L
Journal of Experimental Marine Biology and Ecology 249 (2000) 51–64
www.elsevier.nl / locate / jembe
A long-term mesocosm study on the settlement and survival
of juvenile European lobster Homarus gammarus L. in four
natural substrata
a,b ,* c a
Adrian Linnane , David Mazzoni , John P. Mercer a
National University of Ireland, Galway, Shellfish Research Laboratory, Carna, Galway, Ireland
b
National University of Ireland, Galway, Martin Ryan Marine Science Institute, Galway, Ireland
c
Dipartimento di Protezione e Valorizzazione Agro-Alimentare(DIPROVAL),
Universita’ degli Studi di Bologna, via del Guasto 5 /b, 40126 Bologna, Italy Received 2 April 1998; received in revised form 12 January 2000; accepted 22 February 2000
Abstract
To date, the natural substratum preferences of early benthic phase (EBP) European lobsters (Homarus gammarus) remain largely unknown. This study utilised a large scale mesocosm experiment to determine if the animal favours cobble ground, similar to its American counterpart (Homarus americanus), or has other substratum preferences. Postlarvae were provided with the choice of settling on four natural substrata: sand, coralline algae, mussel shell and cobble. Over a nine month period, the number and size of juveniles on each substratum was recorded, with loss of chelipeds used as an indication of social interaction. After a 30 day period, a non-random distribution of lobsters was observed on the four substrata. Juveniles were more abundant in substrata which provided pre-existing shelter in the form of interstitial spaces, i.e. cobble and mussel shell, than in sand or coralline algae. The survival of individuals from postlarvae to 30 day old juveniles ranged from 5 to 14% with surviving benthic recruits showing a clear mode at 6–8
2
mm carapace length (CL) in size distribution. The density of lobsters per m of cobble remained
2
relatively constant (18 / m ) throughout the study period while the density of juveniles on mussel
2
shell decreased significantly (35 to 5 / m ). The size distribution of lobsters on each substratum also varied with time. By the conclusion of the trial, lobsters found in mussel shell had a mode of 8–10 mm CL within a range of 6–14 mm CL while those in cobble had a mode of 10–12 mm CL within a range of 8–24 mm CL. Overall, the results underline the importance of shelter-providing habitat such as cobble or crevice-type substrata to EBP European lobsters. They also confirm that for a shelter-dwelling animal such as a lobster, the physical structure of the habitat is a key factor in determining both the size and number of its inhabitants. 2000 Elsevier Science B.V. All rights reserved.
Keywords: Homarus gammarus; Settlement; Survival; Preference; Substratum
*Corresponding author. Tel.: 1353-91-524-411; fax: 1353-91-525-005.
E-mail address: [email protected] (A. Linnane)
1. Introduction
Most benthic marine invertebrates have a complex life cycle involving a pelagic dispersal phase followed by settlement onto a preferred substratum associated with their early benthic phase (EBP) habitat (reviewed by Chia and Rice, 1978; Burke, 1983; Crisp, 1984; Morse, 1985; Svane and Young, 1989). The duration of this phase can extend from a few minutes to several months, depending on the species (Mileikovsky, 1971; Scheltema, 1986). Where a suitable site is absent or under conditions unfavourable to subsequent survival and growth, many species have the ability to prolong planktonic life, thereby demonstrating an important degree of selectivity for the settlement substratum (Grosberg, 1981; Botero and Atema, 1982; Cobb et al., 1983; Petersen, 1984; Young, 1989; O’Conner, 1991). Whereas the larval form of the European lobster (Homarus gammarus) has been identified in nature (Tully and O’Ceidigh, 1987), the benthic habitat to which it recruits to remains largely unknown.
Several laboratory studies have examined the substratum preferences of EBP lobsters and many have revealed the animals’ ability to utilise a wide range of habitats of varying structural complexity ranging from unsieved mud to macroalgal covered rocks (Berrill, 1974; Howard and Bennett, 1979; Botero and Atema, 1982; Pottle and Elner, 1982; Barshaw and Bryant-Rich, 1988; Boudreau et al., 1993). However, up to the end of the 1980s there were few quantitative descriptions of newly recruited lobsters in nature (Hudon, 1987; Able et al., 1988) as conventional benthic sampling methods such as cores and grabs were unsuitable for gravel and cobble substrata, areas believed to be prime nursery areas for EBP lobsters. This problem was addressed with the development of the airlift suction sampler (Incze and Wahle, 1991; Wahle and Steneck, 1991). The device was first tested in the United States and proved considerably successful in identifying benthic recruitment habitats and nursery grounds of EBP American lobster (Homarus americanus) within the coastal Gulf of Maine. Here, juveniles were seen to be restricted to the shelter providing habitat of cobble substratum (Wahle and Steneck, 1991) where average
2
population densities were as high as 6.9 individuals / m .
In 1994, a sampling study was undertaken in the west and southwest coasts of Ireland and the Channel Islands, UK, in order to gain some insight into the habitat requirements of EBP European lobsters. To date, there are no published reports of newly settled European lobsters in the wild. Despite being unable to locate juvenile lobsters, the study revealed that the species diversity within cobble habitats in Ireland and the Channel Islands is high, with reptant decapods such as squat lobsters (galatheidae), porcelain crabs (porcellanidae), mud crabs (xanthidae) and snapping shrimp (alpheidae) appearing to dominate (Wahle, 1998). This is in direct contrast to the American situation, where in the Gulf of Maine the diversity is relatively low with Homarus americanus and the rock crab Cancer irroratus being by far the two most abundant species.
2. Materials and methods
2.1. Experimental animals
All lobsters used in the experiment were hatched at the National University of Ireland, Galway, Shellfish Research Laboratory, Carna, County Galway, Ireland, using the methods described by Mercer and Brown (1994). Within 24 h of reaching stage IV, postlarvae were transferred directly from the hatchery to the experimental unit.
2.2. Experimental unit
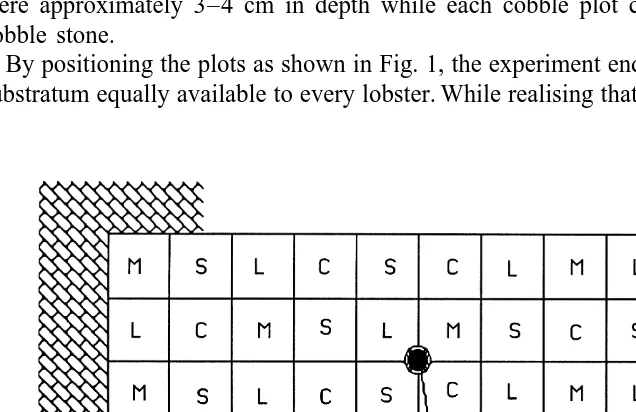
The trials were undertaken in an outdoor rectangular (103431 m) concrete pond
which was lined with black polythene (Fig. 1). The bottom of the unit was covered with
2
a layer of coarse sand approximately 50 mm deep. The pond was divided into 4031 m
plots, each of which was filled with one of the following substrata: coarse sand (1–1.5 mm) as classified by Wentworth (1936), coralline algae (Lithothamnion sp.), mussel shell (Mytilus edulis) or cobble (64–256 mm; Wentworth, (1936)). Thus, each sub-stratum was represented by ten plots. The sand, coralline algae and mussel shell plots were approximately 3–4 cm in depth while each cobble plot contained two layers of cobble stone.
By positioning the plots as shown in Fig. 1, the experiment endeavoured to make each substratum equally available to every lobster. While realising that choice is dependent on
the sequence in which substrata are encountered, the large sample size and the long duration of the study contributed to counteract effects associated with biased settlement pattern related to plot distribution. In addition, research has shown that postlarval lobsters are capable of rapid, directional swimming (Ennis, 1986; Hudon et al., 1986; Cobb et al., 1989) with the function of allowing animals to move into areas suitable for settling. Combined with this capability, the experimental design endeavoured to allow individuals to behaviourally determine their distribution and final settling location.
Seawater entered the pond via an inflow pipe positioned at one corner of the unit. Throughout the experiment the inflow was maintained at approximately 10 l / min. Water exited the system via a central standpipe (70 mm diameter) which was covered with a 2 mm mesh screen to prevent juveniles escaping from the pond.
2.3. Releases
Stage IV juveniles were removed from the rearing unit using a hand net and transferred into a 10 l plastic container filled with seawater. Lobsters were released into the pond from the central standpipe at a rate of 250 individuals every 15 min. For each trial, a total of 4000 postlarvae were used thereby giving an initial density of 100
2
animals / m . Prior to release, the water volume was lowered to approximately 5 cm below the outflow level.
2.4. Sampling protocol
Lobster distribution in different substrata was examined on both a short- and long-term basis. ‘Short-term’ consisted of three separate trials in which postlarvae were released into the pond, allowed to settle and then sampled 30 days later. Sampling consisted of draining the system and manually searching each plot for lobsters. Discovered lobsters were removed from the unit and the number of individuals on the
2
respective plots was recorded. A x test was used to test the null hypothesis that the
observed distribution of settled lobsters would not differ significantly from equal proportions among substrata. The carapace length ((CL), measured using a vernier calipers) and cheliped number of each individual was also recorded. Between trials, the system was drained for a period of 5 days to ensure that undiscovered lobsters did not invalidate the results.
2.5. Feeding
Prior to the introduction of postlarvae into the experimental system, the unit was seeded with brine shrimp (Artemia sp.). Initially, 100 g of hatched cysts were released, with 50 g added every 2 days subsequently. As the temperatures within the system dropped during the long-term study, the feeding ration was reduced to 50 g every 4 days. The Artemia were hatched by placing the cysts into a 50 l conical vessel containing aerated, 1 mm U.V. filtered seawater at a temperature of 188C for a period of 12 h.
2.6. Data analyses
The results were analysed using Chi-square, t and ANOVA tests. Where multiple comparisons were made over the 9 month study, the probability of Type 1 error was
adjusted via the Bonferroni procedure and significance was accepted at P ,0.01. Where
the ANOVA analysis indicated significant differences, a Tukey test (Zar, 1996) was used to examine differences between pairs of treatments. The calculations were performed
with MINITAB (MINITAB Inc., U.S.A) and SYSTAT (Wilkinson et al., 1992)
statistical software.
3. Results
3.1. Survival and distribution within substrata
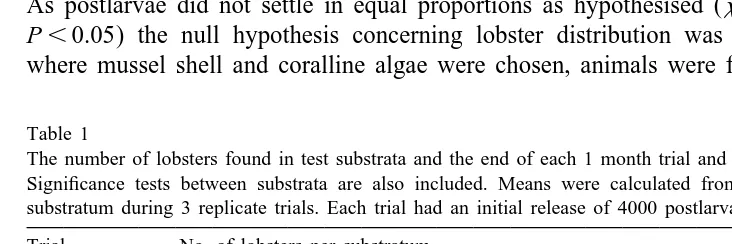
Survival of lobsters from postlarvae to 1 month old ranged from 5% to 14% (Table 1).
2
As postlarvae did not settle in equal proportions as hypothesised (x 549.31, df53,
P,0.05) the null hypothesis concerning lobster distribution was rejected. In cases
where mussel shell and coralline algae were chosen, animals were found sheltering in
Table 1
The number of lobsters found in test substrata and the end of each 1 month trial and overall mean densities. Significance tests between substrata are also included. Means were calculated from 30 quadrats of each substratum during 3 replicate trials. Each trial had an initial release of 4000 postlarvae
Trial No. of lobsters per substratum number
Mussel Cobble Coralline Sand Total Survival
shell algae (%)
1 213 124 47 0 384 9.6
2 78 113 26 0 217 5
3 350 187 9 0 546 14
F * df P
2
Mean n / m 21.3 14.1 2.7 28.8 2 ,0.05
S.E. 2.53 1.44 0.5
*
the interstitial spaces provided by the substratum. Lobsters in cobble, as well as utilising interstitial spaces, constructed burrows in the sand beneath the stones. Frequently, two juveniles were observed sheltering under the same cobble stone.
As there were no differences in the mean size of EBP animals among substrata after 1 month, data from all 3 substrata were pooled. Surviving 1 month benthic recruits formed a clear mode of size classes between 6 and 8 mm carapace length (CL) in the size distributions of lobsters sampled from all three trials (Fig. 2). There was a significant
2
variance (P,0.05) in the density of juveniles per m within mussel shell, cobble, and
coralline algae substrata (Table 1). Post hoc analysis (Tukey test, (Zar, 1996)) identified
a significant variation (P,0.05) between all pairwise mean comparisons of each
substratum.
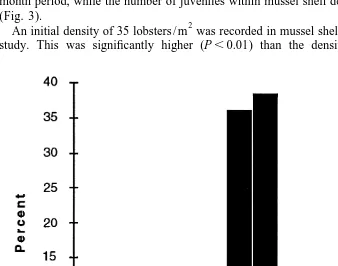
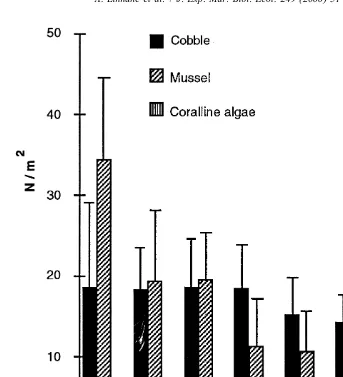
3.2. Long term study – number of lobsters per substratum
2
The number of lobsters / m of cobble remained relatively constant throughout the 9 month period, while the number of juveniles within mussel shell decreased considerably (Fig. 3).
2
An initial density of 35 lobsters / m was recorded in mussel shell after month 1 of the
2
study. This was significantly higher (P,0.01) than the density of 19 lobster / m
Fig. 3. Comparison of mean lobster densities (61 S.D.) on cobble, mussel shell and coralline algae over the 9
2
month study period. No lobsters were found on sand. Means were calculated from 1031 m quadrats of each.
Table 2
Results of t-tests performed on the number of lobsters on cobble and mussel shell substrata over the 9 month study period. Data are presented in Fig. 3
Time (months) df t P
1 18 23.36 0.00
2 18 20.52 0.30
3 18 20.31 0.38
4 18 3.01 0.00
5 18 2.03 0.02
6 18 2.25 0.01
observed in cobble (Table 2). Within months 2 and 3 the density of juveniles within mussel shell substratum decreased to levels comparable with that of cobble (18–19
2
lobsters / m ) and these levels continued to drop significantly in the following counts until the conclusion of the study. Few lobsters were located in coralline algae and after 3 months individuals were no longer sighted in this substratum. No lobsters were recorded in sand.
3.3. Substratum–size relationship
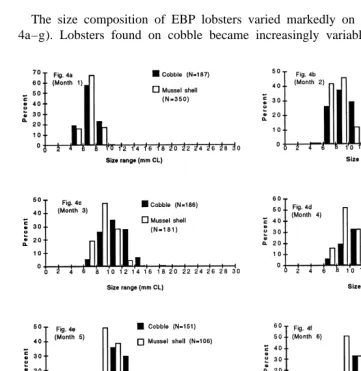
The size composition of EBP lobsters varied markedly on the two substrata (Fig. 4a–g). Lobsters found on cobble became increasingly variable in size as the study
Fig. 5. The mean monthly temperature (6S.D.) as recorded on the surface of the pond throughout the 9 month study period.
progressed. Up to a period of 3 months, lobsters in both substrata showed similar growth trends (Fig. 4a–c). However, after this time period, lobsters in mussel shell were observed to maintain a size mode of 8–10 mm CL within a narrow size range of 6–14 mm (Fig. 4d–f). In month 9, the mode did increase to 10–12 mm but by then the sample number was reduced to 13 individuals (Fig. 4g). By the conclusion of the trial, lobsters in cobble tended to have a central mode of 10–12 mm CL within a range of 8–24 mm CL (Fig. 4g).
3.4. Temperature
Average monthly temperatures as recorded on the surface of the experimental pond followed a clear seasonal thermal regime, with an Autumn cooling leading to a Winter low followed by a Spring warming (Fig. 5).
3.5. Cheliped loss
Data on cheliped loss as an indication of fighting and aggression suggest that agonistic
Table 3
Mean percentage cheliped loss (61 S.D.) on cobble and mussel shell substrata over the 9 month study period.
2
Means were calculated from the number of lobsters missing one or both chelipeds from 1031 m plots of each substratum. Month 1 is excluded from the analyses as the initial number of postlarvae missing chelipeds was unknown
Time (months) 2 3 4 5 6 9
Cobble 15.8610 19.9616. 2567 21.169 18.8611 19.469
encounters were common on both cobble and mussel shell substrata (Table 3). Monthly censuses revealed that 11.3–27% of lobsters recovered were missing either one or both chelipeds. There was no significant difference (t-test, P.0.05) in cheliped loss between substrata.
4. Discussion
The environmental factors affecting postlarval survival were reviewed by Aiken and Waddy (1986) and these included food, light, temperature, salinity, disease, mutilation, social environment and water quality. The number of lobsters present in a substratum at any given time, therefore, are a result of these factors plus an unknown combination of settlement preferences, differential mortality and post-settlement movement. Nonethe-less, since EBP lobsters appeared most abundant in mussel shell and cobble, this indicates a post settlement preference for habitats which provide pre-existing shelter in the form of interstitial spaces. This observation is consistent with previous substratum selection trials on both H. gammarus (Howard and Bennett, 1979; Bertran, 1984) and H. americanus (Botero and Atema, 1982; Pottle and Elner, 1982; Barshaw and Bryant-Rich, 1988; Boudreau et al., 1990).
Caddy (1986) and Fogarty and Idoine (1986) suggested that shelter-providing habitat is a necessary prerequisite for the recruitment of EBP lobsters to the benthos. The strong association of EBP European lobsters in this study with shelter-providing substratum is consistent with this theory and with the findings of field surveys for EBP American lobsters (Hudon, 1987; Incze and Wahle, 1991; Wahle and Steneck, 1991; Wahle, 1993). The mechanisms which reinforce this association are speculative. Various studies suggest that shelter-seeking behaviour is an adaptive response to predation (Lavalli and Barshaw, 1986; Richards and Cobb, 1986; Barshaw and Lavalli, 1988). Wahle and Steneck (1992) have shown by video observation of field predation work, that tethered unsheltered EBP American lobsters were attacked by demersal fish and crabs more frequently than when sheltered by cobble.
The close association of EBP lobsters with sheltering habitats may also be linked to food supply. Cooper and Uzmann (1980) suggested that the walls and roofs of shelters are generally heavily encrusted with soft-bodied invertebrates which may comprise a major food source. Behavioural observations also indicate that EBP lobsters can generate a current through their shelters by pleopod fanning (Barshaw and Bryant-Rich, 1988) allowing them to feed on plankton which are filtered from the incoming water (Barshaw, 1989; Lavalli and Barshaw, 1989).
2
Within cobble plots, the density of juveniles / m remained relatively constant
throughout the course of the study. This finding has also been observed in field research. Wahle and Incze (1997) saturated a series of cobble plots with hatchery reared lobsters to examine whether new recruits or older year classes were near saturation levels for the
2
habitat. From an initial stocking density of 24 individuals / m , densities dropped to
2
with earlier work by Incze and Wahle (1991) who found that while the recruitment density of American lobsters within the Gulf of Maine differed between years, the average postlarval density within cobble remained stable. Clearly, the higher densities of
2
lobsters (approximately 18 / m ) within cobble plots in the current study was influenced by the closed nature of the environment. The population was not affected by emigration and interspecific predation was not a factor. Nonetheless, the study highlights the ability of cobble to support a population of lobsters at a constant density through a large part of its early benthic phase.
Crowding may be one of several factors contributing to the decline in density of lobsters in mussel shell and migration between plots must therefore be considered. In the current study, individuals were allowed to move freely between habitats. It can be suggested, therefore, that the relatively constant density in cobble and the declining density in mussel shell may be the result of equal mortalities in both substrata accompanied by migration from mussel shell to cobble habitat where the size spectrum of niches is larger. The possible lack of niche availability at size within mussel shell could have initiated such a migratory movement to cobble. This conclusion would tend to fit the theory of Caddy (1986) and Caddy and Stamatopoulos (1990) that a crevice-dwelling organism such as a lobster faces a continual reduction in the number of physical niches available to them as they increase in size.
The physical structure of the habitat not only determined the number of its inhabitants, but also their size. One month old juveniles formed a clear mode of size classes between 6 and 8 mm CL in the size distributions of lobsters sampled from all quadrants. This result is comparable to Incze and Wahle (1991) who found that new benthic recruits of the American lobster within the Gulf of Maine showed a size mode of 7–8 mm CL. As the study progressed, however, the size composition of lobsters appeared to differ with substrata. Juveniles in cobble exhibited an increasingly variable size range while lobsters in mussel shell appeared to have a set upper size limit. The effect of substratum controls on the size composition of lobster populations has also been identified in nature. Howard (1980) attributed the small size of lobsters caught in Norfolk, UK, to the fact that the area contained fewer large-scale outcrops and more boulders and cobbles than other fishing grounds. Shelter scaling has also been shown to effect the upper size limits of stomatopods (Moran and Reaka, 1988), reef fish (Anderson et al., 1989) and Caribbean spiny lobster (Eggleston et al., 1990).
Size variation within habitats may also be influenced by social interactions. Decreased growth rates in lobsters as a result of communal rearing are well documented (Stewart and Squires, 1968; Van Olst et al., 1976; Carlberg et al., 1979; Aiken and Waddy, 1988) and usually manifest as a wide variation in the final size range of the cultured animals. The dominance-subordinate relationship which develops among aggressive animals is most likely the cause of this variation (Cobb and Tamm, 1974, 1975). In addition, individuals were seen to experience a high level of cheliped loss indicating frequent physical contact, a factor which may be required to instil a social hierarchy among lobsters (Cobb et al., 1982).
cannot be overlooked. Wahle and Steneck (1991) found that some mussel colonised bedrock sites within the coastal Gulf of Maine harboured EBP lobster densities similar to those of adjacent cobble bottom. This finding should be considered when future sampling for EBP European lobsters is being undertaken.
In conclusion, this study reinforces the association between EBP European lobsters and shelter-providing substrata. It also indicates that such habitat has a strong influence in determining both the density and size of the inhabiting lobsters. However, as the substratum preference of EBP European lobsters remains largely unknown, the results highlight the need to go beyond the single species approach to understand the factors that influence European lobster recruitment. It is important that future studies are designed in order to address this issue.
Acknowledgements
Adrian Linnane received support for this research through a Forbairt Basic Research Award. Part of this work also received funding provided for lobster stock enhancement
´
by Udaras na Gaeltachta and Taighde Mara Teoranta. We would like to thank Rick Wahle, Stanley Cobb and Ingebrigt Uglem for thoughtful comments on the manuscript, Iggy O’Muircheartaigh for advice on statistical procedures and Eamonn Kelly for help with the figures. Further improvements were suggested by two anonymous referees. Finally, a special thank you to all the students at the Shellfish Research Laboratory, Carna who gave up their free time to help in the monthly sampling protocol. [AU]
References
Able, K.W., Heck, Jr. K.L., Fahay, M.P., Roman, C.T., 1988. Use of salt-marsh peat reefs by small juvenile lobsters on Cape Cod, Massachusetts. Estuaries 11, 83–86.
Aiken, D.E., Waddy, S.L., 1986. Environmental influence on recruitment of the American lobster, Homarus
americanus: a perspective. Can. J. Fish. Aquat. Sci. 43, 2258–2270.
Aiken, D.E., Waddy, S.L., 1988. Strategies for maximising growth of communally reared juvenile American lobsters. World Aquacult. 19, 61–63.
Anderson, T.W., DeMartini, E.E., Roberts, D.A., 1989. The relationship between habitat structure, body size and distribution of fishes at a temperate artificial reef. Bull. Mar. Sci. 44, 681–697.
Barshaw, D.E., 1989. Growth and survival of early juvenile American lobsters, Homarus americanus, on a diet of plankton. Fish. Bull. 87, 366–370.
Barshaw, D.E., Bryant-Rich, D.R., 1988. A long-term study on the behaviour and survival of early juvenile American lobster, Homarus americanus, in three naturalistic substrates: eelgrass, mud and rocks. Fish. Bull. 86, 789–796.
Barshaw, D.E., Lavalli, K.I., 1988. Predation upon postlarval lobsters, Homarus americanus, by cunners,
Tautogolabrus adspersus, and mud crabs, Neopanope sayi, on three different substrates: eelgrass, mud and
rocks. Mar. Ecol. Prog. Ser. 48, 119–123.
Berrill, M., 1974. The burrowing behaviour of newly settled lobsters, Homarus vulgaris (Crustacea-Decapoda). J. Mar. Biol. Assoc. U.K. 54, 797–801.
´ ´
Bertran, R., 1984. Selection du substrat et construction d’abris par le jeune homard europeen (Homarus
` ´
Botero, L., Atema, J., 1982. Behaviour and substrate selection during larval settling in the lobster Homarus
americanus. J. Crustacean Biol. 2, 59–69.
Boudreau, B., Bourget, E., Simard, Y., 1990. Benthic invertebrate larval response to substrate characteristics at settlement: shelter preferences of the American lobster Homarus americanus. Mar. Biol. 106, 191–198. Boudreau, B., Bourget, E., Simard, Y., 1993. Effects of age, injury, and predator odors on settlement and
shelter selection by lobster Homarus americanus postlarvae. Mar. Ecol. Prog. Ser. 93, 119–129. Burke, R.D., 1983. The induction of metamorphosis of marine invertebrate larvae: stimulus and response. Can.
J. Zool. 61, 1701–1719.
Caddy, J.F., 1986. Modelling stock-recruitment processes in Crustacea: some practical and theoretical perspectives. Can. J. Fish. Aquat. Sci. 43, 2330–2344.
Caddy, J.F., Stamatopoulos, C., 1990. Mapping growth and mortality rates of crevice-dwelling organisms onto a perforated surface: the relevance of ‘cover’ to the carrying capacity of natural and artificial habitats. Estuar. Coast. Shelf Sci. 31, 87–106.
Carlberg, J.M., Van Olst, J.C., Ford, R.F., 1979. Potential for rearing of the Nephropid lobsters. Proc. World Maricul. Soc. 10, 840–853.
Chia, F.S., Rice, M.E., 1978. Settlement and metamorphosis of marine invertebrate larvae: proceedings of the symposium on settlement and metamorphosis of marine invertebrate larvae. In: Proceedings of the American Zoological Society Meeting, Toronto, December 27–28, 1977, Elsevier, New York.
Cobb, J.S., Tamm, G.R., 1974. Social interactions increase intermolt period in juvenile lobsters Homarus
americanus. J. Fish. Res. Board Can. 32, 1941–1943.
Cobb, J.S., Tamm, G.R., 1975. Dominant status and molt order in lobsters (Homarus americanus). Mar. Behav. Physiol. 3, 119–124.
Cobb, J.S., Tamm, G.R., Wang, D., 1982. Behavioural mechanisms influencing molt frequency in the American lobster Homarus americanus. J. Exp. Mar. Biol. Ecol. 62, 185–200.
Cobb, J.S., Gulbranson, T., Phillips, B.F., Wang, D., Syslo, M., 1983. Behaviour and distribution of larval and early juvenile Homarus americanus. Can. J. Fish. Aquat. Sci. 40, 2184–2188.
Cobb, J.S., Wang, D., Campell, D.B., Rooney, P., 1989. Speed and direction of swimming by postlarvae of the American lobster. Trans. Am. Fish. Soc. 118, 82–86.
Cooper, R.A., Uzmann, J.R., 1980. Ecology of juvenile and adult Homarus. In: Cobb, J.S., Phillips, B.F. (Eds.), Ecology and Management, The Biology and Management of Lobsters, Vol. 2, Academic Press, New York, pp. 97–142.
Crisp, D.J., 1984. Overview of research on marine invertebrate larvae, 1940–1980. In: Costlow, J.D., Tipper, R.C. (Eds.), Marine Biodeterioration: An Interdisciplinary Study, Naval Institute Press, Annapolis, pp. 103–126.
Eggleston, D.B., Lipcius, R.N., Miller, D.L., Coba-Cetina, L., 1990. Shelter scaling regulates survival of juvenile Caribbean spiny lobster Panulirus argus. Mar. Ecol. Prog. Ser. 62, 79–88.
Ennis, G.P., 1986. Swimming ability of larval American lobsters, Homarus americanus, in flowing water. Can. J. Fish. Aquat. Sci. 43, 2177–2183.
Fogarty, M.J., Idoine, J.S., 1986. Recruitment dynamics in an American lobster (Homarus americanus) population. Can. J. Fish. Aquat. Sci. 43, 2368–2376.
Grosberg, R.K., 1981. Competitive ability influences habitat choice in marine invertebrates. Nature 290, 700–702.
Howard, A.E., Bennett, D.B., 1979. The substrate preference and burrowing behaviour of juvenile lobsters
Homarus gammarus. J. Nat. Hist. 13, 433–438.
Howard, A.E., 1980. Substrate controls on the size composition of lobster Homarus gammarus populations. J. Cons. Int. Explor. Mer 39, 130–133.
´
Hudon, C., Fradette, P., Legendre, P., 1986. La repartition horizontale et verticale des larves de homard ˆ
(Homarus americanus) autour de los ı les de la Madeleine, golfe du Saint-Laurent. Can. J. Fish. Aquat. Sci. 43, 2164–2176.
Hudon, C., 1987. Ecology and growth of post-larval and juvenile lobster, Homarus americanus, off Isle de la Madeleine (Quebec). Can. J. Fish. Aquat. Sci. 44, 1855–1869.
Incze, L.S., Wahle, R.A., 1991. Recruitment from pelagic to early benthic phase in lobsters, Homarus
Lavalli, K.L., Barshaw, D.E., 1986. Burrows protect postlarval lobster, Homarus americanus, from predation by the non-burrowing cunner Tautogoglabrus adspersus, but not from the burrowing mud crab, Neopanope
texani. Mar. Ecol. Prog. Ser. 32, 13–16.
Lavalli, K.L., Barshaw, D.E., 1989. Post-larval American lobsters (Homarus gammarus) living in burrows may be suspension feeding. Mar. Behav. Physiol. 15, 255–264.
Mercer, J.P., Brown, R., 1994. Lobster stock enhancement in Ireland. Aquacult. Ireland April–May, 20–24. Mileikovsky, S.A., 1971. Types of larval development in marine bottom invertebrates, their distribution and
ecological significance: a re-evaluation. Mar. Biol. 10, 193–213.
Moran, D.P., Reaka, M.L., 1988. Bioerosion and availability of shelter for benthic reef crustaceans. Mar. Ecol. Prog. Ser. 44, 249–263.
Morse, D.E., 1985. Neuro transmitter–mimetic inducers of larval settlement and metamorphosis. Bull. Mar. Sci. 37, 697–706.
O’Conner, N.J., 1991. Flexibility in timing of the metamorphic molt by fiddler crab megalopae Uca pugilator. Mar. Ecol. Prog. Ser. 68, 243–247.
Petersen, J.H., 1984. Larval settlement behaviour in competing species: Mytilus californianus Conrad and M.
edulis L. J. Exp. Mar. Biol. Ecol. 82, 131–146.
Pottle, R.A., Elner, R.W., 1982. Substrate preference behaviour of juvenile American lobsters, Homarus
americanus in gravel and silt-clay sediments. Can. J. Fish. Aquat. Sci. 39, 928–932.
Richards, R.A., Cobb, J.S., 1986. Competition for shelter between lobsters (Homarus americanus) and Jonah crabs (Cancer borealis): effects of relative size. Can. J. Fish. Aquat. Sci. 43, 2250–2255.
Scheltema, R.S., 1986. On dispersal and planktonic larvae of marine invertebrates: an eclatic overview and summary of problems. Bull. Mar. Sci. 39, 290–322.
Stewart, J.E., Squires, H.J., 1968. Adverse conditions as inhibitors of ecdysis in the lobster Homarus
americanus. J. Fish. Res. Bd. Can. 25, 1763–1774.
Svane, I., Young, C.M., 1989. The ecology and behaviour of ascidian larvae: a review. Oceanogr. Mar. Biol. 27, 45–90.
Tully, O., O’Ceidigh, P., 1987. The seasonal and diel distribution of lobster larvae Homarus gammarus in the neuston of Galway Bay. J. Cons. Int. Explor. Mer 44, 5–9.
van Olst, J.C., Carlberg, J.M., Ford, R.F., 1976. Effects of substrate type and other factors on the growth, survival, and cannibalism of juvenile Homarus americanus in mass rearing systems. Proc. World Maric. Soc. 6, 261–274.
Wahle, R.A., 1993. Recruitment to American lobster populations along an estuarine gradient. Estuaries 16, 731–738.
Wahle, R.A., 1998. A trans-atlantic perspective on Homarus recruitment and enhancement. In: van der Meeren, G.I., Soldal, O. (Eds.), Proceedings of the Seminar on the European lobster Homarus gammarus
˚
(L.), Kvitsøy, Norway, 1995, APEN Press, Bergen, pp. 36–43.
Wahle, R.A., Steneck, R.S., 1991. Recruitment habitats and nursery grounds of the American lobster (Homarus
americanus Milne Edwards): A demographic bottleneck? Mar. Ecol. Prog. Ser. 69, 231–243.
Wahle, R.A., Steneck, R.S., 1992. Habitat restrictions in early benthic life: experiments on habitat selection and in situ predation with the American lobster. J. Exp. Mar. Biol. Ecol. 157, 91–114.
Wahle, R.A., Incze, L.S., 1997. Pre- and post-settlement processes in recruitment of the American lobster. J. Exp. Mar. Biol. Ecol. 217, 179–207.
Wentworth, C.K., 1936. An analysis of shapes of glacial cobbles. J. Sediment. Petrol. 6, 85–96.
Wilkinson, L., Hill, M., Vang, E., 1992. In: SYSTAT: Statistics, version 5.2 edition, SYSTAT Inc, Evanson, Illinois, p. 724.