable isotope labeling of intact plants has revealed that IAA synthesis can also occur from a Trp precursor in Trp-independent pathways (Figure 1; reviewed in [1–3]). Trp-dependent pathways pre-dominate during early embryogenesis and seed germination, whereas Trp-independent pathways predomi-nate during later embryogenesis and vegetative growth [2,3]. IAA is inactivated either reversibly through conjuga-tion, or irreversibly by oxidizing IAA or IAA-conjugates (reviewed in [2,3]). The components of these pathways have been recalcitrant to biochemical approaches and are only now being identified. Here we focus on recent progress in the characterization of IAA metabolism and transport.
Tryptophan as an IAA precursor
In vivolabeling experiments with maize, Arabidopsis, car-rot and duckweed indicate that Trp can be metabolized to IAA ([1–3]); however, its contribution to IAA synthesis
may be overestimated. Rapparini et al. [4••] simultaneous-ly pulse-labeled duckweed fronds with 15N-anthranilate and 2H
5-Trp and monitored incorporation of label into IAA and Trp. Within the first hour, IAA incorporated 15N from anthranilate faster than did Trp, indicating that ini-tial synthesis of IAA was Trp-independent. By two hours post-labeling, Trp was equally labeled with 15N and 2H
5, yet the IAA pool was more enriched with 2H
5 than 15N. Thus, exogenous 2H
5-Trp is more accessible to IAA syn-thesis than denovo synthesized N-Trp. Previous work has suggested that plants have at least two physically separate Trp pools, one of which may be vacuolar [5]. Future stud-ies will need to account for and identify the cellular locations of multiple precursor pools. Further characteri-zation of Trp-dependent IAA synthesis will be facilitated by recently identified indole analogs that inhibit the con-version of 14C-indole to 14C-IAA but not the conversion of 14C-indole to 14C-Trp [6].
Although several pathways have been proposed for Trp-dependent IAA synthesis (Figure 1), both Trp-Trp-dependent and Trp-independent pathways remain poorly defined in terms of the enzymes, their intermediates and cellular locations. Recently, an in vitroassay for Trp-independent conversion of 14C-indole to 14C-IAA has been developed using extracts from maize seedlings [7••]. The pH (8 to 8.5) optimum and requirement for a reducing environment suggest that the activity is plastid-localized.
Establishing a role for nitrilases in
IAA biosynthesis
The indole-3-acetonitrile (IAN) pathway (Figure 1) is apparently limited to three plant families [8] and most of the characterization of this pathway has been done in the Brassicaceae ([9] and references therein). Crucifers can con-vert IAN to IAA, but the redundancy of nitrilase genes confounds genetic analysis [10•] and the potential for non-enzymatic conversion of IAN to IAA complicates biochemical analysis [11]. The cumulative data suggest that if nitrilase converts IAN to IAA in vivo, then the rate-limit-ing step is substrate access. Since the subcellular localization of nitrilase and IAN are not well defined, how and when this pathway is utilized are major unanswered questions. There is some evidence that IAN utilization could be develop-mentally controlled [12•,13] and pathogen-induced [9,13].
Micromolar concentrations of exogenous IAN inhibit Arabidopsis seedling growth in a manner similar to that of excess IAA [10•]. Arabidopsis encodes four differentially expressed nitrilases [13–15] and each converts IAN to IAA in vitro.NIT1 and NIT2 have Km values for IAN in the mM range [13,14,16] and IAN is present in seedlings at ~3–6 nmol/g fresh weight. In vivolabeling studies demon-strated exogenous 13C-IAN conversion to 13C-IAA in
Redundancy as a way of life — IAA metabolism
wild-type Arabidopsis and also in transgenic tobacco and Arabidopsis overexpressing NIT2 [10•,17]. In response to exogenous IAN, wild-type Arabidopsis accumulates nitri-lase protein, and NIT1, NIT2 and NIT4 gene expression is induced 1.4-, 21- and 4.8-fold, respectively [18]. Overexpressing nitrilase genes in Arabidopsis or tobacco also increases nitrilase protein and activity [17,18], yet only NIT2 overexpressing lines show increased sensitivity to exogenous IAN [10•,17]. An Arabidopsis nit1 mutant is resistant to IAN, but metabolizes IAN normally and is morphologically normal in the absence of exogenous IAN [10•]. The existence of three wild-type nitrilases (NIT2–4) may compensate for the nit1 defect, or tissue-specific dif-ferences in IAN metabolism are masked in whole seedlings. Label from 2H
5-Trp is incorporated into both IAN and IAA when added to 5 week-old Arabidopsis root explants [19]. NIT1 is expressed in five-week-old Arabidopsis roots [13,19], but it is important to consider that exogenous Trp can be preferentially utilized for IAA syn-thesis [4••], and that Trp-dependent IAA biosynthetic pathways can be activated by wounding [20,21].
There is correlative evidence for IAN pathway utilization in response to specific developmental and environmental cues. NIT2 is normally expressed at levels four- to eight-fold lower than NIT1 and is restricted to root tips and
siliques [13], but is dramatically induced by pathogenic bacteria [13], exogenous IAN [18], and at the onset of senescence [12•]. In senescing Arabidopsis leaves, free IAA levels increase two-fold, whereas IAN and conjugated IAA levels drop two-fold [12•]. Infection of Arabidopsis or Chinese cabbage with Plasmodiophora brassicae, the causal agent of clubroot disease, increases nitrilase activity [9] and perturbs IAA metabolism [22]. Arabidopsis mutants with diminished indole-3-methyl-glucosinolate levels (a putative IAN precursor, Figure 1) appear unaffected in IAA synthesis and turnover under normal conditions, but upon P. brassicae infection have lower IAA and IAN levels and less severe clubroot symptoms than wild-type [23].
Lastly, two-hybrid screens have identified a nitrilase-associat-ed protein in Arabidopsis (Genbank accession number Z96936), and a nitrilase-like protein in tobacco that interacts with an ethylene-response element binding protein [24]. The functions of these proteins remain to be determined but they are intriguing in light of the need for some event that renders endogenous IAN available to nitrilase for IAA synthesis.
Indole pyruvic acid pathway of
IAA biosynthesis
Indole-3-pyruvic acid (IPA) ([25] and references therein) and aldehyde oxidase (AO) activities that convert Figure 1
Trp biosynthesis de novo IAA biosynthesis IAA inactivation and conjugate hydrolysis
Trp monoxygenase (iaaM*)
Current Opinion in Plant Biology
Simplified diagram of IAA metabolism. IAA can be synthesized via several Trp-dependent pathways (open arrows) that are named after unique intermediates, or by a Trp-independent pathway (dashed arrow) using indole as a precursor. Genes are in italics, microbial genes are marked with an asterisk. Further catabolism of oxidized IAA and
IAA–Asp is reviewed in [3]. IAA–myo-inositol can be further metabolized to other conjugates, many of which can also be hydrolyzed [31•]. It is not known whether IAA–amino acid conjugates
indole-3-acetaldehyde (IAAld) to IAA (Figure 1; [26•] and references therein) have been identified in several plants. As the final step of abscisic acid biosynthesis is also cat-alyzed by an AO [27], it has been difficult to assign in vivo functions to individual isozymes. The Arabidopsis rty mutant, which is allelic to sur1, hls3, ivr, and alf1(Table 1; reviewed in [1]), overproduces IAA and is defective in a putative aminotransferase gene [28]. Roots and hypocotyls of sur1 contain increased AO activity with an apparent preference for IAAld [29••] and accumulate mRNA from
AtAO-1 [26•], one of four differentially expressed AO genes [26•,30]. Although the molecular details of RTY action remain unclear, these observations suggest a role for AtAO-1 in IAA biosynthesis. With the AO genes in hand, it is now possible to screen for insertion mutants. A mutation in AtAO-1 alone may not affect IAA levels if other IAA biosynthetic pathways can compensate; however, the labeling patterns of IAA precursors in such a mutant may be informative.
Increasing free IAA by conjugate hydrolysis
Most of the IAA in plants is conjugated to a variety of amino acids, peptides, sugars, and myo-inositol [45]. The physiological significance of the individual conjugate moieties is yet to be determined, but one theory is that they direct the IAA either to catabolism or to storage for subsequent hydrolosis. Biochemical approaches are being used to isolate IAA-glucose and IAA-inositol hydrolase genes [31•], and genetic approaches have uncovered IAA-amino acid conjugate hydrolases. A screen for insensitivity to IAA-Leu identified the Arabidopsis ILR1 gene [32], which is homologous to the gene defective in iar3, an IAA-Ala insensitive mutant [33••]. Arabidopsis has a family of at least six ILR1/IAR3-like enzymes [33••]. Whereas past screens for conjugate hydrolysis mutants have been based on the toxicity of excess free IAA, IAA-conjugate derivatives with increased phytotoxicity might facilitate future screens [34]. Among the conjugates test-ed, the IAR3 enzyme is specific for IAA-Ala [33••], Table 1
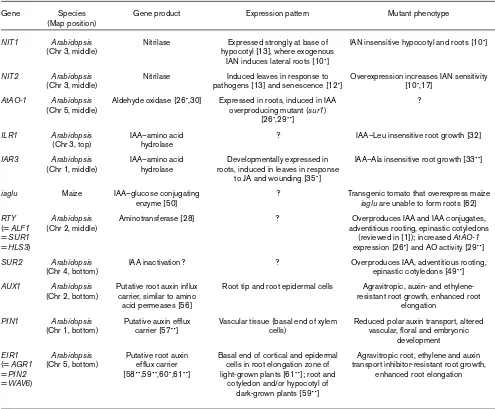
Plant genes potentially involved in IAA metabolism and transport.
Gene Species Gene product Expression pattern Mutant phenotype
(Map position)
NIT1 Arabidopsis Nitrilase Expressed strongly at base of IAN insensitive hypocotyl and roots [10•]
(Chr 3, middle) hypocotyl [13], where exogenous IAN induces lateral roots [10•]
NIT2 Arabidopsis Nitrilase Induced leaves in response to Overexpression increases IAN sensitivity
(Chr 3, middle) pathogens [13] and senescence [12•] [10•,17]
AtAO-1 Arabidopsis Aldehyde oxidase [26•,30] Expressed in roots, induced in IAA ?
(Chr 5, middle) overproducing mutant (sur1) [26•,29••]
ILR1 Arabidopsis IAA–amino acid ? IAA–Leu insensitive root growth [32]
(Chr 3, top) hydrolase
IAR3 Arabidopsis IAA–amino acid Developmentally expressed in IAA–Ala insensitive root growth [33••]
(Chr 1, middle) hydrolase roots, induced in leaves in response to JA and wounding [35•]
iaglu Maize IAA–glucose conjugating ? Transgenic tomato that overexpress maize
enzyme [50] iaglu are unable to form roots [62]
RTY Arabidopsis Aminotransferase [28] ? Overproduces IAA and IAA conjugates,
(= ALF1 (Chr 2, middle) adventitious rooting, epinastic cotyledons
= SUR1 (reviewed in [1]); increased AtAO-1
= HLS3) expression [26•] and AO activity [29••]
SUR2 Arabidopsis IAA inactivation? ? Overproduces IAA, adventitious rooting,
(Chr 4, bottom) epinastic cotyledons [49••]
AUX1 Arabidopsis Putative root auxin influx Root tip and root epidermal cells Agravitropic, auxin- and
ethylene-(Chr 2, bottom) carrier, similar to amino resistant root growth, enhanced root
acid permeases [56] elongation
PIN1 Arabidopsis Putative auxin efflux Vascular tissue (basal end of xylem Reduced polar auxin transport, altered
(Chr 1, bottom) carrier [57••] cells) vascular, floral and embryonic
development
EIR1 Arabidopsis Putative root auxin Basal end of cortical and epidermal Agravitropic root, ethylene and auxin
(= AGR1 (Chr 5, bottom) efflux carrier cells in root elongation zone of transport inhibitor-resistant root growth,
= PIN2 [58••,59••,60•,61••] light-grown plants [61••]; root and enhanced root elongation
= WAV6) cotyledon and/or hypocotyl of
whereas ILR1 prefers IAA-Phe and IAA-Leu to IAA-Ala [32]. Comparison of ILR1 and IAR3 to rice homologs sug-gests that this diversity in specificity arose before the divergence of monocots and dicots. Interestingly, an Arabidopsis cDNA (JR3), that is 96% identical to IAR3, was isolated as a jasmonic acid (JA)-induced gene [35•].
JR3 is also rapidly and transiently induced by wounding, both at the wound site and systemically [35•]. This wound- and induced expression is blocked in the JA-insensitive mutant coi1 and is mediated by a phosphorylation-dependent signal transduction pathway [35•,36••]. As auxin inhibits the expression of some JA-induced genes, JR3 might be involved in feedback inhibition of the JA response by hydrolyzing conjugates and increasing the free IAA concentration [36••]. It will be interesting to determine whether the other conjugate hydrolase genes are similarly regulated and whether the iar3 or iar3 ilr1 mutants show altered responses to JA, wounding, or pathogens.
Like many IAA metabolic enzymes, IAA-conjugate hydrolases are not exclusive to plants. The Enterobacter agglomerans iaaspH gene encodes a hydrolase specific for IAA-Asp [37•]. This enzyme is 20% identical to the
Arabidopsis ILR1 and IAR3 enzymes. IAA-Asp is of par-ticular importance in plants, as it is found in a variety of species and can be an intermediate in IAA catabolism (see below). Thus, overexpressing iaaspH in transgenic plants might short-circuit IAA inactivation.
Differential regulation of redundant IAA
biosynthetic pathways
Plant developmental processes requiring transient, high levels of free IAA may rely on a Trp-dependent pathway, whereas the Trp-independent pathway probably maintains low levels of IAA during vegetative growth. For example, IAA levels are low in unfertilized carrot ovules, increase 80-fold by the late globular and early heart stages and then decline to pre-fertilization levels by the torpedo stage (DM Ribnicky, JD Cohen, W-S Hu, TJ Cooke, personal commu-nication). Conjugated IAA levels also rise, so both synthesis and inactivation rates are changing. Trp-dependent path-ways are active in carrot prior to the onset of somatic embryogenesis and then subsequently decline [38].
Microbes may also differentially regulate multiple IAA biosynthetic pathways. Microbial IAA production is evi-dent during symbiotic nodule formation, in rhizosphere-inhabiting bacteria and in many pathogenic interactions (reviewed in [39–41]). Erwinia herbicola requires the indole-3-acetamide (IAM) pathway for path-ogenicity and the IPA pathway for epiphytic fitness [42•]. Disrupting the IPA decarboxylase gene (ipdC, Figure 1) from E. herbicola does not affect gall size or formation on susceptible plants, whereas disrupting the IAM hydrolase gene (iaaH, Figure 1) decreases gall size. Strains disrupt-ed in either IAA biosynthetic pathway are equally viable when propagated as non-pathogenic epiphytes on wet
bean leaves, but once the leaves dry the decrease in via-bility is an order of magnitude more severe in the ipdC– strain. Reporter gene activity indicates that the iaaH pro-moter is induced dramatically during pathogenic infection, whereas ipdC expression is lower than that of iaaH during pathogenic or non-pathogenic propagation. The regulation of these pathways no doubt determines overall IAA levels, and this may be the key to their differ-ential utilization.
In addition to developmental regulation, IAA metabo-lism can be altered by temperature and light. Hypocotyls of light-grown Arabidopsis seedlings grown at 29°C elon-gate four- to five-fold more than those of control seedlings at 20°C. This elongation is blocked in IAA transport mutants, in auxin response mutants and by the auxin transport inhibitor NPA [43••]. Endogenous free IAA and IAA conjugate levels rise under these condi-tions [43••], implying that increased IAA synthesis and decreased IAA inactivation mediate the elongation. In wild-type duckweed, IAA turnover decreases in continu-ous light compared to darkness, yet IAA levels remain constant, suggesting that biosynthesis also slows in con-tinuous light. The MTR1 mutant, which contains a feedback insensitive anthranilate synthase, maintains a high rate of turnover under all light conditions, but accu-mulates IAA in continuous light [44•]. Perhaps high levels of IAA precursors in this strain prevent the normal light-induced slowing of IAA biosynthesis. As IAA meta-bolic genes are cloned (Table 1), it will be interesting to examine transcriptional regulation by such factors as light and temperature.
IAA inactivation and catabolism
It has long been recognized that higher plants maintain most IAA as conjugates [45]. Lower land plants (e.g. sev-eral liverworts, mosses, a fern, and a lycophyte) contain significant levels of amide- and ester-linked IAA [46•] and conjugate exogenous IAA [47], indicating that the ability to regulate IAA levels via conjugation was an early devel-opment of land plants. The liverworts conjugate exogenous IAA more slowly than the other taxa [46•], sug-gesting that these plants may rely on alternative means of IAA inactivation, such as direct oxidation.
Like the rty mutant, the Arabidopsis superroot2 (sur2) mutant displays phenotypes suggestive of auxin overpro-duction, including epinastic cotyledons and adventitious hypocotyl rooting. sur2 seedlings have elevated free IAA and slightly reduced conjugate levels, suggesting a defect in inactivation [49••], whereas the rty mutant has higher levels of both free and conjugated IAA, suggesting a defect in synthesis (reviewed in [1]). Only one gene directly involved in IAA inactivation has been cloned; the maize iaglu gene that encodes IAA-glucose synthase [50]. Cloning the SUR2 gene should reveal whether it encodes another IAA inactivating enzyme.
IAA transport versus metabolism
Auxin transport assays indicate that free IAA is the pre-ferred substrate for the polar auxin transport machinery [51]. In the absence of details about the localization (or identity) of the proteins responsible for the synthesis, inactivation, activation and transport of IAA it has not been possible to determine the relative contributions of metabolism and transport to the level of IAA in any given cell. While inhibition of auxin transport from the shoot to the root inhibits lateral root formation in Arabidopsis[52•], it is difficult to imagine that polar transport could be the sole source of IAA in complex root systems and large trees. Sundberg and Uggla [53•] replaced the apices of Pinus
sylvestrissaplings with 13C
6-IAA and measured the ratio of labeled, exogenous IAA to unlabeled endogenous IAA in serial stem sections. If the 13C
6-IAA were the sole source of IAA distal to the apex then the ratio of labeled to unla-beled IAA should remain the same throughout the stem. However, the labeled IAA was diluted, indicating IAA synthesis or IAA conjugate hydrolysis in regions other than the apex.
Molecular genetic approaches have recently yielded sever-al genes that may encode the efflux and influx carriers postulated to transport IAA (reviewed in [54•,55•]). AUX1, a putative auxin influx carrier, is similar to amino acid per-meases and the AUX1gene is expressed in root tip and root epidermal cells (Table 1; [56]). PIN1 [57••] localizes to the basal end of stem xylem cells while EIR1[58••], which was also isolated as AGR1[59••,60•] and PIN2[61••], localizes to the basal end of root epidermal cells in the elongation zone (Table 1). In addition to the localization data, genetic and biochemical evidence suggest that PIN1 and EIR1/AGR1/PIN2 may serve as auxin efflux carriers (reviewed in [54•,55•]). These proteins are encoded by gene families, so homologs that localize to other tissues that require auxin transport (e.g. embryos, root cap cells) should be forthcoming. It will be interesting to compare the expression patterns of the auxin carriers with those of enzymes involved in IAA metabolism (Table 1), all of which potentially modulate free IAA levels.
Conclusions and perspectives
A thorough understanding of IAA metabolism will require identification and analysis of the intermediates, enzymes,
and genes involved in IAA biosynthesis, inactivation, catab-olism and transport, as well as of mutants defective in each pathway. Although the enzymes and intermediates in IAA biosynthesis have not yet been definitively established, sub-stantial progress is being made on the biochemical characterization of these pathways. Candidate IAA biosyn-thetic genes have been identified as members of gene families (Table 1) and a variety of approaches are available to assess their substrate specificities and participation in IAA synthesis. The expanding collection of mutants specifically defective in IAA metabolic pathways are valuable analytical tools. The diverse substrate specificities of IAA-conjugate hydrolases [33••] implies that Arabidopsis contains several IAA-amino acid conjugates, making identification of the endogenous conjugates in this species particularly impor-tant. Further characterization of the maize iaglu [50] and the Arabidopsis SUR2 [49••] genes should complement our largely biochemical understanding of IAA inactivation. The identification of gene families that are likely to encode auxin efflux and influx carriers [54•,55•,56,57•• –-59••,60•,61••] represents an enormous leap forward in the characterization of IAA transport and ultimately the manner by which IAA transport ties into IAA metabolism. Now that many of the genes involved in auxin metabolism have been isolated (Table 1), analysis of the substrate specificities of the encoded enzymes, their loss of function phenotypes, and their expression during development and in response to environmental challenges will reveal the roles of particular pathways in regulating free IAA levels.
Acknowledgements
We thank Seiichi Matsuda, John Celenza, and members of the Bartel lab for critical comments on the manuscript. Work in the authors’ laboratories is supported by the DOE (DE-FG02-95ER20204, J Normanly), the NSF (MCB9870798, J Normanly), the NIH (R29 GM54749, B Bartel), and the Robert A Welch Foundation (C-1309, B Bartel).
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest
••of outstanding interest
1. Bartel B: Auxin biosynthesis.Annu Rev Plant Physiol Plant Mol Biol
1997, 48:51-66.
2. Cohen JD, Slovin JP: Recent research advances concerning
indole-3-acetic acid metabolism. In Mechanism of Action of Plant
Hormones. Edited by Palme K, Walden R, Schell J. Berlin: Springer; 1999:in press.
3. Normanly J: Auxin metabolism.Physiologia Plantarum 1997,
100:431-442.
4. Rapparini F, Cohen JD, Slovin JP: Indole-3-acetic acid biosynthesis
•• in Lemna gibba studied using stable isotope labeled anthranilate
and tryptophan. Plant Growth Regulation 1999, in press.
This paper provides further evidence of multiple Trp pools and reveals that exogenous IAA precursors may be preferentially metabolized over their endogenous counterparts. This is an important consideration when assess-ing the relative contributions of various precursors to IAA biosynthesis. 5. Delmer D: Dimethylsulfoxide as a potential tool for analysis of
compartmentation in living plant cells. Plant Physiol 1979,
64:623-629.
6. Ilic N, Östin A, Cohen JD: Differential inhibition of indole-3-acetic acid and tryptophan biosynthesis by indole analogues. I.
Tryptophan dependent IAA biosynthesis.Plant Growth Reg 1999,
7. Östin A, Ilic N, Cohen JD: An in vitrosystem from maize seedlings
•• for tryptophan-independent IAA biosynthesis.Plant Physiol 1999,
119:173-178.
The authors show that Trp-independent conversion of indole to IAA pre-dominates in maize seedlings, whereas Trp-dependent IAA synthesis activi-ty is predominant in maize endosperm, thus providing further evidence for differential utilization of IAA biosynthetic pathways.
8. Thimann KV, Mahadevan S: Nitrilase. I. Occurrence, preparation,
and general properties of the enzyme. Arch Biochem Biophys
1964, 105:133-141.
9. Grsic S, Kirchheim B, Pieper K, Fritsch M, Hilgenberg W, Ludwig-Müller J: Induction of auxin biosynthetic enzymes by jasmonic acid
and in clubroot diseased Chinese cabbage plants. Physiol Plant
1999, 105:521-531.
10. Normanly J, Grisafi P, Fink GR, Bartel B: Arabidopsis mutants
• resistant to the auxin effects of indole-3-acetonitrile are defective
in the nitrilase encoded by the NIT1 gene.Plant Cell 1997,
9:1781-1790.
IAN-insensitive nit1 mutants and IAN-supersensitive NIT2 overexpressors were isolated, demonstrating that both NIT1 and NIT2 can hydrolyze IAN in planta. Stable isotope labeling of intact plants showed that the nit1 mutant metabolized exogenous IAN normally, and the NIT2 overexpressor had increased IAN turnover
11. Ilic N, Normanly J, Cohen JD: Quantification of free plus conjugated
indoleacetic acid in Arabidopsis requires correction for the
nonenzymatic conversion of indolic nitriles.Plant Physiol 1996,
111:781-788.
12. Quirino B, Normanly J, Amasino RM: Diverse range of gene activity
• during Arabidopsis thaliana leaf senescence includes
pathogen-independent induction of defense-related genes.Plant Mol Biol
1999, in press.
A PCR-based screen for Arabidopsis genes that are up-regulated during senescence identified NIT2. Northern analysis indicates that both NIT2 and
NIT4 expression are strongly induced during senescence. At the same time, free IAA levels increase two-fold in senescing leaves, whereas IAN and con-jugated IAA levels drop two-fold.
13. Bartel B, Fink GR: Differential regulation of an auxin-producing
nitrilase gene family in Arabidopsis thaliana.Proc Natl Acad Sci
USA 1994, 91:6649-6653.
14. Bartling D, Seedorf M, Schmidt RC, Weiler EW: Molecular
characterization of two cloned nitrilases from Arabidopsis
thaliana: key enzymes in the biosynthesis of the plant hormone
indole-3-acetic acid.Proc Natl Acad Sci USA 1994, 91:6021-6025.
15. Hillebrand H, Bartling D, Weiler EW: Structural analysis of the
nit2/nit1/nit3 gene cluster encoding nitrilases, enzymes catalyzing the terminal activation step in indole-acetic acid biosynthesis in
Arabidopsis thaliana. Plant Mol Biol 1998, 36:89-99. 16. Bartling D, Seedorf M, Mithöfer A, Weiler EW: Cloning and
expression of an Arabidopsis nitrilase which can convert
indole-3-acetonitrile to the plant hormone, indole-3-acetic acid.
Eur J Biochem 1992, 205:417-424.
17. Schmidt RC, Müller A, Hain R, Bartling D, Weiler EW: Transgenic
tobacco plants expressing the Arabidopsis thaliana nitrilase II
enzyme.Plant J 1996, 9:683-691.
18. Grsic S, Sauerteig S, Neuhaus K, Albrecht M, Rossiter J, Ludwig-Müller J: Physiological analysis of transgenic Arabidopsis thaliana
plants expressing one nitrilase isoform in sense or antisense
direction. J Plant Phys 1998, 153:446-456.
19. Müller A, Hillebrand H, Weiler EW: Indole-3-acetic acid is
synthesized from L-tryptophan in roots of Arabidopsis thaliana.
Planta 1998, 206:362-369.
20. Ribnicky DM, Ilic N, Cohen JD, Cooke TJ: The effects of exogenous auxins on endogenous indole-3-acetic acid metabolism: the
implications for carrot somatic embryogenesis.Plant Physiol
1996, 112:549-558.
21. Koshiba T, Kamiya Y, Iino M: Biosynthesis of indole-3-acetic acid
from L-tryptophan in coleoptile tips of maize (Zea mays L.).Plant
Cell Physiol 1995, 36:1503-1510.
22. Ludwig-Müller J, Epstein E, Hilgenberg W: Auxin-conjugate hydrolysis in Chinese cabbage: characterization of an amidohydrolase and its role during infection with clubroot
disease. Physiologia Plantarum 1996, 96:627-634.
23. Ludwig-Müller J, Pieper K, Ruppel M, Cohen JD, Epstein E, Kiddle G, Bennett R: Indole glucosinolate and auxin biosynthesis in
Arabidopsis thaliana L. glucosinolate mutants and the
development of clubroot disease.Planta 1999, in press.
24. Xu P, Narasimhan ML, Samson T, Coca MA, Huh G-H, Zhou J, Martin GB, Hasegawa PM, Bressan RA: A nitrilase-like protein interacts with GCC box DNA-binding proteins involved in ethylene
and defense responses. Plant Physiol 1998, 118:867-874.
25. Tam YY, Normanly J: Determination of indole-3-pyruvic acid levels in Arabidopsis thaliana by gas chromatography selected ion
monitoring mass spectrometry. J Chromatography 1998,
800:101-108.
26. Sekimoto H, Seo M, Kawakami N, Komano T, Desloire S,
• Liotenberg S, Marion-Poll A, Caboche M, Kamiya Y, Koshiba T:
Molecular cloning and characterization of aldehyde oxidases in
Arabidopsis thaliana. Plant Cell Physiol 1998, 39:433-442.
Arabidopsis encodes at least four aldehyde oxidases. The mRNA for one of these genes, AtAO-1, accumulates in the sur1 (rty) mutant, correlating with increased AO activity and higher IAA levels in this mutant thereby implicat-ing aldehyde oxidase in IAA synthesis.
27. Walker-Simmons M, Kudrna DA, Warner RL: Reduced accumulation of ABA during water stress in a molybdenum cofactor mutant of
barley.Plant Physiol 1989, 90:728-733.
28. Gopalraj M, Tseng T-S, Olszewski N: The Rooty gene of Arabidopsis
encodes a protein with highest similarity to aminotransferases.
Plant Physiol 1996, 111(S):114.
29. Seo M, Akaba S, Oritani T, Delarue M, Bellini C, Caboche M,
•• Koshiba T: Higher activity of an aldehyde oxidase in the
auxin-overproducing superroot1 mutant of Arabidopsis thaliana.Plant
Physiol 1998, 116:687-693.
Aldehyde oxidase activity with a preference for indole acetaldehyde is increased in the sur1 (rty) mutant. IAA levels are also elevated in this mutant, suggesting that the increased aldehyde oxidase activity is responsible for increased IAA synthesis.
30. Hoff T, Frandsen GI, Rocher A, Mundy J: Biochemical and genetic characterization of three molybdenum cofactor hydroxylases in
Arabidopsis thaliana. Biochim Biophys Acta 1998, 1398:397-402. 31. Bandurski RS, Kowalczyk S, Leznicki A, Mekhedov S, Momonoki Y,
• Oguri S: Metabolic targets for control of IAA levels in maize.Plant Growth Regulation Society of America Proceddings of the 25th Annual Meeting 1998, 26:181-186.
The authors review IAA metabolism in maize endosperm, where decades of biochemical experiments have revealed multiple inputs and outputs to the IAA pool in a single tissue.
32. Bartel B, Fink GR: ILR1, an amidohydrolase that releases active
indole-3-acetic acid from conjugates. Science 1995,
268:1745-1748.
33. Davies RT, Goetz DH, Lasswell J, Anderson MN, Bartel B: IAR3
•• encodes an auxin conjugate hydrolase from Arabidopsis.Plant
Cell 1999, 11:365-376.
The IAA-Ala insensitive iar3 mutant was isolated, and the defective gene shown to encode an IAA conjugate hydrolase with specificity for IAA-Ala. The IAR3 gene is similar to the previously isolated ILR1 gene [32] and at least four additional ILR1-like (ILL) genes in Arabidopsis.
34. Slovin JP: Phytotoxic conjugates of indole-3-acetic acid: potential agents for biochemical selection of mutants in conjugate
hydrolysis.Plant Growth Regulation 1997, 21:215-221.
35. Titarenko E, Rojo E, León J, Sánchez-Serrano JJ: Jasmonic acid
• dependent and -independent signaling pathways control
wound-induced gene activation in Arabidopsis thaliana. Plant Physiol
1997, 115:817-826.
The authors use differential display to isolate cDNAs induced by wounding or JA. One of these genes, JR3, is 96% identical to IAR3, which encodes an IAA conjugate hydrolase [33••]. JR3 induction by wounding is blocked
in the JA-insensitive coi1 mutant.
36. Rojo E, Titarenko E, León J, Berger S, Vancanneyt G,
•• Sánchez Serrano JJ: Reversible protein phosphorylation regulates jasmonic acid-dependent and -independent wound signal
transduction in Arabidopsis thaliana.Plant J 1998, 13:153-165.
A pharmacological approach is used to determine that wound signal trans-duction in Arabidopsis involves reversible phosphorylation. JR3 [35•] is
unique among the JA-induced genes studied in that it is induced by cyclo-heximide and its induction by JA is not reversed by auxin. The authors sug-gest that JR3 might dampen the JA response by increasing IAA levels. 37. Chou J-C, Mulbry WW, Cohen JD: The gene for indole-3-acetyl-L
• aspartic acid hydrolase from Enterobacter agglomerans:
molecular cloning, nucleotide sequence, and expression in
Escherichia coli. Mol Gen Genet 1998, 259:172-178.
The bacterial iaaspH gene, encoding an IAA-Asp hydrolase similar to
38. Michalczuk L, Ribnicky DM, Cooke TJ, Cohen JD: Regulation of indole-3-acetic acid biosynthetic pathways in carrot cell cultures.
Plant Physiol 1992, 100:1346-1353.
39. Kawaguchi M, Syono K: The excessive production of indole-3-acetic acid and its significance in studies of the biosynthesis of
this regulator of plant growth and development. Plant Cell Phys
1996, 37:1043-1048.
40. Patten CL, Glick BR: Bacterial biosynthesis of indole-3-acetic acid.
Can J Microbiol 1996, 42:207-220.
41. Costacurta A, Vanderleyden J: Synthesis of phytohormones by
plant-associated bacteria. Crit Rev Microbiology 1995, 21:1-18.
42. Manulis S, Haviv-Chesner A, Brandl MT, Lindow SE, Barash I:
• Differential involvement of indole-3-acetic acid biosynthetic
pathways in pathogenicity and epiphytic fitness of Erwinia herbicola
pv. gypsophialae. Mol Plant–Microbe Int 1998, 11:634-642.
The construction of mutant E. herbicola strains with defects in the IAM path-way, the IPA pathpath-way, or both sheds light upon the differential utilization of these two pathways.
43. Gray WM, Östin A, Sandberg G, Romano CP, Estelle M: High
•• temperature promotes auxin-mediated hypocotyl elongation in
Arabidopsis.Proc Natl Acad Sci USA 1998, 95:7197-7202. High temperature is found to promote hypocotyl elongation in the light, and is accompanied by an increase in free and conjugated IAA levels. The expan-sion is specifically blocked in IAA transport and response mutants. 44. Tam YY, Slovin JP, Cohen JD: Continuous light alters indole-3
• acetic acid metabolism in Lemna gibba. Phytochemistry 1998,
49:17-21.
The authors measured IAA turnover in wild-type and mutant duckweed fronds under various light conditions. For wild-type, IAA levels remain constant in either continuous light or darkness due to adjustments in the rates of biosynthesis and turnover. A feedback-insensitive anthranilate synthase mutant accumulated IAA, presumably because IAA biosynthesis rates were less well modulated. 45. Cohen JD, Bandurski RS: Chemistry and physiology of the bound
auxins. Annu Rev Plant Physiol 1982, 33:403-430.
46. Sztein AE, Cohen JD, García de la Fuente I, Cooke TJ: Auxin
• metabolism in mosses and liverworts.Amer J Bot 1999, in press.
IAA metabolism was characterized in five liverworts, four mosses, and two tracheophytes. The liverworts and mosses have low levels of both free and conjugated IAA, whereas the tracheophhytes have low free IAA but high lev-els of conjugates. Conjugation rates are faster in the mosses and tracheo-phytes than in the liverworts.
47. Sztein AE, Cohen JD, Slovin JP, Cooke TJ: Auxin metabolism in
representative land plants. Amer J Botany 1995, 82:1514-1521.
48. Östin A, Kowalyczk M, Bhalerao RP, Sandberg G: Metabolism of
•• indole-3-acetic acid in Arabidopsis. Plant Physiol 1998, 118:285-296.
The authors fed Arabidopsis seedlings low levels of labeled IAA and fol-lowed its metabolism using MS. Oxidation was most prevalent at low IAA concentrations and formation of conjugates with Asp and Glu was observed at higher IAA concentrations. The authors also show that the IAA-Asp becomes oxidized, indicating that this conjugate can be an intermediate in the irreversible inactivation of IAA in Arabidopsis, as it is in other plants. 49. Delarue M, Prinsen E, Van Onckelen H, Caboche M, Bellini C: Sur2
•• mutations of Arabidopsis thaliana define a new locus involved in
the control of auxin homeostasis. Plant Journal 1998, 14:603-611.
The superroot2 mutant contains elevated levels of free IAA and displays phe-notypes that reflect this increase. The decrease of conjugated IAA in this mutant suggests that it is defective in IAA inactivation.
50. Szerszen JD, Szczyglowski K, Bandurski RS: iaglu, a gene from Zea mays involved in conjugation of growth hormone indole-3-acetic
acid. Science 1994, 265:1699-1701.
51. Lomax TL, Muday GK, Rubery PH: Auxin transport. In Plant Hormones. Edited by Davies PJ . Dordrecht, The Netherlands: Kluwer Academic Publishers; 1995:509-530.
52. Reed RC, Brady SR, Muday GK: Inhibition of auxin movement from
• the shoot to the root inhibits lateral root development in
Arabidopsis. Plant Physiol 1998, 118:1369-1378.
Local applications of an auxin transport inhibitor at the root-shoot junction prevented formation of lateral roots without affecting root elongation, and this inhibition was reversed after application of IAA. These results suggest that IAA from the shoot is required for lateral root induction.
53. Sundberg B, Uggla C: Origin and dynamics of indoleacteic acid
• under polar transport in Pinus sylvestris. Physiologia Plantarum
1998, 104:22-29.
The apical source of IAA in pine saplings was replaced with 13C 6-IAA. The decreasing ratio of labeled to unlabeled IAA in serial stem sections indicates that apical IAA is not the only source of free IAA in these trees.
54. Jones A: Auxin transport: down and out and up again. Science
• 1998, 282:2201-2202.
Review article summarizing the recent cloning of genes likely to be central to polar auxin transport.
55. Estelle M: Polar auxin transport: new support for an old model.
• Plant Cell 1998, 10:1775-1778.
Review article summarizing the recent cloning of genes likely to be central to polar auxin transport.
56. Bennett MJ, Marchant A, Green HG, May ST, Ward SP, Millner PA, Walker AR, Schultz B, Feldmann KA: Arabidopsis AUX1 gene: a
permease-like regulator of root gravitropism. Science 1996,
273:948-950.
57. Gälweiler L, Guan C, Müller A, Wiseman E, Mendgen K, Yephremov A,
•• Palme K: Regulation of polar auxin transport by AtPIN1 in
Arabidopsis vascular tissue. Science 1998, 282:2226-2230. The pin1 mutant, which has a pin-formed inflorescence and defects in embryo symmetry, was previously shown to be defective in polar auxin transport. Here the PIN1 gene was isolated by transposon tagging and shown to be expressed in all organs of the plant. The PIN1 protein was immunolocalized to the basal end of elongated xylem cells in stems, consistent with its pro-posed role as an auxin efflux carrier.
58. Luschnig C, Gaxiola RA, Grisafi P, Fink GR: EIR1, a root-specific
•• protein involved in auxin transport, is required for gravitropism in
Arabidopsis thaliana. Genes Dev 1998, 12:2175-2187.
The eir1 mutant, which was previously isolated based on ethylene insensi-tivity in the root, has agravitropic roots and is allelic with agr1, pin2 and
wav6–52. The EIR1gene was cloned by transposon tagging [58••],
posi-tional cloning [59••,60•], and homology to PIN1[61••], and is expressed in
roots [58••,59••,60•,61••]. Double mutant analysis indicates that eir1roots
have reduced sensitivity to endogenous auxin [58••]. EIR1 expressed in
yeast increases resistance to fluorinated indolic compounds [58,59], con-sistent with its proposed role as a auxin efflux carrier.
59. Chen R, Hilson P, Sedbrook J, Rosen E, Caspar T, Masson PH: The
•• Arabidopsis thaliana AGRAVITROPIC1gene encodes a
component of the polar-auxin-transport efflux carrier. Proc Natl
Acad Sci USA 1998, 95:15112-15117.
See [58••]. AGR1expressed in yeast promotes increased IAA efflux,
con-sistent with its proposed role as an auxin efflux carrier.
60. Utsuno K, Shikanai T, Yamada Y, Hashimoto T: AGR, an Agravitropic
• locus of Arabidopsis thaliana, encodes a novel membrane-protein
family member. Plant Cell Physiol 1998, 39:1111-1118.
See [58••]. Vertically grown agr1roots grow into media containing IAA or
NAA, but not media containing 2,4-D. Since 2,4-D diffuses out of cells whereas IAA and NAA are transported via an efflux carrier, this result sug-gests a specific defect in auxin efflux.
61. Müller A, Guan C, Gälweiler L, Tänzler P, Huijser P, Marchant A,
•• Parry G, Bennett M, Wisman E, Palme K: AtPIN2defines a locus of
Arabidopsis for root gravitropism control. EMBO J 1998,
17:6903-6911.
See [58••]. The PIN2 protein was immunolocalized to the basal end of
epi-dermis cells in the root elongation zone, consistent with its propsed role as an auxin efflux carrier.
62. Iyer M, Cohen JD, Slovin JP: Molecular manipulation of IAA