Chemical ecology of host-plant selection by
herbi-vorous arthropods: a multitrophic perspective
Marcel Dicke
*
Laboratory of Entomology, Wageningen University, P.O. Box 8031, 6700 EH Wageningen, The Netherlands
Received 3 June 1999; accepted 17 August 1999
Abstract
Most herbivorous arthropods are specialists that feed on one or a few related plant species. To understand why this is so, both mechanistic and functional studies have been carried out, predominantly restricted to bitrophic aspects. Host-selection behaviour of herbivorous arthro-pods has been intensively studied and this has provided ample evidence for the role of secondary plant chemicals as source of information in behavioural decisions of herbivores. Many evolutionary studies have regarded co-evolution between plants and herbivores to explain the diversity of secondary plant chemicals and host specialisation of herbivores. However, many cases remain unexplained where herbivores select host plants that are subopti-mal in terms of"tness returns. A stimulating paper by Bernays and Graham [(1988) Ecology 69, 886}892)] has initiated a discussion on the need of a multitrophic perspective to understand the evolution of host-plant specialisation by herbivorous arthropods. However, this has hardly resulted in ecological studies on host-selection behaviour that take a multitrophic perspective. Yet, evidence is accumulating that constitutive and induced infochemicals from natural enemies and competitors can a!ect herbivore behaviour. These cues may constitute important informa-tion on"tness prospects, just as plant cues can do. In this paper I selectively review how information from organisms at di!erent trophic levels varies in space and time and how herbivores can integratively exploit this information during host selection. In doing so, research areas are identi"ed that are likely to provide important new insights to explain several of the questions in herbivore host selection that remain unanswered so far. These research areas are at the interface of evolutionary ecology, behavioural ecology and chemical ecology. ( 2000 Elsevier Science Ltd. All rights reserved.
Keywords:Host-plant selection; Avoidance of competition; Predator avoidance; Herbivore; Tritrophic interactions; Infochemical; Ecology; Optimal foraging
*Fax:#31-317-484821.
E-mail address:[email protected] (M. Dicke)
1. Introduction
Understanding the causes and consequences of variable traits in interactions among individual organisms is a central theme of evolutionary ecology. Variation among individuals may result in di!erences in reproductive success. If this variation has a genetic basis, selection will favour those genotypes that have phenotypes with the largest genetic contributions to the next generation. To maximise reproductive success, individual organisms have to make many&decisions'during their life, many of which revolve around tradeo!s. These decisions comprise e.g. how much to invest in growth relative to defence, when to attract a mate, when and where to reproduce, how many o!spring to allocate to a certain location, whether to search for food or to hide from enemies etc. (Krebs and Davies, 1984; Stephens and Krebs, 1986; Ricklefs, 1990; Price, 1997). Information on biotic and abiotic environmental conditions provides an opportunity to make decisions that are best adapted to current and future circumstan-ces. The information available is subject to variation in time and space. An important challenge for organisms is to adequately interpret this variation in information so as to maximise reproductive success.
This is where evolutionary ecology and chemical ecology meet. Information on biotic environmental conditions is often available through chemical cues that can consist of a mixture of a few to several tens or up to more than a hundred di!erent compounds (e.g. Nordlund et al., 1981; Bell and CardeH, 1984; CardeH and Bell, 1995; Dicke, 1999). The composition of infochemicals (Dicke and Sabelis, 1988) can vary e.g. with genotype of the producer, with biotic or with abiotic conditions. Some of these variations may represent signal, whereas other variation may represent noise to a responding individual, which is dependent on the correlation of cue variation with "tness prospects for the responder. Responding organisms are therefore expected to have evolved the ability to discriminate between signal and noise in infochemical variation. Taking such functional aspects into consideration will be important for the development of chemical ecological approaches.
The value of an infochemical can also depend on contextual variation, e.g. on the simultaneous presence of other cues (Robertson et al., 1995). These other cues may represent an alternative option. For instance, the odour of an inferior host plant may have a di!erent value to a starved herbivore in the absence or in the presence of the odour of a superior host plant. Moreover, the contextual cues may also modulate the intrinsic information value of the primary infochemical. For instance, the odour of a preferred host plant may have a di!erent meaning to a herbivore in the presence or in the absence of cues from competitors.
Schoonhoven et al., 1998). However, considering host-plant selection by herbivores merely as a process of selecting plants with the best quality for development and reproduction, does not provide a complete picture. Furthermore, considering plant}herbivore interactions in a bitrophic context cannot fully explain the evolution of host-plant selection by herbivorous arthropods (Bernays and Graham, 1988; Thompson, 1988b). Herbivores have evolved and function in multitrophic foodwebs and therefore studies of host-plant selection should also consider the importance of defence against enemies and avoidance of competition (Faeth, 1985; Bernays and Graham, 1988; Bernays, 1998). Indeed, host plant selection by herbivores may be a!ected by infochemicals from competitors (e.g., Wood, 1982; Birch, 1984; Prokopy et al., 1984; Schoonhoven 1990) and natural enemies such as carnivores (Ho!meister and Roitberg, 1997; Grostal and Dicke, 1999a), although these cues have received much less emphasis in studies of host-plant selection by herbivores than plant cues. In fact, the in#uence of infochemicals from natural enemies on foraging behaviour of herbivorous arthropods in terrestrial systems is a recent discovery that may have important consequences for future studies on host selection by terrestrial arthropods. In this paper I will emphasise multitrophic aspects of host-plant selection by herbi-vores as mediated by infochemicals. In doing so, I will call attention to both the importance of variation in infochemicals derived from di!erent trophic levels as well as the variation resulting from the integration of di!erent combinations of infochemi-cals on host plant-selection by herbivores. I will restrict this paper to chemical information representing variation in the quality of resources. It should be noted that in addition, quantitative variation in resource availability can also be important (e.g. Roitberg et al., 1999).
2. Variation in plant information
An overwhelmingly large number of so-called secondary plant chemicals have been characterised in plants (Bernays and Chapman, 1994; Gershenzon, 1994; Schoo-nhoven et al., 1998). These secondary chemicals are thought to have a major role in defence against attackers (Fraenkel, 1959) and for many compounds this has been con"rmed experimentally (reviewed by Rosenthal and Berenbaum, 1992; Bernays and Chapman, 1994; Schoonhoven et al., 1998). However, although secondary chemicals may defend plants against generalist herbivores, many secondary chemicals are exploited during host selection as so-called&token stimuli'by specialist herbivores that are not negatively a!ected by the plant chemicals (StaKdler, 1986). For instance, pierid butter#ies use glucosinolates in the plant cuticle to recognise cruciferous host plants on which they oviposit (van Loon et al., 1992; Chew and Renwick, 1995). In addition, plant secondary chemicals may subsequently be exploited by specialist herbivores through sequestration, which can result in protection of the herbivores from their enemies (e.g. Bernays and Graham, 1988; Krischik et al., 1988; Hunter and Schultz, 1993; Rowell-Rahier et al., 1995; Hartmann, 1999).
source}sink relationships (Gershenzon, 1984; Zangerl and Berenbaum, 1990; Herms and Mattson, 1992; Rosenthal and Berenbaum, 1992; Bernays and Chapman, 1994; Honkanen and Haukioja, 1998; Schoonhoven et al., 1998). Herbivores may use this variation during selection of oviposition or feeding sites (Bernays and Chapman 1994; Schoonhoven et al., 1998 and references therein).
Investments in plant defence and plant growth are often negatively correlated and therefore variation in environmental conditions that a!ect a plant's growth rate can also a!ect its investment in defence (Herms and Mattson, 1992; Gershenzon, 1994). Hence, the possibility for herbivores to exploit plant chemicals during host-plant selection may be dependent on e.g. the plant's availability of light and nutrients.
2.1. Herbivore-inducedvariation in plant information
Plant secondary chemicals may be induced by herbivore attack or pathogen infestation and this may have short term (days) or long-term (up to more than a year) e!ects (for reviews see Karban and Myers, 1989; Haukioja, 1990; Tallamy and Raupp, 1991; Karban and Baldwin, 1997; Tollrian and Harvell, 1999). For instance, herbivory induces a drastic increase in nicotine concentration in tobacco plants within several days and this response may vary with abiotic conditions such as nutrient availability (Baldwin, 1999). The induction of secondary chemicals in plants may vary with the herbivore species that damages the plant (Stout et al., 1994). Furthermore, induced changes in chemical composition can occur locally and systemically and may lead to intra-individual variation in addition to constitutive variation. Many of the changes induced by herbivory lead to induced resistance, but induced susceptibility has also been reported (Karban and Baldwin, 1997). Sometimes this induced susceptibility can be explained by responses of specialist herbivores to increased concentrations of&token stimuli'used in host-plant selection (e.g. Stanjek et al., 1997). However, in other cases plant physiological responses such as source-sink relationships being disturbed by defoliation may explain induced susceptibility (Honkanen and Haukioja, 1998).
plant attracts more herbivores. Because many plant species emit a di!erent blend when mechanically damaged (e.g. caused by wind-borne sand or sca"ng against other plants) than when damaged by herbivores, speci"c information on the level of competition is available to herbivores. Therefore, one would expect that herbivores would bene"t from discriminating between volatiles from herbivore-infested plants and mechanically damaged plants (see Dicke and Vet, 1999 for a discussion). How-ever, current experimental data do not provide substantial support for this expecta-tion (Dicke and Vet, 1999). Furthermore, if the induced plant volatiles are speci"c for the herbivore species that damages the plant, they may enable the herbivores to discriminate between plants infested with di!erent types of competitors. This is supported by a study on the response of spider mites to induced plant volatiles (Pallini et al., 1997). The response of herbivores to induced plant volatiles is a research topic that is still in its infancy though, and future studies are expected to elucidate whether our current view is correct and how herbivore responses can be understood from a functional point of view. An interesting study in this respect is that on the cabbage looper moth (Trichoplusia ni). Female moths are attracted to volatiles from cotton plants infested with conspeci"c larvae. After arriving at the infested plants, however, the females do not oviposit on the already infested plants, but search for nearby uninfested plants on which they oviposit (Landolt, 1993). This indicates that volatiles from infested plants provided a cue to locate a patch of host plants and thus made the plants more apparent, but that competition is actively avoided during subsequent foraging decisions.
An induced plant response may also account for a phenomenon that was long thought to involve herbivore-produced cues. Pierid butter#ies avoid oviposition on host plants on which other females have previously oviposited (Schoonhoven, 1990). This was long assumed to be caused by a secretion from the accessory gland that the female deposited during oviposition, and that was termed an oviposition-deterring or host-marking pheromone (Schoonhoven, 1990). However, an in-depth study has shown that a plant cue and not a herbivore product is responsible for the herbivore's behavioural response. Blaakmeer et al. (1994) demonstrated that the plant onto which the female oviposits, responds with the systemic production of chemicals in the leaf cuticle that result in other butter#ies avoiding the plant as an oviposition substrate. This represents an induced response of plants to herbivores that seems to be indepen-dent of the herbivore damaging the plant.
3. Variation in herbivore infochemicals
Products of the herbivore of course represent the most direct information on herbivore presence. These products may comprise herbivore pheromones that are released from glands or as components of faeces (Prokopy et al., 1984; Roessingh et al., 1988; Hilker and Klein, 1989; Schindek and Hilker, 1996). The presence of herbivore pheromones is usually variable in time. They are often emitted during restricted time periods and they can have short half-lives. Adult moths emit sex pheromones during speci"c moments of the day (CardeH and Minks, 1997). The emitted pheromone may adsorb to the foliage and be perceived at a later moment, thus prolonging the time window of pheromone availability (Wall et al., 1981; Noldus et al., 1991). On the other hand, pheromones may be washed away as was shown for host-marking pheromones applied on a host by herbivorous#ies, which shortens the time window (Averill and Prokopy, 1987). It should be noted that in many cases, host-marking pheromones have not been chemically identi"ed (but see Hurter et al., 1987). There is a chance, that, as was found forPieris brassicae, other host-marking pheromones also appear not to be produced by the herbivore, but by the plant in response to the herbivore (Blaakmeer et al., 1994). If the plant is the producer of the herbivore-related cue, the information may be more variable than when the cue is a pheromone produced by the herbivore (Vet and Dicke, 1992).
Herbivore pheromones may also be emitted in response to attack by enemies. Alarm pheromones are well known to be induced in aphids and thrips in response to attack by predators (Pickett et al., 1992; Teerling et al., 1993). Thus, these pheromones not only indicate the presence of competitors, but also the presence of natural enemies. Alarm pheromones can a!ect various herbivore behaviours, among which the aban-doning of a host plant or seeking refuge (McAllister and Roitberg, 1987; Pickett et al., 1992; Pallini et al., 1998). In addition, herbivorous arthropods may also avoid cues related to dead conspeci"cs, which may represent another type of information on the presence of enemies (Rollo et al., 1995; Grostal and Dicke, 1999a).
So, in addition to being indicators of competitors, herbivore cues may also provide information on the presence of carnivorous enemies. However, the more direct information on enemy presence would be derived from the carnivores themselves.
4. Carnivore infochemicals and host selection by herbivores
animal has to cope with (for reviews see e.g. Lima and Dill, 1989; Kats and Dill, 1998). Herbivores may prefer nutritionally inferior host plants on which chances of encoun-tering parasitoids are predictably low over nutritionally superior host plants that are frequently visited by natural enemies (Jaenike, 1985; Ohsaki and Sato, 1994). In addition, there are several examples of herbivores avoiding resources on which their enemies are actually present (e.g. Bernstein, 1984; Prokopy and Duan, 1998) and the avoidance of enemies through infochemicals has been documented for a wide range of organisms (Kats and Dill, 1998; Tollrian and Harvell, 1999). Only recently, evidence for the avoidance of enemies through carnivore infochemicals is becoming available for terrestrial arthropods (Kats and Dill, 1998). For instance, host acceptance by Rhagoletis basiola fruit #ies is negatively a!ected by cues from an egg parasitoid (Ho!meister and Roitberg, 1997) and herbivorous spider mites avoid plant tissue contaminated with an infochemical from phytoseiid predators that incur a large mortality risk (Kriesch and Dicke, 1997; Grostal and Dicke, 1999a). The infochemical responsible for this response of spider mites remained active for at least 4 days after deposition by the predators (Kriesch and Dicke, 1997). Furthermore, the spider mites avoided infochemicals from a wide range of carnivorous mites, including carnivores that would not attack the spider mites such as acarine parasites of chicken or honeybees (Grostal and Dicke, 1999b). However, the spider mites did not respond to cues from fungivorous or pollen-feeding mites (Grostal and Dicke, 1999a,b). More-over, the spider mites were clearly able to distinguish among potential predators: if facultative predators were either fed on spider mites or on pollen, the spider mites had a signi"cantly stronger avoidance of infochemicals from spider mite-fed predators than from infochemicals of the pollen-fed predators (Grostal and Dicke, 1999b). These data are not likely to be an exception given the widespread occurrence of predator avoidance in aquatic systems and in terrestrial vertebrate systems (Kats and Dill, 1998). Therefore, investigating the importance of carnivore infochemicals in host selection by terrestrial arthropod herbivores is likely to be an important and fruitful future research area for chemical ecologists. For example, ants are well known for marking their foraging substrate and they are abundantly available on plants that provide the ants with shelter or alternative food (HoKlldobler and Wilson, 1990; Yano, 1994). Such plants are likely to be contaminated with ant pheromones and herbivores would pro"t from exploiting the infochemicals to avoid running into their predators.
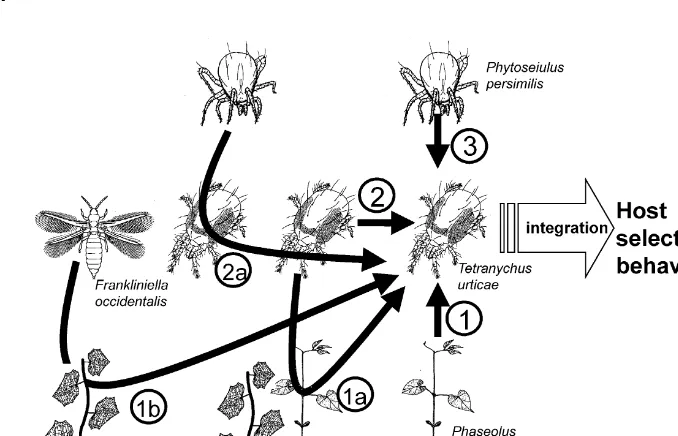
Fig. 1. Example of information from di!erent trophic levels that is known to a!ect host-selection behaviour of the two-spotted spider miteTetranychus urticae(Acari: Tetranychidae). Arrows indicate information that a!ects spider-mite behaviour, while accompanying numbers refer to the trophic level of the organism that produces the information. 1: Volatiles from undamaged Lima bean plants (Phaseolus lunatus) (Dicke, 1986); 1a: spider-mite-induced volatiles emitted by Lima bean plants (Dicke, 1986) or cucumber plants (Pallini et al., 1997); 1b: thrips (Frankliniella accidentalis)-included volatiles emitted by cucumber plants (Pallini et al., 1997); 2: cues from spider mite eggs or adults (Grostal and Dicke, 1999a); 2a: predatory mites (Phytoseiulus persimilis) are likely to induce an alarm pheromone in adult spider mites (Janssen et al., 1997) that a!ects spider mite behaviour (Pallini, 1998); 3: the predatory miteP. persimilisproduces a non-volatile cue that a!ects spider mite foraging behaviour (Kriesch and Dicke, 1997; Grostal and Dicke, 1999a,b) and a volatile cue that can a!ect alarm-pheromone emission by spider mites (Janssen et al., 1997).
5. Correlation between variation in infochemicals from di4erent sources
(Vrieling et al., 1991; Heil et al., 1999). In an analysis of the variation in pyrrolizidine alkaloids inSenecio jacobaeaand associated herbivore numbers, Vrieling et al. (1991) found that plants with high concentrations of pyrrolizidine alkaloids had low num-bers of aphids and aphid-tending ants and vice versa. The aphid-tending ants attack caterpillars of the specialist lepidopteran herbivoreTyria jacobaeaethat is not a!ected by di!erences in pyrrolizidine alkaloids-levels. Thus, plants with low amounts of pyrrolizidine alkaloids may be contaminated with large amounts of carnivore in-fochemicals, while plants with large amounts of pyrrolizidine alkaloids may have low amounts of carnivore cues.
Herbivore pheromones may be positively correlated with certain plant volatiles because these plant cues induce pheromone emission by the herbivore (McNeil and Delisle, 1989; Raina et al., 1991).
A question that has not received attention so far in the literature, is whether plants may respond to the presence of carnivorous arthropods. Plants may constitutively provide shelter and alternative food for carnivores (Koptur, 1992; Walter, 1996) which can result in the presence of carnivores in the absence of herbivores. If plants can perceive the presence of the carnivores, e.g. by perceiving the removal of alternative food or by perceiving faeces of the carnivores, could this have an e!ect on their induced responses to herbivores? For instance, would the investment in induced volatiles be negatively correlated with the presence of carnivorous arthropods? This might enable plants to reduce the exploitation of induced volatiles by herbivores. Such an ability of plants may seem to be far-fetched at "rst. However, the extensive information on the responses of plants to information from competing plants, herbi-vores and pathogens even in the absence of damage (Blaakmeer et al., 1994; Bruin et al., 1995; Karban and Baldwin, 1997; Shulaev et al., 1997; van Loon, 1997; BallareH, 1999) should make us careful not to underestimate the abilities of plants to respond to biotic components in the environment.
6. Integration of information from di4erent trophic levels during host-plant selection
infochemicals to herbivores (Ho!meister and Roitberg, 1997; Grostal and Dicke, 1999a,b) suggests that this will be a fruitful area for further investigation.
Herbivores may integrate information perceived simultaneously from di!erent sources. For instance, the response to sex pheromones can be modulated by in-fochemicals from host plants present (Dickens et al., 1993; Landolt et al., 1994; Lilley and Hardie, 1996). Furthermore, the response by herbivores to infochemicals emitted by herbivores or by herbivore-infested plants can be dependent on the amount of these cues relative to the amount of infochemical from uninfested plants (Dicke 1986; Pettersson et al., 1998).
To foraging herbivores, plants represent a resource that has a value in terms of food, competitors and natural enemies. Food selection has often been studied in a bitrophic context, where the food quality in terms of its e!ect on herbivore "tness was considered. Alternatively, consequences of host-plant selection for defence of herbi-vores against their enemies have been considered (Barbosa, 1988a; Bernays and Graham, 1988; Pasteels et al., 1988; Rowell-Rahier and Pasteels, 1992). It will be important to include studies on herbivore behaviour in situations with con#icting alternatives derived from combinations of costs and bene"ts related to food, competi-tors and enemies. For instance, what decisions does a herbivore make when the alternatives are an inferior host plant without carnivore cues versus a superior host plant with abundant carnivore cues? Are the decisions di!erent for specialist and generalist herbivores? Is the preference of specialist insects for plants containing secondary chemicals that can be exploited in defence against carnivores di!erent in the presence or absence of predator cues? Such research questions are an exciting part of behavioural ecology (e.g. Krebs and Davies, 1984; Courtney, 1986; Godin and Sproul, 1988; Anholt and Werner, 1995; Gotceitas et al., 1995; Bouskila et al., 1998) that deserve the incorporation of a chemical ecological approach on the role of infochemicals in host plant selection by herbivores.
7. Herbivores,information networks and food webs:conclusions
The study of foraging behaviour of carnivorous arthropods has made tremendous progress in the past two decades by proceeding from a bitrophic perspective to a multitrophic perspective. Considering the importance of plant information in foraging strategies of carnivores has created a completely new view on the organisa-tion of food webs (Price, 1981; Dicke et al., 1990; Vet and Dicke, 1992; Tumlinson et al., 1993; Turlings et al., 1993,1995; Bruin et al., 1995; Takabayashi and Dicke, 1996; Janssen et al., 1998; Powell et al., 1998; Dicke and Vet, 1999; Sabelis et al., 1999). It is clear that an information web is superimposed on a food web and that the information web creates many indirect interactions in addition to the mostly trophi-cally based direct interactions (Vet and Dicke, 1992; Janssen et al., 1998; Dicke and Vet, 1999).
acknowledgement that plant chemistry is of signi"cant importance in host selection by herbivorous arthropods, major questions remain unanswered (e.g. Courtney and Kibota, 1990). Alternative approaches to the study of host-plant selection by herbi-vores, such as expressed in the seminal paper by Bernays and Graham (1988) have met with mixed responses (e.g. Barbosa, 1988b; Courtney, 1988; Ehrlich and Murphy, 1988; Jermy, 1988; Schultz, 1988; Thompson, 1988a). However, if an extensive chem-ical information network exists with information on plant presence and nutritional quality, on competitor abundance and identity and on chances of encountering enemies, why would herbivores not exploit this information? At the very least, the importance of this information network for foraging behaviour of herbivores deserves to be investigated. This should be done both in laboratory studies and in"eld studies. A majority of studies on host-plant selection by herbivores has been executed in the laboratory and future laboratory studies will continue to be important to determine the options available to herbivores. In addition,"eld studies should be carried out to reveal to what extent the potential of decision-making that has been recorded in the laboratory can play a role under"eld conditions where conditions are much more variable in many respects. Taking a multitrophic approach to herbivore host-selection behaviour will improve our knowledge and will make an important contribution to solving several of the questions on host selection by herbivores that are still open. In doing so, students of host selection by herbivorous arthropods will make new contributions to evolutionary ecology, behavioural ecology and chemical ecology.
Acknowledgements
The manuscript bene"ted from constructive comments on an earlier version by Joop van Loon, Bernie Roitberg, Louis Schoonhoven and Louise Vet. MD was funded in part by the Uyttenboogaart-Eliasen Foundation, Amsterdam.
References
Agrawal, A.A., Karban, R., 1997. Domatia mediate plant}arthropod mutualism. Nature 387, 562}563. Anholt, B.R., Werner, E.E., 1995. Interaction between food availability and predation mortality mediated
by adaptive behavior. Ecology 76, 2230}2234.
Atsatt, P.R., 1981. Ant-dependent food plant selection by mistletoe butter#yOgyris amaryllis(Lycaenidae). Oecologia 48, 60}63.
Averill, A.L., Prokopy, R.J., 1987. Residual activity of oviposition-deterring pheromone inRhagoletis pomonella(Diptera: tephritidae) and female response to infested fruit. J. Chem. Ecol. 13, 167}177. Baldwin, I.T., 1999. Inducible nicotine production in nativeNicotianaas an example of adaptive phenotypic
plasticity. J. Chem. Ecol. 25, 3}30.
BallareH, C.L., 1999. Keeping up with the neighbours: phytochrome sensing and other signalling mecha-nisms. Trends Plant Science 4, 97}102.
Barbosa, P., 1988a. Natural enemies and herbivore-plant interactions: in#uence of plant allelochemicals and host speci"city. In: Barbosa, P., Letourneau, D.K. (Eds.), In Novel aspects of insect}plant interactions. Wiley, New York, pp. 201}229.
Baylis, M., Pierce, N.E., 1991. The e!ect of host plant quality on the survival of larvae and oviposition by adults of an ant-tended lycaenid butter#y,Jalmenus evagoras. Ecol. Entomol. 16, 1}9.
Bell, W.J., CardeH, R.T. (Eds.), 1984. Chemical Ecology of Insects. Chapman and Hall, London.
Bernays, E.A., 1995. E!ects of experience on host-plant selection. In: Carde, R.T., Bell, W.J. (Eds.), Chemical Ecology of Insects, Vol. 2. Chapman and Hall, New York, pp. 47}64.
Bernays, E.A., 1998. Evolution of feeding behavior in insect herbivores*Success seen as di!erent ways to eat without being eaten. Bioscience 48, 35}44.
Bernays, E.A., Chapman, R.L., 1994. Host-Plant Selection by Phytophagous Insects. Chapman and Hall, New York.
Bernays, E., Graham, M., 1988. On the evolution of host speci"city in phytophagous arthropods. Ecology 69, 886}892.
Bernstein, C., 1984. Prey and predator emigration responses in the acarine systemTetranychus urticae} Phytoseiulus persimilis. Oecologia 61, 134}142.
Birch, M.C., 1984. Aggregation in bark beetles. In: Bell, W.J., CardeH, R.T. (Eds.), Chemical Ecology of Insects. Chapman and Hall, New York, pp. 331}353.
Blaakmeer, A., Hagenbeek, D., van Beek, T.A., Groot, A.E. de, Schoonhoven, L.M., van Loon, J.J.A., 1994. Plant response to eggs vs. host marking pheromone as factors inhibiting oviposition byPieris brassicae. J. Chem. Ecol. 20, 1657}1665.
Bolter, C.J., Dicke, M., van Loon, J.J.A., Visser, J.H., Posthumus, M.A., 1997. Attraction of Colorado potato beetle to herbivore damaged plants during herbivory and after its termination. J. Chem. Ecol. 23, 1003}1023.
Bouskila, A., Robinson, M.E., Roitberg, B.D., Tenhumberg, B., 1998. Life-history decisions under predation risk: importance of a game perspective. Evol. Ecol. 12, 701}715.
Bruin, J., Sabelis, M.W., Dicke, M., 1995. Do plants tap SOS signals from their infested neighbours? Trends Ecol. Evol. 10, 167}170.
CardeH, R.T., Bell, W.J. (Eds.) (1995). Chemical Ecology of Insects, Vol. 2. Chapman and Hall, New York.
CardeH, R.T., Minks, A.K. (Eds.) (1997). Insect Pheromone Research: New Directions. Chapman and Hall, New York.
Chew, F.S., Renwick, J.A.A., 1995. Host plant choice inPierisbutter#ies. In: Carde, R.T., Bell, W.J. (Eds.), Chemical Ecology of Insects, Vol. 2. Chapman and Hall, New York, pp. 214}238.
Courtney, S.P., 1986. Why insects move between host patches: some comments on&risk spreading'. Oikos 47, 1.
Courtney, S.P., 1988. If it's not coevolution, it must be predation? Ecology 69, 910}911.
Courtney, S.P., Kibota, T.T., 1990. Mother doesn't know best: Selection of hosts by ovipositing insects. In: Bernays, E.A. (Ed.), Insect-plant interactions, Vol. 2. CRC Press, Florida, pp. 161}189.
Denno, R.F., McClure, M.S. (Eds.) (1983). Variable plants and herbivores in natural and managed systems. Academic Press, New York.
Dicke, M., 1986. Volatile spider-mite pheromone and host-plant kairomone, involved in spaced-out gregariousness in the spider mite¹etranychus urticae. Physiol. Entomol. 11, 251}262.
Dicke, M., 1994. Local and systemic production of volatile herbivore-induced terpenoids: Their role in plant-carnivore mutualism. J. Plant Physiol 143, 465}472.
Dicke, M., 1999. Evolution of induced indirect defence of plants. In: Tollrian, R., Harvell, C.D. (Eds.), The Ecology and Evolution of Inducible Defenses. Princeton University Press, Princeton, NJ, pp. 62}88.
Dicke, M., Sabelis, M.W., 1988. Infochemical terminology: based on cost-bene"t analysis rather than origin of compounds? Funct. Ecol. 2, 131}139.
Dicke, M., Takabayashi, J., Posthumus, M.A., SchuKtte, C., Krips, O.E., 1998. Plant-phytoseiid interactions mediated by prey-induced plant volatiles: variation in production of cues and variation in responses of predatory mites. Exp. Appl. Acarol. 22, 311}333.
Dicke, M., Vet, L.E.M., 1999. Plant}carnivore interactions: evolutionary and ecological consequences for plant, herbivore and carnivore. In: Ol!, H., Brown, V.K., Drent, R.H. (Eds.), Herbivores: Between Plants and Predators. Blackwell Science, Oxford, UK, pp. 483}520.
Dickens, J.C., Smith, J.W., Light, D.M., 1993. Green leaf volatiles enhance sex attractant pheromone of the tobacco budworm.Heliothis virescens(Lep.: Noctuidae). Chemoecology 4, 175}177.
Dukas, R., 1998. Ecological relevance of associative learning in fruit#y larvae. Behav. Ecol. Sociobiol. 19, 195}200.
Ehrlich, P.R., Murphy, D.D., 1988. Plant chemistry and host range in insect herbivores. Ecology 69, 908}909.
Faeth, S.H., 1985. Host leaf selection by leaf miners: interactions among three trophic levels. Ecology 66, 870}875.
Fraenkel, G.S., 1959. The raison d'e(tre of secondary plant substances. Science 129, 1466}1470.
Gershenzon, J., 1984. Changes in the levels of plant secondary metabolites under water and nutrient stress. In: Timmermans, N., Steelink, C., Loewus, F.A. (Eds.), Rec. Adv. Phytochem. 18, 273}320.
Gershenzon, J., 1994. The cost of plant chemical defense against herbivory: a biochemical perspective. In: Bernays, E.A. (Ed.), Insect}Plant Interactions, Vol. 5. CRC Press, Boca Raton, Florida, pp. 105}173.
Godin, J.J., Sproul, C.D., 1988. Risk taking in parasitized sticklebacks under threat of predation: e!ects of energetic need and food availability. Can. J. Zool. 66, 2360}2367.
Gotceitas, V., Fraser, S., Brown, J.A., 1995. Habitat use by juvenile atlantic cod (Gadus morhua) in the presence of an actively foraging and non-foraging predator. Marine Biology 123, 421}430.
Grostal, P., Dicke, M., 1999a. Direct and indirect cues of predation risk in#uence behavior and reproduc-tion of prey: a case for acarine interacreproduc-tions. Behav. Ecol., 10, 422}427.
Grostal, P., Dicke, M., 1999b. Recognising one's enemies: a functional apprach to risk assessment by prey. Behav. Ecol. Sociobiol., in press.
Grostal, P., O'Dowd, D.J., 1994. Plants, mites and mutualism: leaf domatia and the abundance and reproduction of mites onViburnum tinus(Caprifoliaceae). Oecologia 97, 308}315.
Hartmann, T., 1999. Chemical ecology of pyrrolizidine alkaloids. Planta 207, 483}495. Haukioja, E., 1990. Induction of defenses in trees. Annu. Rev. Entomol. 36, 25}42.
Hay, M.E., 1996. Marine chemical ecology: what's known and what's next? J. Exp. Marine Biol. Ecol. 200, 103}134.
Heil, M., Fiala, B., Linsenmair, K.E., 1999. Reduced chitinase activities in ant plants of the genus
Macaranga. Naturwissenschaften 86, 146}149.
Herms, D.A., Mattson, W.J., 1992. The dilemma of plants: to grow or to defend. Q. Rev. Biol. 67, 283}335.
Hilker, M., Klein, B., 1989. Investigation of oviposition deterrent in larval frass ofSpodoptera littoralis (Boisd.). J. Chem. Ecol. 15, 929}937.
Ho!meister, T.S., Roitberg, B.D., 1997. Counterespionage in an insect herbivore-parasitoid system. Natur-wissenschaften 84, 117}119.
HoKlldobler, B., Wilson, E.O., 1990. The ants. Springer Verlag, Berlin.
Honkanen, T., Haukioja, E., 1998. Intra-plant regulation of growth and plant-herbivore interactions. Ecoscience 5, 470}479.
Hunter, M.D., Schultz, J.C., 1993. Induced plant defenses breached? Phytochemical induction protects an herbivore from disease. Oecologia 94, 195}203.
Hurter, J., Boller, E.F., StaKdler, E., Blattmann, B., Buser, H.R., Bosshard, N.U., Damm, L., Kozlowski, M.W., Schoni, R., Raschdorf, F., Dahinden, R., Schlumpf, E., Fritz, H., Richter, W.J., Schreiber, J., 1987. Oviposition-deterring pheromone in Rhagoletis cerasi l * puri"cation and determination of the chemical constitution. Experientia 43, 157}164.
Huxley, C.R., Cutler, D.F. (Eds.), 1991. Ant}Plant Interactions. Oxford University Press, Oxford. Jaenike, J., 1985. Parasite pressure and the evolution of amanitin tolerance inDrosophila. Evolution 39,
1295}1301.
Jaenike, J., Papaj, D.R., 1992. Behavioral plasticity and patterns of host use by insects. In: Roitberg, B.D., Isman, M.B. (Eds.), Chemical Ecology of Insects: an Evolutionary Approach. Chapman and Hall, New York, pp. 245}264.
Janssen, A., Bruin, J., Jacobs, G., Schraag, R., Sabelis, M.W., 1997. Predators use volatiles to avoid prey patches with conspeci"cs. J. Anim. Ecol. 66, 223}232.
Janssen, A., Pallini, A., Venzon, M., Sabelis, M.W., 1998. Behaviour and indirect food web interactions among plant inhabiting arthropods. Exp. Appl. Acarol. 22, 497}521.
Jermy, T., 1988. Can predation lead to narrow food specialization in phytophagous insects? Ecology 69, 902}904.
Karban, R., Baldwin, I.T. (1997). Induced responses to herbivory. Chicago University Press, Chicago. Karban, R., Myers, J.H., 1989. Induced plant responses to herbivory. Annu. Rev. Ecol. Syst. 20,
331}348.
Kats, L.B. and Dill, L.M. (1998). The scent of death: chemosensory assessment of predation risk by prey animals. Ecoscience 5, 361}394.
Koptur, S., 1992. Extra#oral nectary-mediated interactions between insects and plants. In: Bernays, E.A. (Ed.), Insect}Plant Interactions, Vol. 4. CRC Press, Boca Raton, Florida, pp. 81}129.
Krebs, J.R., Davies, N.B., 1984. Behavioural ecology, an evolutionary approach, 2nd Edition. Blackwell Scienti"c Publications, Oxford 493.
Kriesch, S., Dicke, M., 1997. Avoidance of predatory mites by the two-spotted spider miteTetranychus urticae: the role of infochemicals. Proc. Exp. Appl. Entomol. 8, 121}126.
Krischik, V.A., Barbosa, P., Reichelderfer, C.F., 1988. Three trophic level interactions: allelochemicals,
Manduca sexta(L.), andBacillus thuringiensisvar. kurstaki Berliner. Environ. Entomol. 17, 476}482. Landolt, P.J., 1993. E!ects of host plant leaf damage on cabbage looper moth attraction and oviposition.
Entomol. Exp. Appl. 67, 79}85.
Landolt, P.J., Heath, R.R., Millar, J.G., Davis-Hernandez, K.M., Dueben, B.D., Ward, K.E., 1994. E!ects of host plant,Gossypium hirsutumL, on sexual attraction of cabbage looper moths, ¹richoplusia ni (Hubner) (Lepidoptera: Noctuidae). J. Chem. Ecol. 20, 2959}2974.
Lilley, R., Hardie, J., 1996. Cereal aphid responses to sex pheromones and host-plant odours in the laboratory. Physiol. Entomol. 21, 304}308.
Lima, S.L., Dill, L.M., 1989. Behavioral decisions made under the risk of predation: a review and prospectus. Can. J. Zool. 68, 619}640.
Loughrin, J.H., Potter, D.A., Hamilton-Kemp, T.R., 1995. Volatile compounds induced by herbivory act as aggregation kairomones for the Japanese beetle (Popilia japonica Newman). J. Chem. Ecol. 21, 1457}1467.
Mallet, J., Longino, J.T., Murawski, D., Murawski, A., De Gamboa, A.S., 1987. Handling e!ects in
Heliconius. Where do all the butter#ies go? J. Anim. Ecol. 56, 377}386.
McAllister, M.K., Roitberg, B.D., 1987. Adaptive suicidal behaviour in pea aphids. Nature 328, 797}799. McNeil, J.N., Delisle, J. 1989. Are host plants important in pheromone-mediated mating systems of
Lepidoptera? Experientia 45, 236}240.
MuKller, C.B., Godfray, H.C.J., 1999. Predators and mutualists in#uence the exclusion of aphid species from natural communities. Oecologia 119, 120}125.
Noldus, L.P.J.J., Potting, R.P.J., Barendregt, H.E., 1991. Moth sex pheromone adsorption to leaf surface: bridge in time for chemical spies. Physiol. Entomol. 16, 329}344.
Nordlund, D.A., Jones, R.L., Lewis, W.J. (Eds.), 1981. Semiochemicals, their role in pest control. Wiley, New York.
Ohsaki, N., Sato, Y., 1994. Food plant choice ofPieris butter#ies as a trade-o! between parasitoid avoidance and quality of plants. Ecology 75, 59}68.
Pallini, A., 1998. Odour-mediated Indirect Interactions in an Arthropod Food Web. University of Amster-dam, Amsterdam.
Pallini, A., Janssen, A., Sabelis, M.W., 1997. Odour-mediated responses of phytophagous mites to con-speci"c and heterospeci"c competitors. Oecologia 100, 179}185.
Papaj, D.R., Lewis, A.C. (Eds.), 1993. Insect Learning: Ecological and Evolutionary Perspectives. Chapman and Hall, New York.
Papaj, D.R., Prokopy, R.J., 1989. Ecological and evolutionary aspects of learning in phytophagous insects. Annu. Rev. Entomol. 34, 315}350.
Pasteels, J.M., Rowell-Rahier, M., Raupp, M.J., 1988. Plant-derived defense in Chrysomelid beetles. In: Barbosa, P., Letourneau, D.K. (Eds.), Novel aspects of Insect}Plant Interactions. Wiley, New York, pp. 235}272.
Pettersson, J., Karunaratne, S., Ahmed, E., Kumar, V., 1998. The cowpea aphid,Aphis craccivora, host plant odours and pheromones. Entomol. Exp. Appl. 88, 177}184.
Pickett, J.A., Wadhams, L.J., Woodcock, C.M., Hardie, J., 1992. The chemical ecology of aphids. Annu. Rev. Entomol. 37, 67}90.
Pierce, N.E., Elgar, M.A., 1985. The in#uence of ants on host plant selection byJalmenus evagoras, a myrmecophilous lycaenid butter#y. Behav. Ecol. Sociobiol. 16, 209}222.
Pierce, N.E., Young, W.R., 1986. Lycaenid butter#ies and ants: two-species stable equilibria in mutualistic, commensal, and parasitic interactions. Am. Nat. 128, 216}227.
Powell, W., Pennacchio, F., Poppy, G.M., Tremblay, E., 1998. Strategies involved in the location of hosts by the parasitoidAphidius erviHaliday (Hymenoptera: Braconidae: Aphidiinae). Biol. Control 11, 104}112. Price, P.W., 1981. Semiochemicals in evolutionary time. In: Nordlund, D.A., Jones, R.L., Lewis, W.J. (Eds.),
Semiochemicals, their role in pest control. Wiley, New York, pp. 251}271. Price, P.W., 1997. Insect Ecology. Academic Press, New York.
Prokopy, R.J., Duan, J.J., 1998. Socially facilitated egglaying behavior in Mediterranean fruit#ies. Behav. Ecol. Sociobiol. 42, 117}122.
Prokopy, R.J., Roitberg, B.D., Averill, A.L., 1984. Resource partitioning. In: Bell, W.J., CardeH, R.T. (Eds.), Chemical Ecology of Insects. Chapman and Hall, New York, pp. 301}330.
Quiroz, A., Pettersson, J., Pickett, J.A., Wadhams, L.J., Niemeyer, H.M., 1997. Semiochemicals mediating spacing behavior of bird cherry-oat aphidRhopalosiphum padifeeding on cereals. J. Chem. Ecol. 23, 2599}2607.
Raina, A.K., Kingan, T.G., Mattoo, A.K., 1991. Chemical signals from host plant and sexual behavior in a moth. Science 255, 592}594.
Ricklefs, R.E., 1990. Ecology. W.H. Freeman & Co., New York.
Robertson, I.C., Roitberg, B.D., Williamson, I., Senger, S.E., 1995. Contextual chemical ecology: an evolutionary approach to the chemical ecology of insects. Am. Entomol. 41, 237}239.
Roessingh, P., Peterson, S.C., Fitzgerald, T.D., 1988. The sensory basis of trail following in some lepidopter-ous larvae: contact chemoreception. Physiol. Entomol. 13, 219}224.
Roitberg, B.D., Robertson, I.C., Tyerman, J.G.A., 1999. Vive la variance: a functional oviposition theory for insect herbivores. Entomol. Exp. Appl. 91, 187}194.
Rollo, C.D., Borden, J.H., Casey, I.B., 1995. Endogenously produced repellent from American cockroach (Blattaria: Blattidae): Function in death recognition. Environ. Entomol. 24, 116}124.
Rosenthal, G.A. and Berenbaum, M.R. (eds.) (1992). Herbivores: their Interaction with Secondary Plant Metabolites, 2nd Edition. Academic Press, New York.
Rowell-Rahier, M., Pasteels, J.M., 1992. Third trophic level in#uences of plant allelochemics. In: Rosenthal, G.A., Berenbaum, M.R. (Eds.), Herbivores: their Interaction with Secondary Plant Metabolites, 2nd Edition, Vol. 2. Academic Press, New York, pp. 243}277.
Rowell-Rahier, M., Pasteels, J.M., Alonso-Mejia, A., Brower, L.P., 1995. Relative unpalatability of leaf beetles with either biosynthesized or sequestered chemical defence. Anim. Behav. 49, 709}714. Sabelis, M.W., van Baalen, M., Bakker, F.M., Bruin, J., Drukker, B., Egas, M., Janssen, A.R.M., Lesna, I.K.,
Pels, B., Van Rijn, P., Scutareanu, P., 1999. The evolution of direct and indirect plant defence against herbivorous arthropods. In: Ol!, H., Brown, V.K., Drent, R.H. (Eds.), Herbivores: Between Plants and Predators. Blackwell Science, Oxford, pp. 109}166.
Schindek, R., Hilker, M., 1996. In#uence of larvae ofGastrophysa viridulaon the distribution of conspeci"c adults in the"eld. Ecol. Entomol. 21, 370}376.
Schoonhoven, L.M., Jermy, T., Van Loon, J.J.A., 1998. Insect}Plant Biology. From Physiology to Evolu-tion. Chapman and Hall, London.
Schultz, J.C., 1988. Many factors in#uence the evolution of herbivore diets, but plant chemistry is central. Ecology 69, 896}897.
Shulaev, V., Silverman, P., Raskin, I., 1997. Airborne signalling by methyl salicylate in plant pathogen resistance. Nature 385, 718}721.
StaKdler, E., 1986. Oviposition and feeding stimuli in leaf surface waxes. In Insects and the Plant Surface. In: Juniper, B.E., Southwood, T.R.E (Eds.), Edward Arnold, London, pp. 105}121.
Stanjek, V., Herhaus, C., Ritgen, U., Boland, W., Stadler, E., 1997. Changes in the leaf surface ofApium graveolens(Apiaceae) stimulated by jasmonic acid and perceived by a specialist insect. Helv. Chim. Acta 80, 1408}1420.
Stephens, D.W., Krebs, J.R., 1986. Foraging Theory. Princeton University Press, Princeton.
Stout, M.J., Workman, J., Du!ey, S.S., 1994. Di!erential induction of tomato foliar proteins by arthropod herbivores. J. Chem. Ecol. 20, 2575}2594.
Szentesi, A., Jermy, T., 1990. The role of experience in host plant choice by phytophagous insects. In: Bernays, E.A. (Ed.), Insect}Plant Interactions, Vol. 2. CRC Press, Boca Raton, Florida, pp. 39}75. Takabayashi, J., Dicke, M., 1996. Plant-carnivore mutualism through herbivore-induced carnivore
attrac-tants. Trends Plant Sci. 1, 109}113.
Tallamy, D.W., Raupp, M.J. (eds.), 1991. Phytochemical induction by herbivores. Wiley, New York. Teerling, C.R., Pierce Jr., H.D., Borden, J.H., Gillespie, D.R., 1993. Identi"cation and bioactivity of alarm
pheromone in the western#ower thrip,Frankliniella occidentalis. J. Chem. Ecol. 19, 681}697. Thompson, J.N., 1988a. Coevolution and alternative hypotheses on insect/plant interactions. Ecology 69,
893}895.
Thompson, J.N., 1988b. Evolutionay ecology of the relationship between oviposition preference and performance of o!spring in phytophagous insects. Entomol. Exp. Appl. 47, 3}14.
Tollrian, R., Harvell, C.D. (Eds.), 1999. The Ecology and Evolution of Inducible Defenses. Princeton University Press, Princeton NJ.
Tumlinson, J.H., Lewis, W.J., Vet, L.E.M., 1993. How parasitic wasps"nd their hosts. Sci. Am. 268, 100}106.
Turlings, T.C.J., Loughrin, J.H., McCall, P.J., Rose, U.S.R., Lewis, W.J., Tumlinson, J.H., 1995. How caterpillar-damaged plants protect themselves by attracting parasitic wasps. Proc. Natl. Acad. Sci. USA 92, 4169}4174.
Turlings, T.C.J., WaKckers, F.L., Vet, L.E.M., Lewis, W.J., Tumlinson, J.H., 1993. Learning of host-"nding cues by Hymenopterous parasitoids. In: Papaj, D.R., Lewis, A.C. (Eds.), Insect learning: Ecological and Evolutionary Perspectives. Chapman and Hall, New York, pp. 51}78.
van Loon, J.J.A., Blaakmeer, A., Griepink, F.C., van Beek, T.A., Schoonhoven, L.M., de Groot, A.E., 1992. Leaf surface compound fromBrassica oleracea(Cruciferae) induces oviposition byPieris brassicae
(Lepidoptera: Pieridae). Chemoecology 3, 39}44.
van Loon, L.C., 1997. Induced resistance in plants and the role of pathogenesis-related proteins. Eur. J. Plant Pathol. 103, 753}765.
Vet, L.E.M., Dicke, M., 1992. Ecology of infochemical use by natural enemies in a tritrophic context. Annu. Rev. Entomol. 37, 141}172.
Vet, L.E.M., Lewis, W.J., Carde, R.T., 1995. Parasitoid foraging and learning. In: Carde, R.T., Bell, W.J. (Eds.), Chemical Ecology of Insects, Vol. 2. Chapman and Hall, New York, pp. 65}101.
Visser, J.H., 1986. Host odor perception in phytophagous insects. Annu. Rev. Entomol. 31, 121}144. Vrieling, K., Smit, W., van der Meijden, E., 1991. Tritrophic interactions between aphids (Aphis jacobaeae
Schrank), ant species,Tyria jacobaeae L. and Senecio jacobaea L. lead to maintenance of genetic variation in pyrrolizidine alkaloid concentration. Oecologia 86, 177}182.
Wagner, D., Kurina, L., 1997. The in#uence of ants and water availability on oviposition behaviour and survivorship of a facultatively ant-tended herbivore. Ecol. Entomol. 22, 352}360.
Walter, D.E., 1996. Living on leaves: mites, tomenta, and leaf domatia. Annu. Rev. Entomol. 41, 101}114. Wood, D.L., 1982. The role of pheromones, kairomones, and allomones in the host selection and
colonization behavior of bark beetles. Annu. Rev. Entomol. 27, 411}446.
Yano, S., 1994. Flower nectar of an autogamous perennialRorippa indicaas an indirect defense mechanism against herbivorous insects. Res. Popul. Ecol. 36, 63}71.