www.elsevier.nlrlocateraqua-online
Negative correlation between aneuploidy and
growth in the Pacific oyster, Crassostrea gigas:
ten years of evidence
Alexandra Leitao
˜
a, Pierre Boudry
b, Catherine Thiriot-Quievreux
´
a,)a
ObserÕatoire Oceanologique, Uni´ Õersite P. et M. Curie — CNRS — INSU, BP 28,´
06230 Villefranche sur-Mer, France
b
Station IFREMER, Laboratoire de Genetique et Pathologie, BP 133, 17390 La Tremblade, France´ ´
Received 25 March 2000; received in revised form 10 July 2000; accepted 18 July 2000
Abstract
This study unites previous and new observations that chromosome loss in somatic cells of juveniles of the Pacific oyster Crassostrea gigas is associated with reduced growth rate. New data were obtained from four hatchery-produced progenies. Within each progeny, oysters of identical age were sampled and classified as fast-growing or slow-growing according to their shell length, or live weight. Chromosome numbers were individually scored from 30 randomly chosen metaphases from gill tissue. Although the diploid chromosome number in C. gigas is 2 Ns20, hypodiploid cells of 2 Ns19, 18 or 17 were observed in all progenies. The difference in percentage of aneuploidy between fast- and slow-growing oysters ranged from 5% to 22% and
Ž 2 .
was highly significant. In one progeny, a highly significant negative correlation R s0.58 was observed between individual level of aneuploidy and growth performances individually recorded under common controlled conditions. The consistency of the negative relationship between growth rate and aneuploidy was obvious in all populations studied between 1988 and 1999. However, when comparing aneuploidy between fast- and slow-growing oysters, two different groups of populations can be clearly distinguished. The question remains to determine the cause of these differences.q2001 Elsevier Science B.V. All rights reserved.
Keywords: Aneuploidy; Growth; Chromosome; Oyster; Crassostrea gigas
)Corresponding author. Tel.:q33-4-93-76-38-25; fax:q33-4-93-76-38-93.
Ž .
E-mail address: [email protected] C. Thiriot-Quievreux .´
0044-8486r01r$ - see front matterq2001 Elsevier Science B.V. All rights reserved.
Ž .
1. Introduction
High growth rate variability has been often observed in bivalves. Even among individuals with the same age and grown under common conditions, a great variation in
Ž
shell size and total weight can be observed e.g. Singh and Zouros, 1978; Mallet and .
Haley, 1983 . Several factors have been found to be associated with bivalve growth rate,
Ž .
including physical and nutritional environment e.g. Askew, 1972; Utting, 1986 ,
Ž . Ž
broodstock conditioning e.g. Gallager and Mann, 1986 , physiological Bayne et al.,
. Ž
1999 and genetic parameters e.g. Newkirk, 1980; Gaffney, 1988; Hedgecock et al., .
1996 . Positive correlations have been observed between heterozygosity and growth, as well as between heterozygosity and other fitness related traits such as viability, fecundity
Ž .
and developmental stability e.g. Mitton and Grant, 1984; Zouros and Foltz, 1987 . The Ž
general significance of these correlations has been extensively debated e.g. Pogson and .
Zouros, 1994; David et al., 1995 .
Cytogenetic abnormalities, such as aneuploidy, are known to be common in bivalve Ž
populations Longwell et al., 1968; Ahmed and Sparks, 1967; Dixon, 1982; Thiriot-Quievreux, 1986; Martinez-Exposito et al., 1992; Cornet, 1993; Li and Havenhand,
´
. Ž
1997 . Aneuploidy has been also observed in triploid and tetraploid oysters Guo and .
Allen, 1994; Wang et al., 1999 .
This phenomenon which mainly originates from the non-disjunction of chromosomes
Ž .
during mitosis or meiosis e.g. Bond and Chandley, 1983; Martin and Rademaker, 1990 is often lethal in higher animals, such as mammals, or is associated with growth
Ž .
retardation Vig and Sandberg, 1987 . But the effects of aneuploidy seem less
deleteri-Ž .
ous in plants and lower animals Verma, 1990 . A negative correlation between somatic aneuploidy and growth rate has been described in offspring of cultivated Crassostrea
Ž .
gigas oysters Thiriot-Quievreux et al., 1988, 1992 and in natural populations of the
´
Ž .
same species Zouros et al., 1996 , i.e. in a single cohort, fast-growing animals show
Ž .
fewer aneuploid cells than slow-growing animals Table 1 . No relationship was seen Ž between allozyme heterozygosity and either shell length or chromosome loss
Thiriot-. Quievreux et al., 1992; Zouros et al., 1996 .
´
In this paper, we present recent data from populations of hatchery-produced C. gigas, and a global survey of all populations studied since 1988, to assess the consistency of the growth-aneuploidy relationship.
2. Materials and methods
2.1. Origin of the studied oysters
Ž .
Oysters from two IFREMER hatcheries, La Tremblade Charente Maritime, France
Ž . Ž
and Argenton Finistere, France , were studied. In La Tremblade, parental oysters five
`
.
males and five females per site originating from three sites located along the French
Ž .
Atlantic coast Arcachon, Port-des-Barques and Bonne-Anse were crossed to generate
Ž .
three progenies, as described by Collet 1998 . In Argenton, parental oysters originating from a Scottish hatchery were reproduced by mass spawning. Larvae and spat were
Ž .
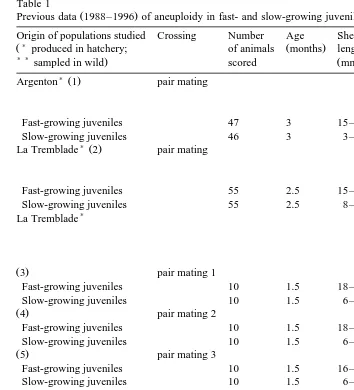
Table 1
Ž .
Previous data 1988–1996 of aneuploidy in fast- and slow-growing juvenile oysters
Origin of populations studied Crossing Number Age Shell Aneuploidy Reference )
Ž produced in hatchery; of animals Žmonths. length Ž .%
) )sampled in wild. scored Žmm.
)Ž .
Argenton 1 pair mating
Thiriot-Quievreux´ Ž .
et al. 1988
Fast-growing juveniles 47 3 15–20 5
Slow-growing juveniles 46 3 3–5 19
)Ž .
La Tremblade 2 pair mating
Thiriot-Quievreux´ Ž .
et al. 1988
Fast-growing juveniles 55 2.5 15–20 10
Slow-growing juveniles 55 2.5 8–10 27
)
Fast-growing juveniles 10 1.5 18–22 6
Slow-growing juveniles 10 1.5 6–8 14
Ž .4 pair mating 2
Fast-growing juveniles 10 1.5 18–22 9
Slow-growing juveniles 10 1.5 6–8 25
Ž .5 pair mating 3
Fast-growing juveniles 10 1.5 16–21 7
Slow-growing juveniles 10 1.5 6–8 21
Ž .6 mass mating
Fast-growing juveniles 10 1.5 15–21 12
Slow-growing juveniles 10 1.5 7–8 34
)Ž .
La Tremblade 7 pair mating
Thiriot-Quievreux´ Ž .
et al. 1992
Fast-growing juveniles 39 6 20–25 11
Slow-growing juveniles 45 6 10–15 27
) )Ž .
Bonne Anse 8 mass mating Zouros
Ž .
et al. 1996
Fast-growing juveniles 40 6 25–29 16
Slow-growing juveniles 40 6 9–16 21
Ž .:numbering of populations for Fig. 2.
Ž .
facilities at Bouin Vendee, France
´
where they were grown with Skeletonemacostatum-enriched seawater.
2.2. Growth monitoring
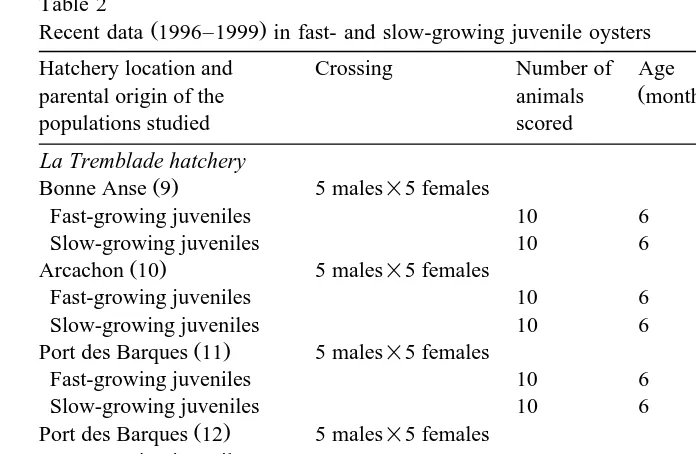
Table 2
Ž .
Recent data 1996–1999 in fast- and slow-growing juvenile oysters
Hatchery location and Crossing Number of Age Shell lengthr Aneuploidy
Ž . Ž .
parental origin of the animals months weight %
populations studied scored
La Tremblade hatchery
Ž .
Bonne Anse 9 5 males=5 females
Fast-growing juveniles 10 6 40–50 mm 19
Slow-growing juveniles 10 6 20–35 mm 25
Ž .
Arcachon 10 5 males=5 females
Fast-growing juveniles 10 6 40–50 mm 22
Slow-growing juveniles 10 6 20–35 mm 30
Ž .
Port des Barques 11 5 males=5 females
Fast-growing juveniles 10 6 40–50 mm 18
Slow-growing juveniles 10 6 20–35 mm 25
Ž .
Port des Barques 12 5 males=5 females
Fast-growing juveniles 12 20 74.7–94.2 gr 6
Medium-growing juveniles 12 20 46.9–59.3 gr 17
Fast-growing juveniles 12 20 22.9–36.8 gr 22
Argenton hatchery
Ž .
Scotland 13 mass mating
Fast-growing juveniles 15 17 78–96 mm 5
Slow-growing juveniles 15 17 45–66 mm 16
Ž .: numbering of populations for Fig. 2.
Ž .
length or live weight Table 2 . Additionally, 36 1-year-old oysters, representative of the range of growth performance present in the Port-des-Barques progeny, were individually labelled and their live weight was measured at 12, 15 and 20 months in order to examine the aneuploidy-growth relationship at the individual level.
2.3. Chromosome scoring
The animals were incubated for 8–10 h in seawater containing 0.005% colchicine. Then gills were dissected in seawater, treated for 30 min in 0.9% sodium citrate and
Ž .
fixed in a freshly prepared mixture of absolute alcohol–acetic acid 3:1 with three 20 min changes. Slides were made from one individual gill following the air drying
Ž .
technique of Thiriot-Quievreux and Ayraud 1982 . The preparation was stained for 10
´
Ž .
min with Giemsa 4%, pH 6.8 . Chromosome counts were made directly by microscope
Ž .
observation Zeiss III photomicroscope on apparently intact and well-spread metaphases.
2.4. Data analyses
The level of aneuploidy was estimated by counting 30 randomly chosen metaphases per individual showing a similar good chromosome spread. This is the minimal
Ž
statistical number usually accepted in cytogenetic studies Stallard et al., 1981; Wenger .
Since the number of aneuploid metaphases per studied individual was the same in all
Ž .
the studied material 30 per individual , it was therefore possible to test for size andror population effects using one- and two-way analyses of variance. Differences in aneu-ploidy between fast- and slow-growing oysters in the populations studied were also tested by analysis of covariance. Statistical analyses were computed using SYSTAT 9.0 by SPSS.
3. Results
Ž
Although the Pacific oyster has a diploid chromosome number of 20 Ahmed and .
Sparks, 1967 , hypodiploid cells of 2 Ns19, 18 or 17 were observed in all of the four
Ž .
recent progenies studied Table 2 . The level of aneuploidy was significantly different
Ž .
between populations Fs12.454; P-0.001 , the oysters of Scottish origin being less aneuploid than those of the other three populations. The size class was tested as a
Ž .
within-population effect and found to be highly significant Fs8.703; P-0.001 . Individual aneuploidy levels were scored for 36 20-month-old oysters from the
APort-des-BarquesBprogeny. To give a concise view of the extent of aneuploidy in this Ž progeny, oysters were grouped intoAslow-B, Amedium-B andAfast-growingBgroups 12
.
oysters in each . The number of aneuploid cells with 2 Ns19, 18 or 17 was calculated
Ž .
for each group Table 3 . Highly significant linear negative correlations were observed
Ž 2
between aneuploidy and total weight in 12-, 15- and 20-month-old oysters R s0.40,
. Ž .
0.53 and 0.58; P-0.001 , respectively Fig. 1 .
Ž Looking at the 13 populations studied since 1988, including the present ones, Tables
.
1 and 2, Fig. 2 , slow-growing oysters always showed higher levels of aneuploidy than fast-growing oysters. The difference in aneuploidy between fast- and slow-growing oysters ranged from 5% to 22%. Statistical analyses of the unpublished data obtained in
Ž . Ž
1988 four populations revealed significant between-population Fs10.553; P
-. Ž .
0.001 and within-population size effects Fs21.536; P-0.001 .
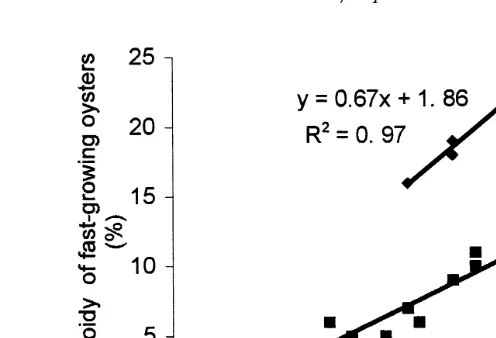
When comparing aneuploidy between fast- and slow-growing oysters in the 13 populations studied, two different groups can be clearly distinguished: populations 8, 9,
Ž . Ž . Ž .
10 and 11 in one group 1 and the other populations in a second group 2 Fig. 3 . In populations of group 1, fast growers are 0.72–0.76 times less aneuploid than slow
Table 3
Observed aneuploidy of individually labelled oysters from theAPort-des-BarquesB pogeny
Growth Mean Total number Number of cells observed with Total number Aneuploidy
Fig. 1. Correlation between individual aneuploidy level and total weight in 20-month-old oysters of the
APort-des-BarquesB population.
Ž .
growers, while in group 2, this ratio is much lower 0.26–0.43 . In other words, the difference in aneuploidy between fast and slow growers was twice as much in group 2 as in group 1. An analysis of covariance revealed no significant difference in the slopes of linear regression between aneuploidy of fast- and slow-growing oysters between these
Ž . Ž .
Fig. 2. Comparison of % aneuploidy between fast- black and slow-growing white oysters in the 13
Ž .
Fig. 3. Relationship between mean aneuploidy of slow-growing and fast-growing oysters in the 13 populations studied.
Ž .
two groups of populations Fs2.47; Ps0.15 . However, this analysis showed a
Ž .
significant group effect Fs199.7; P-0.001 .
4. Discussion
The occurrence of hypodiploid cells was observed in all experimental populations of
C. gigas studied here. The consistency of the negative relationship between growth rate
Ž .
and aneuploidy in C. gigas is obvious Fig. 2 . Fast-growing animals always showed less aneuploidy than slow-growing animals in all the cultivated and natural populations studied over this 10-year survey. Such growth retardation of aneuploids was not
Ž
observed in triploid and tetraploid Pacific oysters Guo and Allen, 1994; Wang et al.,
. Ž .
1999 , or in the Pacific abalone Fujino et al., 1990 . Nevertheless, aneuploidy in higher
Ž .
animals has often been associated with growth retardation Vig and Sandberg, 1987 . In humans, growth retardation in Turner’s syndrome has been associated with hypodiploidy ŽHaverkamp et al., 1999 ..
The causes of the observed relationship between growth and aneuploidy are unclear,
Ž .
but a genetic basis Zouros et al., 1996 and environmental factors, such as pollution ŽDixon, 1982 or viral disease e.g. Maroun et al., 1986 have been suggested. Zouros et. Ž .
Ž .
al. 1996 proposed that the aneuploidy–growth relationship is due to the unmasking of deleterious recessive genes caused by the progressive haploidization of somatic cells. This progressive haploidization will, in turn, result in the unmasking of mutations affecting growth rate and may cause a retardation of growth. This hypothesis has been
Ž .
When comparing the populations reported in the present paper, differences in the level of aneuploidy were observed among populations and two different groups can be
Ž .
distinguished Fig. 3 . The causes of the observed inter-population variation on the relationship between growth and aneuploidy are unknown. However, a point
worth-not-Ž
ing is that three out of the four populations of the first group in which the difference in . aneuploidy between fast and slow growers was half as much as in the second group were produced simultaneously and experienced abnormal mortalities during early life
Ž .
stages Collet, 1998 . Unfortunately, no information about mortality of the fourth Ž
population of this group is available as it was sampled from the wild Zouros et al., .
1996 . The question remains to determine the respective importance of these presumed causative factors to explain the observed variation, both within and among population levels.
Ž .
Wang et al. 1999 reported aneuploidy in triploids but discounted it in normal diploid C. gigas, assuming that diploid metaphases were due to artifacts. However, for these authors, a chromosome number was accepted for an oyster if 5–10 metaphases produced the same chromosome count. We suggest that the probability of accurately estimating aneuploidy using only five or 10 metaphases is considerably reduced. In our study, the probability of aneuploid cells being the result of artifacts of the air-drying methods of chromosome preparation is reduced by the higher number of metaphases
Ž .
studied Wenger et al., 1984 and by the selection of similar well-spread metaphases. A study of the reliability of this method for aneuploidy scoring has been performed by
Ž .
Thiriot-Quievreux 1986 .
´
One limitation of our assessment of aneuploidy, however, is that we have not identified the missing chromosomes. This can now be performed by G-banding tech-niques, as these have recently allowed the individual identification of each homologous
Ž .
chromosome pair in C. gigas Leitao et al., 1999 . The identification of missing
˜
chromosomes will be a fundamental step towards a better understanding of the aneu-ploidy phenomenon in oysters.
Acknowledgements
Part of this research was supported by the EU AGENEPHYSB contract FAIR PL 95.421. We thank Dr A. Gerard for the management of this project, S. Heurtebise for
´
technical assistance and the teams in Bouin, Argenton and La Tremblade for the hatchery and nursery work. We are grateful to Dr H. McCombie for English editing.
References
Ž
Ahmed, M., Sparks, A.K., 1967. A preliminary study of two species of oysters Ostrea lurida and Crassostrea
.
gigas . J. Fish. Res. Board Can. 24, 2155–2159.
Askew, C.G., 1972. The growth of Ostrea edulis and Crassostrea gigas in Ensworth Harbour. Aquaculture 1, 237–259.
Bayne, B.L., Svensson, S., Nell, J.A., 1999. The physiological basis for faster growth in the Sydney rock
Ž .
Bond, D.J., Chandley, A.C., 1983. Aneuploidy. Oxford Univ. Press, Oxford.
Collet, B., 1998. Bases genetiques des caracteres physiologiques impliques dans la croissance chez l’huıtre´ ´ ` ´ ˆ
creuse Crassostrea gigas. These de Doctorat de l’Institut National Agronomique Paris-Grignon, 208 pp.`
Cornet, M., 1993. A short-term culture method for chromosome preparation from somatic tissues of adult
Ž .
mussel Mytilus edulis . Experientia 49, 87–90.
David, P., Delay, B., Berthou, P., Jarne, P., 1995. Alternative models for allozyme-associated heterosis in the marine bivalve Spisula oÕalis. Genetics 139, 1719–1726.
Ž .
Dixon, D.R., 1982. Aneuploidy in mussel embryos Mytilus edulis L. originating from a polluted dock. Mar. Biol. Lett. 3, 155–161.
Fujino, K., Arai, K., Iwadare, K., Yoshida, T., Nakajima, S., 1990. Induction of gynogenetic diploid by inhibiting second meiosis in the Pacific abalone. Nippon Suisan Gakkaishi 56, 1755–1763.
Gaffney, P.M., 1988. Genetic improvement of cultured bivalve species. J. Shellfish Res. 7, 158–159. Gallager, S.M., Mann, R., 1986. Growth and survival of larvae of Mercenaria mercenaria and Crassostrea
Õirginica relative to broodstock conditioning and lipid contents of eggs. Aquaculture 56, 105–123.
Ž .
Guo, X.M., Allen, S.K., 1994. Viable tetraploids in the pacific oyster Crassostrea gigas Thunberg produced
Ž .
by inhibiting polar body I in eggs from triploids. Mol. Mar. Biol. Biotechnol. 3 1 , 42–50.
Haverkamp, E., Wolfle, J., Zerres, K., Butenandt, Q., Amendt, P., Hauffa, B.P., Weimann, E., Bettendorf, M., Keller, E., Muhlenberg, R., Partsch, C.J., Sippel, W.G., Hoppe, C., 1999. Growth retardation in Turner syndrome: aneuploidy, rather than specific gene loss, may explain growth failure. J. Clin. Endocrinol. Metab. 84, 4578–4582.
Hedgecock, D., McGoldrick, D.J., Manahan, D.T., Vavra, J., Appelmans, N., Bayne, B.L., 1996. Quantitative and molecular genetic analyses of heterosis in bivalve molluscs. J. Exp. Mar. Biol. Ecol. 203, 49–59. Launey, S., Hedgecock, D., 1999. Genetic load causes segregation distorsion in oysters: mapping at 6 hours.
Plant and Animal Genome VII Conference. Abstract W 14.
Leitao, A., Thiriot-Quievreux, C., Boudry, P., Malheiro, I., 1999. G-chromosome banding study of three˜ ´ Ž
species of cupped oysters: Crassostrea gigas, Crassostrea angulata and CrassostreaÕirginica Mollusca:
.
Bivalvia . Genet. Sel. Evol. 31, 519–527.
Li, X.X., Havenhand, J.N., 1997. Karyotype, nucleolus organizer regions and constitutive heterochromatin in
Ž .
Ostrea angasi Mollusca: Bivalvia : evidence of taxonomic relationship within Ostreidae. Mar. Biol. 127,
443–448.
Longwell, A.C., Stiles, S.S., Smith, D.G., 1968. Chromosome complement of the American oyster
Cras-sostreaÕirginica as seen in meiotic and cleaving eggs. Can. J. Genet. Cytol. 9, 845–856.
Mallet, A.L., Haley, L.E., 1983. Growth rate and survival in pure population matings and crosses of the oyster
CrassostreaÕirginica. Can. J. Fish. Aquat. Sci. 40, 948–954.
Maroun, L.E., Degner, M., Mourey, M.E., Rowan, D.F., Amankwah, K.S., 1986. Increased mortality and aneuploidy in embryos from rabbits injected with Newcastle disease virus at the time of ovulation and conception. Teratology 34, 201–206.
Martin, R.H., Rademaker, A., 1990. The frequency of aneuploidy among individual chromosomes in 6,821 human sperm chromosome complements. Cytogenet. Cell Genet. 53, 103–107.
Martinez-Exposito, M.J., Martinez-Lage, A.A., Pasantes, J.J., Mendez, J., 1992. A preliminary study of aneuploidy in natural populations in the genus Mytilus. Cuad. Area Cienc. Mar. Semin. Estud. Galegos 6, 49–55.
Mitton, J.B., Grant, M.C., 1984. Associations among protein heterozygosity, growth rate and developmental stability. Annu. Rev. Ecol. Syst. 15, 479–499.
Newkirk, G.F., 1980. Review of the genetics and the potential for selective breeding of commercially important bivalves. Aquaculture 19, 209–228.
Pogson, G.H., Zouros, E., 1994. Allozyme and RFLP heterozygoties as correlates of growth in the scallop
Placopecten magellanicus: a test of the associate overdominance hypothesis. Genetics 137, 221–231.
Singh, S.M., Zouros, E., 1978. Genetic variation associated with growth rate in the American oyster
ŽCrassostreaÕirginica . Evolution 32, 342–353..
Stallard, R., Haney, N.R., Frank, P.A., Styron, P., Juberg, R.C., 1981. Leukocyte chromosomes from parents of cytogenetically abnormal offspring: preliminary observations. Cytogenet. Cell Genet. 30, 50–53.
´ Ž .
Thiriot-Quievreux, C., 1986. Etude de l’aneuploıdie dans differents naissains d’Ostreidae Bivalvia . Genetica´ ¨
Thiriot-Quievreux, C., Ayraud, N., 1982. Les caryotypes de quelques especes de Bivalves et de Gasteropodes´ ` ´
marins. Mar. Biol. 70, 165–172.
Thiriot-Quievreux, C., Noel, T., Bougrier, S., Dallot, S., 1988. Relationships between aneuploidy and growth´ ¨
rate in pair matings of the oyster Crassostrea gigas. Aquaculture 75, 89–96.
Thiriot-Quievreux, C., Pogson, G.H., Zouros, E., 1992. Genetics of growth rate variation in bivalves:´
aneuploidy and heterozygosity effects in a Crassostrea family. Genome 35, 39–45.
Utting, S.D., 1986. A preliminary study on growth of Crassostrea gigas larvae and spat in relation to dietary protein. Aquaculture 56, 123–138.
Verma, R.S., 1990. The Genome. VCH Publishers.
Vig, B.K., Sandberg, A.A., 1987. Aneuploidy: Part A. Incidence and Etiology. Alan R. Liss, New York.
Ž .
Walne, P.R. Ed. , 1974. Culture of Bivalve Molluscs: Fifty Years Experience at Conwy. Whitefriars Press, London, 189 pp.
Ž .
Wang, Z., Guo, X., Allen, S.K., Wang, R., 1999. Aneuploid pacific oyster Crassostrea gigas Thunberg as incidentals from triploid production. Aquaculture 173, 347–357.
Wenger, S.L., Golden, W.L., Dennis, S.P., Steele, M.W., 1984. Are the occasional aneuploid cells in peripheral blood cultures significant? Am. J. Med. Genet. 19, 715–719.
Zouros, E., Foltz, D.W., 1987. Minimal selection requirements for the correlation between heterozygosity and growth, and for the deficiency of heterozygotes, in oyster populations. Dev. Genet. 4, 393–405. Zouros, E., Thiriot-Quievreux, C., Kotoulas, G., 1996. The negative correlation between somatic aneuploidy´