Impaired renal vascular endothelial function in vitro in
experimental hypercholesterolemia
John M. Stulak
a, Amir Lerman
b, James A. Caccitolo
d, Stephanie H. Wilson
b,
J. Carlos Romero
a, Hartzell V. Schaff
d, Martin Rodriguez Porcel
b,
Lilach O. Lerman
c,*
aDepartment of Physiology and Biophysics,Mayo Clinic and Foundation,200First Street SW,Rochester,MN55905,USA bDepartment of Internal Medicine,Di6ision of Cardio6ascular Diseases,Mayo Clinic and Foundation,200First Street SW,Rochester,
MN55905,USA
cDepartment of Internal Medicine,Di6ision of Hypertension,Mayo Clinic and Foundation,200First Street SW,Rochester,MN55905,USA dDepartment of Surgery,Mayo Clinic and Foundation,200First Street SW,Rochester,MN55905,USA
Received 13 May 1999; received in revised form 7 February 2000; accepted 2 March 2000
Abstract
Hypercholesterolemia (HC) induces alterations in systemic vascular reactivity, which can manifest as an attenuated endothe-lium-dependent relaxation, partly consequent to an impairment in nitric oxide (NO) activity. To determine whether experimental HC has a similar effect on renal vascular function, renal artery segments obtained from pigs fed a HC (n=5) or normal (n=5) diet were studied in vitro. Endothelium-dependent relaxation was examined using increasing concentrations of acetylcholine (Ach), calcium ionophore A23187, and Ach following pre-incubation with NG-monomethyl-L-arginine or L-arginine (L-ARG). The NO-donor diethylamine (DEA) was used to examine smooth muscle relaxation response and cyclic GMP generation in endothelium-denuded vessels. The expression of endothelial NO synthase (eNOS) in the renal arteries was examined using Western blotting. Endothelium-dependent relaxation to Ach was significantly attenuated in the HC group compared to normal (53.399.1 vs. 98.893.7%, PB0.005), but normalized after pre-incubation with L-ARG (82.3913.8%, P=0.21). Receptor-independent endothelium-dependent relaxation to A23187 was also significantly blunted in HC (75.2910.5 vs. 115.594.2%, PB0.017). Smooth muscle relaxation and cyclic GMP generation in response to DEA were greater in denuded HC vessels, while relaxation of intact vessels to nitroprusside was unaltered. In the HC vessels eNOS was almost undetectable. In conclusion, experimental HC attenuates in vitro endothelium-dependent relaxation of the porcine renal artery, possibly due to low bioavailability of NO. These vascular alterations in HC could play a role in the pathogenesis of renal disease or hypertension, supporting a role for HC as a risk factor for renovascular disease. © 2001 Elsevier Science Ireland Ltd. All rights reserved.
Keywords:Hypercholesterolemia; Kidney; Renal artery; Endothelium; Nitric oxide
www.elsevier.com/locate/atherosclerosis
1. Introduction
Hypercholesterolemia (HC) is a major health risk associated with an increased risk of coronary artery disease and cardiac events [1]. Even short term experi-mental HC induced by cholesterol feeding has been shown to lead to dysfunction of the vascular endothe-lial layer, which characterizes early atherosclerosis [2].
The intact endothelium releases nitric oxide (NO), which serves to regulate both basal and reactive vascu-lar tone, and to balance the stimulation of the blood vessel by vasoconstrictor substances [3]. However, the bioavailability of NO in HC states may be reduced, most likely due to an endothelial impairment, while systemic levels of vasoconstrictors may increase [4]. Consequently, endothelium-dependent relaxation is attenuated. The cholesterol fraction most deleterious to normal vascular reactivity is the low-density lipo-protein (LDL) fraction, most probably in its oxidized form.
* Corresponding author. Tel.: +1-507-2551929; fax: + 1-507-2551935.
E-mail address:[email protected] (L.O. Lerman).
Despite a large body of evidence demonstrating the injurious effects of HC on the systemic and coronary vasculature, data pertaining to its effects on the renal vasculature are rather limited. The renal vasculature shows unique responses to various vasoactive sub-stances [5,6], and may have a differential response to risk factors compared to other vascular beds [7,8]. Hence, the effect of HC on the renal vasculature may conceivably differ from that observed in other vascular beds, where endothelial dysfunction is consistently observed. Moreover, maladjusted responses of the renal vasculature and/or a decrease in NO bio-availability, in addition to direct cellular effects of HC, may have grave repercussions on the wide spec-trum of the homeostatic and endocrine functions of the kidney.
Renal synthesis of NO is governed by the NO syn-thase family of enzymes, of which the inducible form is expressed in epithelial cells along the nephron [9], while the endothelial (eNOS) isoenzyme is expressed constitu-tively in blood vessels. The renal consequences of HC-induced endothelial dysfunction or low bioavailability of NO may be multifold, and may potentially con-tribute to development of renal damage and hyperten-sion [10,11]. Under normal conditions, NO plays a critical role in the local regulation of renal hemodynam-ics and excretory function [11]. NO contributes to the modulation of afferent arteriolar tone and mesangial relaxation, thus regulating the glomerular microcircula-tion, as well as renal vascular resistance [12,13]. In extreme cases, impaired NO synthesis may cause marked renal vasoconstriction, which may lead to outer medullary ischemia, tubular anoxia, and acute renal failure [14]. Ribiero et al. demonstrated that chronic (15 days) inhibition of NO synthase in rats was associated with decreased renal perfusion, hypertension, and accel-erated glomerulosclerosis [15]. NO also plays a role in regulating renal sodium excretion and renin release, as well as prevention of thrombosis. Consequently, any process that compromises endothelial function and NO activity can contribute to a progression of renal disease and hypertension [16]. Nevertheless, it is yet unclear whether renal vascular function is impaired in HC, and whether NO is involved in this impairment.
Therefore, the aim of this study was to define if HC has a deleterious effect on renal vascular function in the swine kidney, and whether NO availability played a role in any alterations observed. For this purpose, renal vascular function was studied in isolated arterial seg-ments harvested from both normal pigs and pigs fed a high-cholesterol diet for 3 months. This experimental model has been previously shown to develop im-pairments in vascular reactivity in other vascular beds [17].
2. Methods
All procedures and handling of the animals in this study in this were in compliance with the ‘Guide for the Care and Use of Laboratory Animals’ formulated by the US National Institutes of Health, and approved by the Institutional Animal Care and Use Committee. Five domestic pigs (60 – 70 kg) were fed for 3 months a 2% cholesterol diet, including 15% Lard (Harlan Teklad, Madison, WI), euthanized with an intravenous over-dose of pentobarbital sodium (Sleepaway®
, 30 mg/kg, Fort Dodge Laboratories), and their kidneys removed. For controls, kidneys were removed from five normal domestic pigs maintained on regular pig chow. Kidneys of all animals were immersed in cool, oxygenated phys-iological salt solution (Kreb’s) of the following millimo-lar composition: NaCl, 118.3; KCl, 4.7; MgSO4, 1.2; KH2PO4, 1.22; CaCl2, 2.5; NaHCO3, 25.0; and glucose, 11.1 (control solution). Main branches of the main renal artery (3 – 4 mm in diameter) were dissected from the kidneys under magnification and placed in control solution, with care taken to remove as much of the connective tissue as possible. Vessels were sectioned into rings 5 – 6 mm in length. In some of the rings, the endothelium was mechanically removed by inserting the tip of a watchmaker’s forceps and scraping gently, a technique that has been previously described [18].
2.1. In 6itro relaxation
Rings with or without endothelium were suspended in 25 ml organ chambers (one ring from each animal in each chamber) filled with Kreb’s solution, maintained at 37°C, and aerated with 95% oxygen and 5% carbon dioxide (pH 7.4). Each ring was suspended by two stainless clips passed through the vessel lumen. One clip was attached to a stationary post, and the other was attached to a strain gauge (Statham Gould UC 2, Viggo Spectamed, Critical Care Division, Oxnard, CA) for the measurement of isometric force. Rings were placed at the optimal point of their length-tension relationship by progressively stretching them until the contraction to potassium chloride (20 mM) was maxi-mal. In all experiments, the presence of functioning endothelium was confirmed by the response to acetyl-choline (Ach, 1×10−6 M) following precontraction with potassium ions (20 mM). After optimal tension was determined and the presence or absence of en-dothelium confirmed, the rings were allowed to equili-brate for 30 min before performance of any further experiments. All experiments were performed in the presence of indomethacin (1×10−5 M) in order to block endogenous production of prostaglandins. En-dothelial-dependent relaxation after precontraction with endothelin-1 (ET-1, 10−7
alone, Ach in the presence of NG -monomethyl-L-arginine (L-NMMA, 10−4 M), Ach in the presence of L-arginine (L-ARG, 10−4 M), and calcium ionophore A23187 (10−9– 10−5 M). Responses of the vascular smooth muscle were examined in endothelium-denuded vessels exposed to increasing doses of diethylamine (DEA, 10−9– 10−5 M). At the end of each experiment, each ring was exposed to sodium nitroprusside (SNP, 10−4 M) to establish a maximal relaxation response. The following drugs were used in conducting the experiments: Ach, calcium ionophore A23187, DEA, indomethacin, and SNP (Sigma, St. Louis, MO); L-ARG and L-NMMA (Calbiochem, San Diego, CA); and ET-1 (Phoenix Pharmaceuticals). All powdered drugs were prepared with distilled water except in-domethacin, which was dissolved in Na2CO3. Stock solutions of each agent were prepared every day. Due to the buffering effect of the Kreb’s solution and aera-tion with 5% CO2, addiaera-tion of L-NMMA and L-ARG did not alter organ bath pH. All drug concentrations are expressed as final concentration in organ chambers.
2.2. Cyclic GMP production
As a measure of NO activity, cyclic guanosine-3%,5% -monophosphate (cGMP) was measured in the follow-ing manner: vascular rfollow-ings were collected from each group and placed in an organ chamber filled with Kreb’s solution. After 1 h of incubation, 146 ml of 3-isobuthyl-1-methyl-xanthine 10−4 M
/l and 100 ml of indomethacin 10−5 M
/l were added to the solution in the organ chamber for 30 min. Samples were then randomized to either standards (controls) or DEA (10−6 M/l) treatment for 1 min, and then shock-frozen.
Samples were then placed on dry ice, 2 ml of abso-lute alcohol added, and the samples homogenized. Af-ter being allowed to sit for 5 min, they were centrifuged for 10 min at 4000 RPM at 10°C. Superna-tant was placed separately for posterior protein assay. Samples were dried with Nitrogen evapo-rack and 500 ml of Tris buffer added, after which they were cen-trifuged as before and placed on ice. Following prepa-ration of standards, 50ml of radioactive dye and 50 ml of antiserum were subsequently added to both samples and standards. After vortex mixing, tubes were placed on ice and refrigerated for 1.5 h. After incubation, 1 ml of ammonium sulfate was added, and vortex and sam-ples were allowed to sit for 5 min. The samsam-ples were subsequently centrifuged for 10 min at 4000 RPM at 10°C. Supernatant was discarded, 1.1 ml of cold DI water added, and the samples were vortexed again. Finally, 1 ml of the mixture was placed in a plastic scintillation vial and 15 ml of Opti-flur added. The samples were then mounted on the scintillator counter and tested.
2.3. Western Blotting for eNOS
Arteries from four HC and five normal pigs were snap frozen in liquid nitrogen. Care was taken to ensure that the endothelial layer was untouched and undamaged. The vessels were subsequently homoge-nized in lysis buffer (50 mM Tris HCl, pH 8.0, 150 mM NaCl, 0.02% sodium azide, 0.1% SDS, PMSF 100 mg/ml, aprotinin 1 mg/ml 1% NP-40, 0.5% sodium de-oxycholate) using a tissue homogenizer. The lysate was analyzed for protein content using a Bradford assay (Bio-Rad, CA, USA). Equal amounts of protein were resolved under reducing conditions on a 12% SDS-polyacrylamide gel. Immuno-blotting was performed using a monoclonal antibody to eNOS (Signal Trans-duction Laboratory, Lexington, KY) at a dilution of 1:1000 in a non-fat milk/Tris buffer. The membrane was subsequently probed with a secondary anti-mouse antibody conjugated to horseradish peroxidase (Amer-sham Life Sciences, IL) at a dilution of 1:5000 and developed with chemiluminescence (Pierce, IL). The membrane was then exposed to X-ray film (Kodak, NY) which was subsequently developed.
2.4. Statistical analysis
Vascular tensions in response to acetylcholine, A23187, and DEA are expressed as the percent change from the maximal contraction level of each renal arte-rial ring following pre-contraction with ET-1 [18]. The results were expressed as means9standard error of the mean (S.E.M.). Statistical evaluation of the data was performed with ANOVA or the Student’s t-test for paired or unpaired observations. Values were consid-ered statistically significant when the P value was less than 0.05.
3. Results
3.1. Serum cholesterol le6els and systemic
hemodynamics
In normal pigs total and LDL cholesterol levels were 74.2918.5 and 33.8918.9 mg/dl, respectively. The total serum cholesterol level in the cholesterol-fed pigs was 454.2948.5 mg/dl (P=0.002 compared to nor-mal), and the LDL cholesterol fraction was 370.09
Fig. 1. Endothelial-dependent relaxation of renal arterial segments removed from normal and hypercholesterolemic (HC) pigs in re-sponse to increasing doses of acetylcholine (Ach) (top) and calcium ionophore (A23187) (bottom). *PB0.05 compared to normal arte-rial segments.
3.2. Endothelial function
The maximal contractile response to ET-1 (10−7 M) achieved during pre-contraction were similar between normal rings and HC rings (P=0.2). Renal arterial rings removed from HC pigs demonstrated a signifi-cantly attenuated maximal relaxation response to in-creasing doses of Ach compared to normal (53.399.1 vs. 98.893.7%, respectively, PB0.005) (Fig. 1, top panel). Relaxation in response to calcium ionophore A23187 was also attenuated in the HC group compared to normal (75.2910.5 vs. 115.594.2%, respectively,
PB0.017) (Fig. 1, bottom panel). On the other hand, the maximal endothelium-independent relaxation re-sponse to SNP (10−4 M) was similar between normal and HC rings (P=0.2).
3.3. Role of NO
Pre-incubation with L-ARG prior to relaxation with Ach enhanced and normalized endothelial function in the HC group (82.3913.8%, P=0.21 and P=0.66 compared to normal and normal pre-incubated with L-ARG, respectively) (Fig. 2), but not in the normal group. The relaxation of the pre-incubated HC vessels was consequently significantly greater than that of HC rings exposed to Ach alone (PB0.027). Pre-incubation of normal vessels with L-NMMA resulted in a signifi-cantly attenuated response to Ach (25.6911.1 vs. 98.893.7%,P=0.01 compared to normal vessels with-out L-NMMA). Pre-incubation with L-NMMA of ves-sels obtained from the HC group led to a smaller and non-significant further attenuation of relaxation to Ach (31.499.6 vs. 53.399.1%, P=0.21 compared to HC vessels without L-NMMA), indicating that the endoge-nous NO bioavailability in HC vessels was reduced. After pre-incubation with L-NMMA, the response to Ach was very similar in the HC and normal groups (P=0.92) (Fig. 2). Subtraction of the L-NMMA pre-incubated Ach responses from the Ach responses showed that in the normal group 71.1923.4% of the vascular relaxation was attributable to NO, while in the HC group this amounted to only 28.2925.1% (PB
0.05).
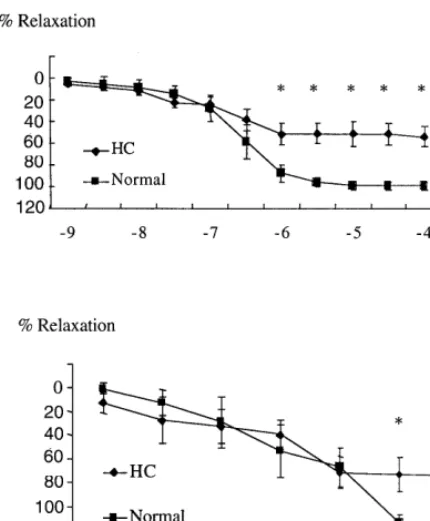
Relaxation responses of endothelium-denuded arte-rial segments to the NO-donor DEA were significantly greater in the HC group (114.099.9%) compared to control rings (75.993.1%, PB0.022) (Fig. 3). Genera-tion of cGMP under baseline condiGenera-tions in HC vessels was similar to normal (3.692.4 and 0.9190.27 pmol/ mg protein, respectively,P=0.18), but was significantly greater in response to DEA (98.3910.2 vs. 6.391.3 pmol/mg protein,P=0.0004). The relative change (per-cent increase) in cGMP generation in response to DEA was 5-fold greater in HC than in normal pigs (P= 0.0006).
Fig. 2. Maximal relaxation of the renal arterial segments in response to acetylcholine (Ach). Shown are normal rings, normal rings pre-in-cubated with NG-monomethyl-L-arginine (L-NMMA), high
Fig. 3. Renal vascular smooth muscle relaxation in response to increasing doses of diethylamine (DEA). *PB0.05 compared to normal arterial segments.
dothelium or elsewhere [20]. Endothelial dysfunction in the coronary and systemic vasculatures has been shown to be an early event in the development of atherosclero-sis, preceding any structural alterations [21]. Many mechanisms have been implicated in this early impair-ment of endothelial function, including uncoupling of Gi-receptor interactions, decrease in L-ARG availabil-ity due to co-factor deficiency, reduction in NOS protein due to excess oxidized LDL levels, increased NO degradation due to an increase in superoxide for-mation, and production of peroxynitrite that can lead to lipoprotein oxidation.
This study extends previous observations made in other vascular beds, and demonstrates that diet-induced HC in pigs imposes a deleterious insult on the renal vascular endothelium, likely due to reduced NO bioavailability, resulting in a functional impairment of the renal artery. Endothelium-dependent relaxation in response to both receptor-mediated (Ach) and non receptor-mediated (calcium ionophore A23187) en-dothelium-dependent vasodilators was significantly blunted in the HC group compared with controls. This impairment was normalized when the arterial segments were incubated with L-ARG prior to exposure to Ach, supporting a role for low bioavailability of NO, and implying that at least at the early phase of diet-induced HC the functional abnormalities may be reversible. The mechanism by which L-ARG restored renal endothelial function in the setting of HC may be multifactorial. In HC states, transport of L-ARG into the cells may be decreased due to the accumulation of competitive in-hibitors of L-ARG, such as oxidized LDL [22]. In addition, there is accumulation of competitive in-hibitors of NOS activity, such as asymmetric dimethyl arginine (ADMA) [23]. Since this inhibition is competi-tive, NO production can be restored by excess supply of L-ARG [24]. Although this has been documented in other vascular beds [25], this is the first such demon-stration in the renal vasculature.
The role of the NO pathway in this vascular dysfunc-tion was further underscored by the observadysfunc-tion of hyper-responsiveness of denuded HC renal arterial seg-ments to DEA. Relaxation in response to the NO-donor DEA was significantly augmented in HC arterial rings compared to normal, and cGMP production was markedly augmented. This may reflect adaptation of the smooth muscle to become more sensitive to NO in NO-deficient states such as HC. This hypothesis is supported by previous studies, which demonstrated that removal of the endothelial NO synthesis pathway by denuding the endothelium resulted in an augmented response to NO-donors [26]. Moreover, blockade of the residual NO synthesis using L-NMMA had a relatively smaller effect on HC rings compared to normal rings in further attenuating the vasorelaxation response to Ach. Fig. 4. Immunoblot of renal artery tissue homogenates for endothelial
NO synthase (eNOS) protein (100 mg protein/lane) obtained from five normal (N) animals and four hypercholesterolemic (HC) pigs. The Western blot demonstrates a band in the 140 kDa range in all the renal arteries obtained from the normal group, but in none of the HC group. Molecular weight markers of 218 and 125 kDa are shown.
As Fig. 4 demonstrates, Western blotting preformed in four HC renal arteries has shown markedly reduced eNOS expression compared to five normal renal arteries.
4. Discussion
en-Thus, the observation that pincubation of HC re-nal vessels with L-NMMA did not significantly atten-uate their response to Ach any further supports the hypothesis that HC in the renal circulation is associ-ated with a decrease in endogenous NO activity.
Notably, an enhanced response of denuded vessels to the NO-donor DEA was not accompanied by en-hanced responses of intact vessels to the NO-donor SNP. It is likely that the endothelial dysfunction ob-served in this study and others resulted not only from a decrease in NO bioavailability, but also from a concurrent increased release [7] or response to [27] endothelium-derived vasoconstrictors, which shifted the vasodilator/vasoconstrictor balance that controls vascular tone [28] in favor of the latter. Thus, en-hanced sensitivity of the vessel to NO may be appar-ent only in the absence of the endothelium, which may also explain why this phenomenon is not ob-served in vivo.
Previous studies in HC rats have demonstrated im-paired in vivo renal vasodilatory responses to acetyl-choline [29], while other investigators have witnessed no change in single-nephron glomerular filtration rate and renal afferent plasma flow, but an increase in glomerular capillary pressure [30]. In cholesterol-fed rabbits, the in vivo renal vasodilatory response to acetylcholine was unaltered [31] after 8 weeks of diet, but the response of the renal artery in vitro was slightly impaired [8]. Indeed, the discrepancy among those observations may be related to the different an-imal models used, as well as the duration or content of the HC diet. The finding of decreased eNOS levels in the renal arteries of HC pigs also agrees with the decrease in eNOS immunoreactivity observed in the coronary arteries of HC pigs [32]. However, it con-trasts with a recent study, showing decreased NO re-lease in response to an endothelin B-receptor antagonist (BQ-3020) in the isolated, perfused kidney of HC rats, but immunostaining of eNOS comparable to that of normal controls [33]. Again, the dis-crepancy among these observations may be related to the different animal models used, or to the different methodology used to determine eNOS expression.
Interestingly, although chronic administration of NO inhibitors is associated with elevated blood pres-sure resembling the characteristics of essential hyper-tension [34], decreased NO-mediated responses and eNOS expression in the HC pigs was not associated with development of hypertension. The reason for this can be only speculated. The decrease in NO bioavailability in the peripheral circulation in this early phase of HC may possibly not be as complete as that obtained with L-NMMA administration. Be-cause many regulatory mechanisms contribute to de-termination of basal vascular tone, it may be well-balanced under resting conditions, and the effects
on vascular tone often apparent only in stimulated situations [35]. Moreover, in vivo blunted renal perfu-sion response to acetylcholine was recently observed in HC pigs [36], but enhanced renal tubular response and sodium excretion. The reason behind the aug-mented sodium excretion in response to acetylcholine is yet unclear, but by this mechanism early diet-in-duced HC may hypothetically blunt development of hypertension in this model, despite decreased bioavailability of NO.
Nevertheless, the alteration of renovascular en-dothelial function in this study may well have func-tional implications, and it is not unlikely that longer duration of HC in animals or in humans would lead to glomerulosclerosis [37,38] or an increase in blood pressure [16]. Furthermore, endothelial dysfunction in the renal arteries may represent a more extensive pro-cess that involves the renal microcirculation as well. Hence, partly through interference with the NO path-way [37], HC may lead to intra-renal hemodynamic and functional abnormalities, and may potentially cul-minate in permanent renal damage.
In summary, this study demonstrated that experi-mental HC in pigs resulted in renal vascular endothe-lial dysfunction in vitro, likely due to reduced NO bioavailability. This impairment was restored to near control values when the precursor of NO was sup-plied, suggesting a reversible NO-deficient state in early HC. Further evidence that supports this notion was obtained with NO-synthesis inhibition, during which normal renal arterial segments reacted to Ach similarly to those rings taken from HC pigs. In HC rings, NO-synthesis inhibition had a much smaller ef-fect on the already attenuated Ach-induced endothe-lium-dependent relaxation, while the sensitivity of the vascular smooth muscle to an NO-donor was signifi-cantly augmented. This functional impairment of the renal vasculature may lead to maladaptive responses of the kidney to physiological or pharmacological challenges. Furthermore, as speculated regarding the coronary circulation, this renal dysfunction may pos-sibly precede the development of overt atherosclerotic processes in the renal macro and microvasculature, eventuating in renal artery stenosis, glomerulosclero-sis, and/or hypertension.
Acknowledgements
References
[1] Multiple Risk Factor Intervention Trial Research Group. Multi-ple risk factor intervention trial. Risk factor changes and mortal-ity results. J Am Med Assoc 1982;248:1465 – 1477.
[2] Shimokawa H, Tomoike H, Nabeyama S, Yamamoto H, Araki H, Nakamura M, Ishii Y, Tanaka K. Coronary artery spasm induced in atherosclerotic miniature swine. Science 1983;221:560 – 2.
[3] Cocks TM, Angus JA. Endothelium-dependent relaxation of coronary arteries by noradrenaline and serotonin. Nature 1983;305:627 – 30.
[4] Lerman A, Edwards BS, Hallett JW, Heublein DM, Sandberg SM, Burnett JC, Jr. Circulating and tissue endothelin immunore-activity in advanced atherosclerosis. N Engl J Med 1991;325:997 – 1001.
[5] Elkayam U, Mehra A, Cohen G, Tummala PP, Karaalp IS, Wani OR, Canetti M. Renal circulatory effects of adenosine in patients with chronic heart failure. J Am Coll Cardiol 1998;32:211 – 5.
[6] Conger JD, Falk SA, Yuan BH, Schrier RW. Atrial natriuretic peptide and dopamine in a rat model of ischemic acute renal failure. Kidney Int 1989;35:1126 – 32.
[7] Luscher TF, Diederich D, Weber E, Vanhoutte PM, Buhler FR. Endothelium-dependent responses in carotid and renal arteries of normotensive and hypertensive rats. Hypertension 1988;11:573 – 8.
[8] Stewart-Lee AL, Ferns GA, Anggard EE. Differences in onset of impaired endothelial responses and in effects of vitamin E in the hypercholesterolemic rabbit carotid and renal arteries. J Cardio-vasc Pharmacol 1995;25:906 – 13.
[9] Kone BC, Baylis C. Biosynthesis and homeostatic roles of nitric oxide in the normal kidney. Am J Physiol 1997;272:F561 – 78. [10] Quyyumi AA. Endothelial function in health and disease: new
insights into the genesis of cardiovascular disease. Am J Med 1998;105:32S – 9S.
[11] Luscher TF, Bock HA. The endothelial L-arginine/nitric oxide pathway and the renal circulation. Klin Wochenschr 1991;69:603 – 9.
[12] Beierwaltes WH, Sigmon DH, Carretero OA. Endothelium mod-ulates renal blood flow but not autoregulation. Am J Physiol 1992;262:F943 – 9.
[13] Deng X, Welch WJ, Wilcox CS. Role of nitric oxide in short-term and prolonged effects of angiotensin II on renal hemody-namics. Hypertension 1996;27:1173 – 9.
[14] Brezis M, Heyman SN, Dinour D, Epstein FH, Rosen S. Role of nitric oxide in renal medullary oxygenation. J Clin Invest 1991;88:390 – 5.
[15] Ribiero MO, Antunes E, Muscara MN, De Nucci G, Zatz R. Nifedipine prevents renal injury in rats with chronic nitric oxide inhibition. Hypertension 1995;26:150 – 5.
[16] Mu¨ller, JFM, Franz, I-W. Is plasma LDL-cholesterol level corre-lated with blood pressure in normotensive and hypertensive patients? J Am Coll Cardiol 1999;Abstracts:283A.
[17] Best PJ, McKenna CJ, Hasdai D, Holmes DR, Jr, Lerman A. Chronic endothelin receptor antagonism preserves coronary en-dothelial function in experimental hypercholesterolemia. Circula-tion 1999;99:1747 – 52.
[18] Hasdai D, Rizza RA, Holmes DR, Jr, Richardson DM, Cohen P, Lerman A. Insulin and insulin-like growth factor-I cause coronary vasorelaxation in vitro. Hypertension 1998;32:228 – 34. [19] Moncada S, Higgs A. The L-arginine/nitric oxide pathway. N
Engl J Med 1993;329:2002 – 11.
[20] Palmer RMJ, Ferrige AG, Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived
relax-ing factor. Nature 1987;327:534 – 6.
[21] Sorenson KE, Celermajer DS, Georgakopolous D, Hatcher G, Betteridge DJ, Deanfield JE. Impairment of endothelium-depen-dent dilation is an early event in children with familial hyperc-holesterolemia and is related to the lipoprotein(a) level. J Clin Invest 1994;93:50 – 5.
[22] Stroes E, Kastelein J, Cosentino F, Erkelens W, Wever R, Koomans H, Lusher T, Rabelink T. Tetrahydrobiopterin re-stores endothelial function in hypercholesterolemia. J Clin Invest 1997;99:41 – 6.
[23] Bode-Boger SM, Boger RH, Kienke S, Junker W, Frolich JC. Elevated L-arginine/dimethylarginine ratio contributes to en-hanced systemic NO production by dietary L-arginine in hyperc-holesterolemic rabbits. Biochem Biophys Res Commun 1996;219:598 – 603.
[24] Chen LY, Mehta P, Mehta JL. Oxidized LDL decreases L-arginine uptake and nitric oxide synthase protein expression in human platelets: relevance of the effect of oxidized LDL on platelet function. Circulation 1996;93:1740 – 6.
[25] Girerd XJ, Hirsch AT, Cooke JP, Dzau VJ, Creager MA. L-arginine augments endothelium dependent vasodilation in cholesterol-fed rabbits. Circ Res 1990;67:1301 – 8.
[26] Moncada S, Rees DD, Schulz R, Palmer RM. Development and mechanism of a specific supersensitivity to nitrovasodilators after inhibition of vascular nitric oxide synthesis in vivo. Proc Natl Acad Sci USA 1991;88:2166 – 70.
[27] Heistad DD, Armstrong ML, Marcus ML, Piegors DJ, Mark AL. Augmented responses to vasoconstrictor stimuli in hyperc-holesterolemic and atherosclerotic monkeys. Circ Res 1984;54:711 – 8.
[28] Lerman A, Burnett JC, Jr. Intact and altered endothelium in regulation of vasomotion. Circulation 1992;86:12 – 9.
[29] Bank N, Aynedjian HS. Role of thromboxane in impaired renal vasodilatation response to acetylcholine in hypercholesterolemic rats. J Clin Invest 1992;89:1636 – 42.
[30] Kasiske BL, O’Donnell MP, Schmitz PG, Kim Y, Keane WF. Renal injury of diet-induced hypercholesterolemia in rats. Kid-ney Int 1990;37:880 – 91.
[31] Carroll JF, Mizelle HL, Cockrell K, Reckelhoff JF, Clower BR, Granger JP. Cholesterol feeding does not alter renal hemody-namic response to acetylcholine and angiotensin II in rabbits. Am J Physiol 1997;272:R940 – 7.
[32] Mathew V, Cannan CR, Miller VM, Barber DA, Hasdai D, Schwartz RS, Holmes DR, Jr, Lerman A. Enhanced endothelin-mediated coronary vasoconstriction and attenuated basal nitric oxide activity in experimental hypercholesterolemia. Circulation 1997;96:1930 – 6.
[33] Kakoki M, Hirata Y, Hayakawa H, Tojo A, Nagata D, Suzuki E, Kimura K, Goto A, Kikuchi K, Nagano T, Omata M. Effects of hypertension, diabetes mellitus, and hypercholesterolemia on endothelin type B receptor-mediated nitric oxide release from rat kidney. Circulation 1999;99:1242 – 8.
[34] Romero JC, Lahera V, Salom MG, Biondi ML. Role of the endothelium-dependent relaxing factor nitric oxide on renal function. J Am Soc Nephrol 1992;2:1371 – 87.
[35] Cardillo C, Kilcoyne CM, Cannon RO, 3rd, Quyyumi AA, Panza JA. Xanthine oxidase inhibition with oxypurinol improves endothelial vasodilator function in hypercholesterolemic but not in hypertensive patients. Hypertension 1997;30:57 – 63.
[36] Feldstein A, Krier JD, Hershman Sarafov M, Lerman A, Best PJM, Wilson SH, Lerman LO. Renal perfusion and function in hypercholesterolemic pigs in vivo. Hypertension 1999;34:859 – 64. [37] Bank N. Renal hemodynamic consequences of hyperlipidemia.
Miner Electrolyte Metab 1993;19:165 – 72.
[38] Lee HS, Kim YS. Identification of oxidized low density lipo-protein in human renal biopsies. Kidney Int 1998;54:848 – 56.