Redox state and peroxidase system in sunflower plants exposed to
ozone
Annamaria Ranieri *, Francesco Petacco, Antonella Castagna, Gian Franco Soldatini
Dipartimento di Chimica e Biotecnologie Agrarie,Uni6ersita` di Pisa,6ia del Borghetto80,56124Pisa,Italy Received 22 May 2000; received in revised form 24 July 2000; accepted 24 July 2000
Abstract
Sunflower plants subjected to a short-term fumigation with O3(150 ppb for 4 h repeated for 4 days) exhibited an increase in
total ascorbate content, accompanied by a marked oxidation of ascorbate, leading to a decrease in its redox state, either at intracellular or extracellular level. O3 exposure induced a rise in free extracellular peroxidase (POD) activity, assayed by
syringaldazine as electron donor, as well as in the ionically and covalently cell wall bound PODs. On the contrary, the activity of both extracellular and intracellular guaiacol – POD did not show significant changes as a consequence of the pollutant exposure. The stimulation of syringaldazine – POD activities may be related to the effect of ozone on the growth of the cells, inducing an early senescence through the activation or acceleration of lignification processes. Beside, the reduced plasticity of the cell wall may oppose an unspecific mechanical resistance against the abiotic stress induced by the ozone exposure. © 2000 Elsevier Science Ireland Ltd. All rights reserved.
Keywords:Ascorbate; Ozone; Oxidative stress; Peroxidases; Redox state; Sunflower
www.elsevier.com/locate/plantsci
1. Introduction
Ozone (O3) is perceived to be one of the most
ubiquitous and damaging air pollutant to which vegetation is exposed [1]. Current ground-level concentrations of the pollutant are known to have adverse effects on the vitality of natural and agro-nomic ecosystems in many parts of the industri-alised world.
Ozone can affect biochemical processes even in plants showing no visible sign of injury [2]. The influence of ozone on vegetation is dependent on concentration and the duration of exposure to the pollutant, the genetic backgrounds and the growth phases of the plants [3,4]. The cuticle is practically impermeable to O3, so the flux of the pollutant to
the leaf interior is predominantly controlled by stomatal aperture [5]. Once inside the leaf, ozone is in contact with the aqueous phase of the cell wall (the apoplast) where the reaction of O3 with
constituents of the apoplast generates various re-active oxygen species (ROS) like free radicals (OH, O2−) and peroxides (H2O2, R2O2) [6 – 8],
which can damage the components of plas-mamembranes, such as proteins and lipids.
Among ROS, the radical OH is the most reac-tive one, able to attack all the biomolecular spe-cies. On the other side, O2− and H2O2 are less
reactive but equally dangerous for their possibility of diffusion in all cellular compartments.
Thus, according to Luwe et al. [9], the aqueous phase of the apoplast seems to represent the site where O3 and its highly toxic reactive
intermedi-ates must be detoxified if they are to be prevented from reacting with the plasmamembranes and cy-toplasmic components. Detoxification systems in this microenvironment, either enzymatic or
non-* Corresponding author. Tel.: +39-50-571557/58; fax: + 39-50-598614.
E-mail address:[email protected] (A. Ranieri).
enzymatic, able to prevent or to mitigate O3injury
have been reported [10]. Among antioxidant metabolites, ascorbic acid, in addition to playing a key role in detoxification of H2O2 as a specific
substrate for ascorbate peroxidase (APX), is capa-ble of direct scavenging of O3 molecules and O3
-derived ROS [7,8,11 – 13]. Although the pool of ascorbate present in the apoplast is only a small proportion of the total leaf content, it is suggested that its increase following ozone exposure may afford protection against the dangerous effect of this pollutant [12,14].
In the process of detoxification of H2O2 is
par-ticularly important the large family of peroxidases (POD), which includes either the specific ascorbate peroxidase enzyme (APX) and the so called unspe-cific peroxidases (POD). Previous data, obtained in our laboratory on sunflower plants subjected to O3exposure, have shown a differential stimulation
of APX isoforms, indicating an enhancement in the activity at both the apoplastic and symplastic level, while stromal and thylakoid-bound chloro-plastic APX activity remained unchanged [15]. The unspecific POD, besides their antioxidant role, is involved in many metabolic functions. In the cell wall, PODs are present in soluble, ionically- and covalently-bound forms and, in addition to a detoxificant role as scavengers of H2O2, they have
been reported to be involved in a number of physiological processes which regulate cell growth by catalysing the formation of cross-links between extensin and feruloyated polysaccarides and the polymerisation of lignin precursors [16 – 20]. The cell wall stiffening has been attributed mainly to peroxidases whose activity can be detected by using, in the enzymatic assay mixture, syringal-dazine as a specific substrate [16].
In the present work, the apoplastic ascorbate pool content of sunflower plants exposed to O3
and the cell redox state, measured as reduced versus total ascorbate ratio, was determined. Be-side, the behaviour of unspecific PODs following O3 exposure was tested, measuring their activity
by using different electron donors such as guaiacol and syringaldazine, both in the extracellular (IWF) and in the intracellular (RCM) leaf fluids, as well as in the soluble (S), ionically (IB) and covalently (CB) cell wall-bound fractions. In addition, the POD isoforms of each fraction, along with the apoplastic and symplastic ones, were separated by isoelectrofocusing analysis.
2. Materials and methods
2.1. Plant material
Sterilised sunflower seeds (Heliantus annuus L., cv Hor) were germinated, in the dark, in Petri dishes for 3 days and the seedlings were grown in perlite for a week. After this period, plants were transferred and grown for 4 weeks in a greenhouse at 17 – 25°C (night – day), R.H. between 60 and 80%, with a 14 h photoperiod and a photosyn-thetic photon flux density of 530 mmol m−2 s−1
(PAR: 400 – 700 nm).
Only uniform plants with eight fully expanded leaves were selected (:35 days after sowing). All biochemical analyses were carried out on fully expanded middle-aged leaves from control and treated plants.
2.2. Fumigation treatment
O3fumigation was performed in air conditioned
chambers (0.48 m3). Temperature was maintained
at 2091°C and R.H. at 8595%. A photon flux
density at plant height of 530 mmol m−2 s−1was
provided by incandescent lamps. O3was generated
by electric discharge passing pure oxygen through a Fisher ozone generator 500 (Fisher Labor und
Verfahrenstechnik, Meckenheim, Germany).
Ozone concentration in the fumigation chambers was continuously monitored with a Monitor Labs Analyzer mod. 8810 (Monitor Labs, San Diego, CA) operating on the principle of UV absorption and interfaced with a personal computer. Control plants were grown in charcoal-filtered air cham-bers under the same conditions. Plants were pre-adapted to the chamber conditions for 48 h and
half of them were exposed to 150 ppb O3 for 4
days (4 h per day), while the remainders, un-treated, were used as a control.
2.3. Preparation of the apoplastic fluid
Freshly-harvested intact leaves (10 g) were rinsed with distilled water and vacuum infiltrated (−65 kPa, three cycles of 30 s each) in 50 ml of 66 mM K-phosphate buffer (pH 7.0) and 100 mM KCl. After infiltration, the leaves were wiped and
centrifuged (1500×g for 10 min at 4°C). The
[21]. The residual cell material (RCM) was
im-mersed in liquid nitrogen and stored at −80°C
until use.
2.4. Extraction of enzymes from residual cell material
For POD determination, the RCM was crushed in liquid nitrogen and homogenised at 4°C in 0.22
M Tris – HCl pH 7.4, PMSF (50 mg/ml), 10 mM
b-mercaptoethanol and 0.1% insoluble PVP. After centrifugation at 24 000×g for 10 min at 4°C, the supernatant was recovered and dialysed overnight against 2 mM Tris – HCl (pH 7.4) and tested for enzyme activity [22].
To test the activity of the cytoplasmatic and chloroplastic enzyme markers, glucose-6-P dehy-drogenase (G6PDH, EC 1.1.1.49) and
glyceralde-hyde-3-P dehydrogenase (GAPDH, EC 1.2.1.12),
the RCM was homogenised with liquid nitrogen in a cold buffer solution (1:4, w/v) consisting of 50 mM HEPES (pH 7.4) containing 0.25 M sucrose,
70 mM KCl, 10 mM MgCl2, 1 mM EDTA, 1%
(w/v) BSA, 10 mMb-mercaptoethanol (bME) and
phenylmethylsulfonyl fluoride (PMSF) (50 mg
ml−1). NH
4SO2 (70% of saturation) at 4°C was
added to the surnatant obtained after centrifuga-tion of the homogenate at 39 000×g for 15 min at 4°C, to precipitate the proteins. After centrifu-gation (17 000×g for 15 min at 4°C), the pellet obtained was resuspended in 10 mM HEPES
buffer at pH 7.4, containing 10 mM MgCl2, 50
mM KCl, 0.5 mM EDTA and 1 mM bME and
dialysed overnight. The extract obtained was utilised for the analysis of the enzymatic activity [15].
2.5. Marker enzyme acti6ity
The incubation medium for the G6PDH activity
determination consisted of 86.3 mM
tri-etanolamine-HCl buffer (pH 7.6), 6.7 mM MgCl2,
12 mM glucose-6-P, 0.37 mM NADP+ and a
suitable aliquot of IWF or RCM. The absorbance was read at 340 nm. The activity was expressed as mmol of NADP+ reduced per minute and referred
to the protein content of the extract. The activity assay for GAPDH was carried out at 25°C, recording the decreasing of the absorbance at 340 nm. The reaction mixture contained 50 mM Tris –
HCl buffer (pH 7.8), 10 mM MgCl2, 5 mM
EDTA, 3 mM ATP, 100 mM NADPH, 5 mM
3-P-glycerate, 0.45 U ml−1 P-glycerate KINASE
and an aliquot of RCM or IWF [15].
2.6. Separation of soluble, ionically and co6alently-bound peroxidases
The separation of soluble, ionically- and cova-lently-bound POD was carried out according to Grison and Pilet [23]. Freshly harvested leaves were homogenised at 4°C with 66 mM Na-phos-phate pH 6.1 and centrifuged at 800×gfor 5 min.
The supernatant was again centrifuged at
10 000×g for 5 min and the recovered second
supernatant was considered the soluble fraction. The first pellet was washed twice with phosphate buffer, twice with water and after shaking continu-ously for 1 h at 4°C in Triton X-100, it was again rinsed five times with water. The pellet obtained
was treated with 1 M CaCl2 for 1 h and
cen-trifuged at 800×g for 10 min at 4°C. The result-ing supernatant was the ionically-bound fraction. The pellet was washed several times with distilled water and incubated at room temperature for 16 h with 0.3% cellulase, 0.3% macerase and 0.3% cellu-lolysin in 50 mM Na-acetate buffer (pH 5.5) to obtain the covalently-bound fraction, after cen-trifugation at 800×g for 10 min. The residual cell wall material was dried at 80°C and weighed.
2.7. POD assay
POD activities were measured spectrophotomet-rically using two different substrates, guaiacol and syringaldazine.
The incubation mixture for the determination of guaiacol – POD activity consisted of 20 mM Na-acetate buffer (pH 5.0), 2 mM guaiacol, 30 mM H2O2 and a suitable portion of plant tissues
ex-tract. Increase in absorbance was recorded at 470 nm [22].
Syringaldazine – POD activity was assayed by the increase in absorbance at 530 nm in a reaction medium containing 200 mM Na-K phosphate buffer (pH 6.0), 2.5 mM H2O2and 2 mM
2.8. Protein content determination
The protein content of IWF and RCM was determined by the protein – dye binding method of Bradford [25], using bovine serum albumin as standard. The spectrofotometrical reading was measured at 595 nm.
2.9. POD isoenzyme determination
Isoperoxidases were resolved by isoelectric fo-cusing (IEF), in a horizontal slab apparatus (Bio-Rad), on acrylamide gel using a pH gradient of 3.5 – 10. Samples were dialysed overnight in 0.05 Na-phosphate buffer (pH 7.0), lyophilised and then dissolved in 1% glycine. The resuspended samples, after filtration with 0.45 mm pore size filters (Whatman), were loaded on 5% polyacry-lamide gel, containing 4.8% acrypolyacry-lamide, 0.2% N,N%-methylene-bis-acrylamide, 5% glycerol, 5%
ampholine pH 3.5 – 10. To the solution (10 ml)
were added 3 ml of TEMED and 70 ml of 10%
APS. To estimate the pI of separated isoenzymes, standard proteins of known pI (Sigma) were run on the same gels.
The IEF run was carried out at constant voltage of 100 V for 15 min, followed by 15 min at 200 V and 45 min at 450 V. The POD isoenzymes were visualised by incubating gel in 0.5% benzidine and 0.03% H2O2 in 4.5% acetic acid (modified from
Ref. [26]) and after the reaction was stopped by immersing the gel in 7% acetic acid for 30 s, gels were immediately photographed. This benzidine stain was chosen since this substrate is slightly less selective towards peroxidase isoforms than other hydrogen donors and rapidly precipitates on gels.
2.10. Ascorbate extraction and determination
For determination of ascorbic (ASA) and dehy-droascorbic (DHA) acid, the residual cell material was homogenised in a mortar with liquid nitrogen, quartz sand and a solution of 5% metaphosphoric acid (1:2, w/v) and centrifuged at 14 000×g for 20 min.
The total amount of ASA+DHA in
intercellu-lar washing fluid was quantified immediately after its extraction to minimise the ascorbate oxidase (AAO)-dependent oxidation of ASA during mea-surements. The absence of such oxidation of ASA was confirmed by adding 10 mM sodium azide (an
inhibitor of AAO) to the buffer used to obtain IWF [27]. The quantitative determination was car-ried out according to Okamura [28] and Law et al. [29]. An aliquot of supernatant or IWF was added
to 10% TCA (w/v) and after addition of 5 M
NaOH, the mixture was centrifuged at 12 000×g for 2 min. To quantify ASA, 150 mM phosphate buffer (pH 7.4) was added to the supernatant. For
the total amount of ASA+DHA, 10 mM DTT
was supplied, after incubation with 10 mM DTT for 15 min at room temperature, 0.5% of N-ethyl-maleimide solution was added. Then, each sample was supplied with 10% TCA (w/v), 44% H3PO4
solution (v/v), 4% a-a%dipyridyl (w/v) in 70%
methanol and 3% FeCl3 (w/v). After vigorous
stirring, the samples were kept at 37°C for 60 min and then the absorbance was read at 525 nm against a standard curve of pure ASA (Sigma) in the 0 – 40 nmol range.
2.11. Statistic
A minimum of 12 plants per treatment were used in all experiments. Values shown in the tables
are the means of eight determinations9S.D.
Comparison between means was evaluated by
t-test and the P=0.05 level of error.
3. Results
At the end of ozone exposure, sunflower plants did not show any visible symptoms of injury on leaf surfaces, confirming previous data on the likeness of chlorophyll content of O3-treated and
untreated leaves [30].
Accidental contamination of IWF with intracel-lular proteins during the infiltration procedure was tested by analysing the activity of the cytosolic and chloroplastic enzyme markers G6PDH and GAPDH respectively. Regardless of the treatment, the relative activity of these marker enzymes in the
IWF was always B0.1% of the total activity in
the RCM (data not shown).
3.1. Redox state
At apoplastic level the total ascorbate content raised (+112%) as a consequence of O3
signifi-Table 1
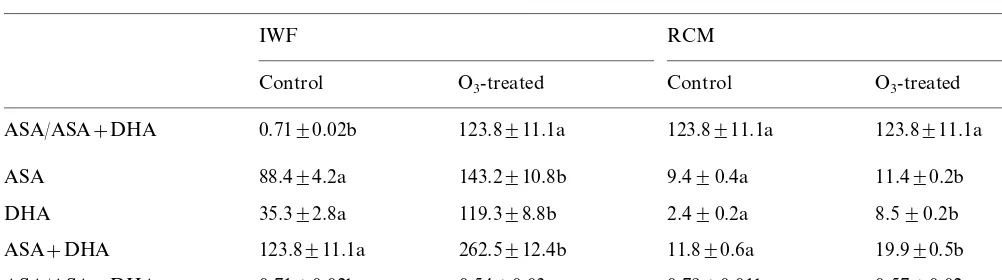
Reduced (ASA), oxidized (DHA), total (ASA+DHA) ascorbate content and redox state of ascorbate (ASA/ASA+DHA) of extracellular (IWF) and intracellular (RCM) extracts of control and O3-treateed sunflower plantsa
RCM IWF
Control O3-treated Control O3-treated
ASA/ASA+DHA 0.7190.02b 123.8911.1a 123.8911.1a 123.8911.1a
ASA 88.494.2a 143.2910.8b 9.490.4a 11.490.2b
119.398.8b 2.490.2a
35.392.8a 8.590.2b
DHA
262.5912.4b
ASA+DHA 123.8911.1a 11.890.6a 19.990.5b
0.5490.03a 0.7990.01b 0.5790.02a ASA/ASA+DHA 0.7190.02b
aValues are expressed as nmol g−1fw andmmol g−1fw for extracellular and intracellular samples, respectively. Within each
fraction (IWF or RCM) values followed by different letters are statistically different atP=0.05 (n=5).
ing O3 exposure, while the syringaldazine – POD
activity underwent a significant increase by 23.4% (Table 2).
At simplastic level, the guaiacol – POD activity showed no significant variation in the ozonated plants as compared with the untreated ones (Table 2).
The activity of soluble, ionically- and cova-lently-bound PODs was assessed using syringal-dazine as electron donor, owing to the preferential involvement of these fractions in the lignification process. All three fractions tested showed a signifi-cant similar increase in Syr – POD activity
follow-ing O3 fumigation, with the soluble POD
increasing by 94%, the ionically-bound POD by 112% and the covalently-bound POD by 82%, respectively (Table 3).
cant increase, DHA showing a more pronounced
stimulation than ASA (+238 and +62% in
com-parison to their respective controls). As a conse-quence of these different increments the redox state changed from 0.71 in the control to 0.54 in the ozonated plants (−24%). Similar reduction in the redox state of ascorbate was also exhibited at symplastic level (−29%) where the oxidised and the reduced form underwent an increase, respec-tively, by 255 and 21% in the fumigated sunflower plants (Table 1).
3.2. POD acti6ity
With regard to the extracellular unspecific POD, the guaiacol – POD activity did not change
follow-Table 2
Guaiacol (G–POD) and syringaldazine (Syr–POD) peroxidase activity in the intercellular washing fluid (IWF) and intracel-lular soluble fraction (RCM) of sunflower leaves treated with O3 (150 ppb, 4 d, 4 h per day)a
Enzyme activity
IWF RCM
G–POD
Samples Syr–POD G–POD
1.4790.2a 4.0490.20a
Control 90.491.5a
3.9890.17a
O3-treated 111.594.3b 1.2790.2a
aEnzyme activity is expressed as DA
470 (guaiacol–POD)
min−1 mg−1 proteins and DA
530 (syringaldazine–POD)
min−1mg−1 proteins. Within each fraction (IWF or RCM)
values followed by different letters are statistically different at
P=0.05 (n=5).
Table 3
Effect of fumigation with O3 (150 ppb, 4d, 4h per day) on
soluble (S), ionically (IB) and covalently (CB) cell wall bound peroxidases of sunflower leavesa
Samples Enzyme activity
I.B.
Soluble C.B.
34.591.7a 7.490.6a 145.196.7a Control
67.091.1b 15.790.7b
O3-treated 264.1911.5b
aEnzyme activity, determined using syringaldazine as
elec-tron donor, is expressed as DA530min−1mg−1proteins, with
the exception of CB activity, which is expressed asDA min−1
mg−1dry weight of residual cell wall material. Within each
Fig. 1. Benzidine staining of peroxidase isoforms of extracel-lular (IWF) and intracelextracel-lular (RCM) leaf fluids of control (C) and O3-treated (O3) sunflower plants. A total of 50 mg of
proteins for IWF and 30mg of proteins for RCM fractions were resolved by a pH 3.5 – 10 isoelectrofocusing gel. Isolec-trophoretic analysis was performed in triplicate.
untreated ones, particularly evident for the most acidic and the most basic isoforms. Beside, an additional anionic band seems to be present as a
consequence of O3 exposure. In the
ionically-bound fraction, only a few bands with POD activ-ity are visible. Also, in this fraction, the increase in the staining intensity was accompanied by qualita-tive differences between the two treatments be-cause of the presence of an anionic protein band in the O3-treated sample that, on the contrary, was
not visible in the control. As regards the cova-lently-bound POD profiles, only slight quantitative differences in the staining intensity were evident between treated and untreated plants.
4. Discussion and conclusions
Sunflower plants, subjected to a short-term fu-migation with O3 (150 ppb for 4 h repeated for 4
days), exhibited an increase in the levels of re-duced form of ascorbic acid either inside the cell (+21%) and in the extracellular matrix (+62%). According to some authors [7,11 – 13], this increase at apoplastic level might indicate an important role for ascorbic acid in the direct scavenging of O3 molecules before they penetrate the cell wall
and cause damage. This seems the case for sunflower plants, where no visible symptoms of injury were present at the end of fumigation, as reported by Polle et al. [14] in O3-exposed spruce.
Owing to a leaf water content of 80% and assum-ing that cell wall water is 10% of the leaf fresh 3.3. Isoenzyme pattern determination
Regarding the total POD, separated by IEF and visualised by benzidine gel staining, a generalised higher staining intensity of all the bands was evi-dent in the IWF of O3-treated plants, as compared
to the control, whereas at RCM level the POD isoenzymatic pattern did not exhibit evident differ-ences between the two samples (Fig. 1).
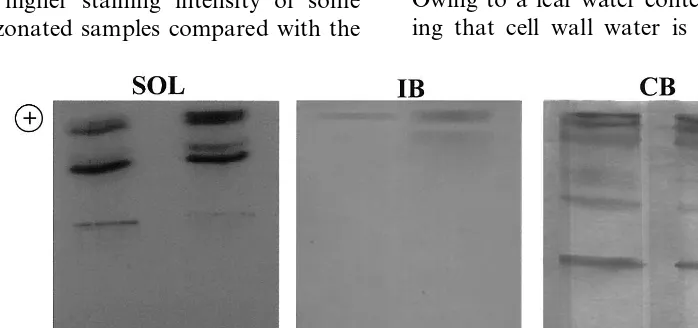
The isoenzyme profile of the three fractions of soluble, ionically- and covalently-bound POD is shown in Fig. 2. In the soluble fraction, there is evidence of a higher staining intensity of some bands in the ozonated samples compared with the
Fig. 2. Benzidine staining of peroxidase isoforms of soluble (S), ionically- (IB) and covalently- (CB) cell wall bound fractions of control (C) and O3-treated (O3) sunflower plants. A total of 50 mg of proteins for soluble fractions and 30 mg of proteins for
weight [13], the ascorbate concentration in the apoplast of sunflower leaves ranged from 1.1 mM in the control to 1.8 mM in the O3-treated plants.
Depending on the cell wall thickness, a similar ascorbate concentration may permit the direct detoxification of at least half of the ozone imping-ing the wall surface, in accordance to that reported by Chameides [7] and Moldau et al. [11]. More-over ASA, in addition to its role as direct antioxi-dant, represents the substrate for Apx, whose activity was found to increase in sunflower plants following O3 fumigation, either at extracellular
and intracellular level [15]. This stimulation of Apx activity contributed to the rise in the levels of
the oxidized form of ascorbate (+238 and +
255%, respectively in IWF and RCM samples) and, in turn, to lower its redox state, according to the results previously obtained on O3-treated
pumpkin plants [2]. When an active oxidation of ASA takes place in the extracellular ambient, newly-formed ASA can be exported from the cyto-sol and DHA originated may be retransported to the cytoplasm where it can be reduced by DHA reductase, as reported by Rautenkranz et al. [31] and Horemans et al. [32]. It has been suggested that the translocation of the oxidised DHA to the cytoplasm is a necessary step in the regeneration of extracellular reduced ascorbate [32]. Recent data [33] have indicated the presence of either DHA reductase and reduced glutathione (GSH) in the apoplastic environment, so pointing to the presence of a regeneration system for ascorbic acid also in the apoplast.
In addition to stimulation of the antioxidant metabolite ascorbic acid, O3 exposure induced a
rise in free extracellular unspecific POD activity, assayed by the utilization of syringaldazine as electron donor. Differently to Apx, specifically involved in the H2O2 detoxification, the functions
of unspecific PODs are generally less well-defined because they catalyze the oxidation of a wide range of phenolic substrates [34,35].
The syringaldazine has often been considered a specific substrate for PODs present at extracellular level and involved in the cell wall lignification process [17] catalyzing the oxidation of phenolic acids by hydrogen peroxide [36], produced through the oxidation of NADH [37,38]. However, doubts about their function, derived from some discrepan-cies reported by Goldberg [39] between hystochem-ical and biochemhystochem-ical observations are still present.
Along with the soluble POD, also the ionically and covalently cell wall bound PODs, reacting with syringaldazine as electron donor, underwent a significant rise in their activity in ozonated plants. As with the free PODs, the wall-bound peroxidases are thought to be involved in the lignification process of the cell wall [40]. Inside these POD pool, overall the ionically-bound ones are reported to catalyze the (a) polymerization of lignin precursors; (b) suberization; and (c) the cross-linking of proteins, polysaccharides or other molecules with wall material, so regulating cell elongation through the formation of interchain covalent bonds between various cell wall polymers [41,42]. In sunflower plants, despite the activity of syringaldazine, POD increased in all fractions tested, the higher stimulation was, in fact, found in the ionically-bound fraction (+112%).
Under stress conditions, the enhanced perox-idase activity in the intercellular spaces can proba-bly lead to reduction of cell growth, stimulating cell wall stiffening [43]. So, the enhancement of the activity of either free and bound syringaldazine – peroxidases in sunflower plants may be related to the effect of ozone on the growth of the cells, inducing an early senescence through the activa-tion or acceleraactiva-tion of lignificaactiva-tion processes [44,45]. Beside, as a consequence of the increase, in particular, of cell wall-bound POD activity, the cell wall may reduce its plasticity with the result of
opposing an unspecific mechanical resistance
against the abiotic stress induced by the ozone exposure, according to the suggestions reported by Castillo [46].
The data obtained on the enzymatic analysis of syringaldazine – POD are coherent with the results related to the isoelectrophoretic separation of POD isoenzymes followed by benzidine staining procedure. Generally, the soluble and ionically cell-wall bound fractions showed an enhanced in-tensity of band staining in the O3-treated plants in
respect to the control ones. Besides, O3 exposure
In contrast to what happened for syringal-dazine – POD, the activity of extracellular guaia-col – POD did not show significant changes as a consequence of pollutant exposure, as reported also by Polle et al. [48] in total extract of Picea abies. Different roles have been reported for guaia-col – POD at apoplastic level; (i) oxidation of ascorbate through the formation of phenoxy radi-cals [49]; (ii) detoxification of H2O2at the interface
cell wall/ plasmalemma [50]; and (iii) cross-linking of lignin precursors, similarly to syringaldazine – POD [41,51].
As what happened for extracellular PODs, at intracellular level the guaiacol – PODs did not show any modification in their activity following
O3 treatment, a result confirmed by the IEF
separation.
Previous data obtained by spectrophotometric and electrophoretic analysis have revealed, both at apoplastic and symplastic level, an enhancement of ascorbate-dependent peroxidase activity (Apx) in the O3-treated sunflower plants [15]. These data,
along with the lack of stimulation of guaiacol – POD, seem to ascribe a selective role to APx activity in detoxifying the H2O2 generated as a
consequence of O3 exposure.
In conclusion, in sunflower plants, the stimula-tion of unspecific POD activity seems to be re-stricted only to syringaldazine – PODs, which, inducing molecular alterations at cell wall struc-ture level, are probably involved in a rapid adap-tation of the plant cells to changes in their environment.
Acknowledgements
This research was supported by a Grant from MURST (National Project), Rome, Italy.
References
[1] R.L. Heath, G.E. Taylor Jr, Physiological processes and plant responses to ozone exposure, in: H. Sandermann Jr, A.R. Wellburn, R.L. Heath (Eds.), Forest Decline and Ozone, Springer – Verlag, Berlin, 1997, p. 317.
[2] A. Ranieri, G. D’Urso, C. Nali, G. Lorenzini, G.F. Soldatini, Ozone stimulates apoplastic systems in pump-kin leaves, Physiol. Plant. 97 (1996) 381 – 387.
[3] R.L. Heath, Possible mechanism for the inhibition of photosynthesis by ozone, Photosynth. Res. 39 (1994) 439 – 451.
[4] S.E. Benes, T.M. Murphy, P.D. Anderson, J.L.J. Houpis, Relationship of antioxidant enzymes to ozone tolerance in branches of mature ponderosa pine trees exposed to long term, low concentration, ozone fumigation and acid precipitation, Physiol. Plant. 94 (1995) 124 – 134. [5] V.C. Runeckles, Uptake of ozone by vegetation, in: A.S.
Lefohn (Ed.), Surface Level Ozone Exposures and their Effects on Vegetation, Lewis, Chelsea, 1992, pp. 157 – 188. [6] J. Kanofsky, P. Sima, Singlet oxygen production from the reaction of ozone with biological molecules, J. Biol. Chem. 266 (1991) 9039 – 9042.
[7] W.L. Chameides, The chemistry of ozone deposition on plant leaves: role of ascorbic acid, Environ. Sci. Technol. 23 (1989) 595 – 600.
[8] H. Moldau, Ozone detoxification in the mesophyll cell wall during a stimulated oxidative burst, Free Rad. Res. 31 (1999) 19 – 24.
[9] M.W.F. Luwe, U. Takahama, U. Heber, Role of ascor-bate in detoxifying ozone in the apoplast of spinach (Spinacia oleracea L.) leaves, Plant Physiol. 101 (1993) 969 – 976.
[10] R.L. Heath, Biochemical mechanisms of pollutant stress, in: W.W. Heck, O.C. Taylor, D.T. Tingey (Eds.), Assess-ment of Crop Loss from Air Pollutants, Elsevier Applied Science, London, 1988, pp. 259 – 286.
[11] H. Moldau, Hierarchy of ozone scavenging reactions in the plant cell wall, Physiol. Plant. 104 (1998) 617 – 622. [12] F.J. Castillo, H. Greppin, Extracellular ascorbic acid and
enzyme activities related to ascorbic acid metabolism in
Sedum album L. leaves after ozone exposure, Environ. Exp. Bot. 28 (1988) 231 – 238.
[13] A. Ranieri, A. Castagna, E. Padu, H. Moldau, M. Rahi, G.F. Soldatini, The decay of O3through direct reaction
with cell wall ascorbate is not sufficient to explain the different degrees of O3-sensitivity in two poplar clones, J.
Plant Physiol. 154 (1999) 250 – 255.
[14] A. Polle, G. Wieser, W.M. Havranek, Quantification of ozone influx and apoplastic ascorbate content in needles of Norway spruce trees (Picea abiesL., Karst) at high altitude, Plant Cell Environ. 18 (1995) 681 – 688. [15] A. Ranieri, A. Castagna, G.F. Soldatini, Differential
stimulation of ascorbate peroxidase isoforms by ozone exposure in sunflower plants, J. Plant Physiol. 156 (2000) 266 – 271.
[16] R. Goldberg, A.M. Catesson, Y. Czaninski, Some prop-erties of syringaldazine oxidase, a peroxidase specifically involved in the lignification process, Z. Pflanzenphysiol. 110 (1983) 267 – 279.
[17] A. Imberty, R. Goldberg, A.M. Catesson, Isolation and characterization of Populus isoperoxidases involved in the last step of lignin formation, Planta 164 (1985) 221 – 226.
[18] F. Abeles, C. Biles, Characterization of peroxidases in lignifying peach fruit endocarp, Plant Physiol. 95 (1991) 269 – 273.
[19] A. Polle, T. Otter, F. Seifert, Apoplastic peroxidases and lignification in needles of Norway spruce (Picea abiesL.), Plant Physiol. 106 (1994) 53 – 60.
[21] F.J. Castillo, H. Greppin, Balance between anionic and cationic extracellular peroxidase activities in Sedum al-bum leaves after ozone exposure. Analysis by high-per-formance liquid chromatography, Physiol. Plant. 68 (1986) 201 – 208.
[22] A. Ranieri, G. Schenone, L. Lencioni, G.F. Soldatini, Detoxificant enzymes in pumpkin grown in polluted ambient air, J. Environ. Qual. 23 (1994) 360 – 364. [23] R. Grison, P.E. Pilet, Cytoplasmic and wall
isoperoxi-dases in growing maize roots, J. Plant Physiol. 118 (1985) 189 – 199.
[24] T. Pandolfini, R. Gabbrielli, C. Comparini, Nickel toxic-ity and peroxidase activtoxic-ity in seedlings ofTriticum aes
-ti6umL, Plant Cell Environ. 15 (1992) 719 – 725.
[25] M.M. Bradford, A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding, Anal. Biochem. 72 (1976) 248 – 254.
[26] A. Ros Barcelo´, R. Mun˜oz, F. Sabater, Lupin peroxi-dases. I. Isolation and characterization of cell wall-bound isoperoxidase activity, Physiol. Plant. 71 (1987) 448 – 454. [27] U. Takahama, T. Oniki, The association of ascorbate and ascorbate oxidase in the apoplast with IAA-enhanced elongation of epicotyls fromVigna angularis, Plant Cell Physiol. 35 (1994) 257 – 266.
[28] M. Okamura, An improved method for determination of
L-ascorbic acid and L-dehydroascorbic acid in blood
plasma, Clin. Chim. Acta 103 (1980) 259 – 268.
[29] M.J. Law, A.S. Charles, B. Halliwell, Glutathione and ascorbic acid in spinach (Spinacia oleraceae) chloroplasts, Biochem. J. 210 (1983) 899 – 903.
[30] A. Castagna, A. Ranieri, G.F. Soldatini, Influence of ozone treatment on pigment content and composition in sunflower plants, in: P. Mathis (Ed.), Photosynthesis: from Light to Biosphere, vol. 9, Kluwer Academic, Dordrecht, 1995, pp. 123 – 126.
[31] A.A.F. Rautenkranz, L. Li, F. Machler, E. Martinoia, J.J. Oertli, Transport of ascorbic acid and dehydroascor-bic acid across protoplast and vacuole membranes iso-lated from barley (Hordeum6ulgareL. cv Gerbel), Plant
Physiol. 106 (1994) 187 – 193.
[32] N. Horemans, H. Asard, P. Van Gestelen, R.J. Caubers, Facilitated diffusion drives transport of oxidised ascor-bate molecules into purified plasma membrane vesicles of
Phaseolus6ulgaris, Physiol. Plant. 104 (1998) 783 – 789. [33] H. Vanacker, T.L.W. Carver, C.H. Foyer,
Pathogen-in-duced changes in the antioxidant status of the apoplast in barley leaves, Plant Physiol. 117 (1998) 1103 – 1114. [34] T. Otter, A. Polle, Characterization of acid and basic
apoplastic peroxidases from needles of Norway spruce (Picea abiesL., Karsten) with respect to lignifying sub-strates, Plant Cell Physiol. 38 (1997) 595 – 602.
[35] B.Z. Siegel, Plant peroxidases — an organismic perspec-tive, Plant Growth Regul. 12 (1993) 303 – 312.
[36] L.M. Lagrimini, Wound-induced deposition of polyphe-nols in transgenic plants overexpressing peroxidase, Plant Physiol. 96 (1991) 577 – 583.
[37] E. Elstner, A. Heupel, Formation of hydrogen peroxide by isolated cell walls from horseradish (Armoracia lap
-athifoliaGilib.), Planta 130 (1976) 175 – 180.
[38] B. Halliwell, Lignin synthesis: the generation of hydro-gen peroxide and superoxide by horseradish peroxidase and its stimulation by manganese (II) and phenols, Planta 140 (1978) 81 – 88.
[39] R. Goldberg, A. Pang, C. Roland, C. Francesch, A.M. Catesson, Cell wall peroxidases and lignification: tissue and substrate specificity, in: J. Lobarzewski, H. Greppin, C. Perrel, T. Gaspar (Eds.), Biochemical, Molecular and Physiological Aspects of Plant Peroxidases, University M. Curie, Skolodowska Lublin, 1991, pp. 209 – 220 Uni-versity of Geneva.
[40] H. Grisebach, Lignins, in: E.E. Conn (Ed.), The Bio-chemistry of Plants, vol. 7, Academic Press, New York, 1981, pp. 457 – 478.
[41] S.C. Fry, Cross-linking of matrix polymers in the grow-ing cell wall of angiosperms, Annu. Rev. Plant Physiol. 37 (1986) 165 – 186.
[42] D.J. Bradley, P. Kjillbom, C.J. Lamb, Elicitor and wound induced oxidative cross linking of a proline rich plant cell wall protein: a novel, rapid defence response, Cell 70 (1992) 20 – 30.
[43] T. Gaspar, C. Penel, F.J. Castillo, H. Greppin, A two-step control of basic and acidic peroxidases and its significance for growth and development, Physiol. Plant. 64 (1985) 418 – 423.
[44] F.R. Tadeo, E. Primo Millo, Peroxidase activity changes and lignin deposition during the senescense process in citrus stigmas and styles, Plant Sci. 68 (1990) 47 – 56. [45] E.J. Pell, C.D. Schlagnhaufer, R.N. Arteca,
Ozone-in-duced oxidative stress: mechanism of action and reac-tion, Physiol. Plant. 100 (1997) 264 – 273.
[46] F.J. Castillo, Extracellular peroxidases are markers of stress?, in: H. Greppin, C. Penel, T. Gaspar (Eds.), Molecular and Physiological Aspects of Plant Peroxi-dases, University of Geneva, Geneva, 1986, pp. 419 – 426.
[47] L.M. Lagrimini, J. Vaughn, A. Erb, S.A. Miller, Perox-idase overproduction in tomato: wound-induced polyphenol deposition and disease resistance, Hort Sci. 28 (1993) 218 – 221.
[48] A. Polle, T. Pfirrmann, S. Chakrabarti, H. Rennenberg, The effects of enhanced ozone and enhanced carbon dioxide concentrations on biomass, pigments and an-tioxidative enzymes in spruce needles (Picea abies L.), Plant Cell Environ. 16 (1993) 311 – 316.
[49] U. Takahama, T. Oniki, Regulation of peroxidase-de-pendent oxidation of phenolics in the apoplast of spinach leaves by ascorbate, Plant Cell Physiol. 33 (1992) 379 – 387.
[50] A. Vianello, M. Zancani, G. Nagy, F. Macrı`, Guaiacol peroxidase associated to soybean root plasma membrane oxidized ascorbate, J. Plant Physiol. 150 (1997) 573 – 577. [51] K. Iiyama, B.T. Lam, B.A. Stone, Covalent cross-link in
the cell wall, Plant Physiol. 104 (1994) 315 – 320.