Summary Maturation in conifers includes several distinct and persistent changes in the growth habits of apical meristems. Despite many studies on maturation in conifers, there are still many aspects of the process that have not been elucidated. For example, it is not known why maturation of cotyledon-derived tissue culture plantlets is rapid, whereas the natural maturation process is gradual. Also, it is not known whether rejuvenation occurs as a result of mature cells reverting to the juvenile state, or whether rejuvenation results from selective multiplication of cells that have never matured. In this paper, I review the primary causes of maturation in woody plants, with emphasis on gene expression and its role in cell determination during the maturation process. Recent experiments demonstrating both accelerated maturation and apparent rejuvenation in several woody species are discussed with reference to several matura-tion models.
Keywords: apical meristems, cell determination, gene expres-sion, growth, maturation models, tissue culture plantlets.
Introduction
Maturation (also called phase change or ontogenetic aging) in woody plants has received renewed attention over the last 15 years as a result of efforts to effect either flowering or vegeta-tive propagation of woody plants. These efforts have met with limited success because vegetative propagation of woody plants becomes increasingly difficult with age and juvenile trees can rarely be induced to flower. Thus, a detailed under-standing of the maturation process is essential to the develop-ment and evaluation of methods for rejuvenating tissue and enhancing flowering. Maturation is an integral part of the life cycle of all vascular plants. Four phases of maturation have been recognized (Greenwood 1987, Poethig 1990): (1) the embryonic phase, (2) the post-embryonic juvenile vegetative phase, (3) the adult vegetative phase, and (4) the adult repro-ductive phase. In woody plants, maturation is associated with decreased growth rates during phases 2--4 (which often persist in vegetative propagules), increased plagiotropism, and changes in reproductive competence, branching characteristics and foliar morphology. In addition to morphological changes, there are numerous physiological and biochemical changes that accompany the transition to the adult phases (see reviews by Hackett 1985, Zimmerman et al. 1985, Greenwood 1987, Pierik 1990, Poethig 1990, Haffner et al. 1991, Bonga and von
Aderkas 1993, Greenwood and Hutchinson 1993).
Maturational changes are particularly persistent and difficult to reverse in conifers, as has been demonstrated in several species by following the growth of grafted scions from ortets of different ages for several years. In these studies, matura-tional characteristics were observed without the confounding effects of size, because the grafted plants were initially the same height. In radiata pine (Pinus radiata D. Don), loblolly pine (Pinus taeda L.), eastern larch (Larix laricina (Du Roi) C. Koch) and Douglas-fir (Pseudotsuga menziesii (Mirb.) Franco), height, diameter growth and number of branches per unit of stem length all decrease with increasing ortet age, with the most pronounced decline occurring between ages 1 and 4 years (Sweet 1973, Greenwood 1984, Greenwood et al. 1989, Ritchie and Keeley 1994). The proximity of mature scions to juvenile rootstocks does not result in rejuvenation, because some mature characteristics persist several years after grafting. Thus, these grafting studies demonstrate that maturation re-sults in changes in the growth habits of the apical meristem that persist even when the mature meristem is re-exposed to physi-ological conditions associated with a young plant, including inputs from a juvenile rootstock. Trewavas (1983) argues that meristems ‘‘behave very much like individual organisms,’’ and the studies described above show that the mature behavior of grafted meristems is to some degree autonomous and resists the effects of changed environmental inputs. Thus, a plant can be considered a colony of semi-autonomous meristems, simul-taneously competing with one another, but serving the whole plant over time by subtly altering their behavior.
Maturation: meristems change their habits
Maturation involves changes in the habitual behavior of mer-istems, where habit is defined as a behavior pattern that devel-ops in response to a particular set of physiological inputs. By definition, habits tend to resist change, but nonetheless can be altered in response to varying environmental inputs. The earli-est maturation event is the polarization of the embryo into roots and shoots, where each meristem, starting with the same genes, acquires unique habits adapted to different environments. In shoots, the meristems continue to develop new habits as the plant grows. Over time, the meristems of conifers change their behavior, losing regenerative potential and capacity for vege-tative growth, but gaining reproductive competence and more massive leaves, which are better adapted to the increasing
Juvenility and maturation in conifers: current concepts
MICHAEL S. GREENWOOD
Department of Forest Ecosystem Science, College of Natural Resources, Forestry and Agriculture, University of Maine, Orono, ME 04469, USA
Received August 10, 1994
environmental stresses associated with increased height (Hutchison et al. 1990). Insect and amphibian metamorphoses provide spectacular examples of adaptive morphological and behavioral change in the living world, and occur in response to environmental and hormonal cues. The causes of maturation in plants are less well defined.
Attempts to reverse maturation and restore the regenerative competence associated with the juvenile state have met with varying degrees of success (Greenwood 1987, Bonga and von Aderkas 1993). Although regenerative competence has been temporarily restored by a variety of cultural treatments, other maturational traits may remain unchanged. Hackett (1985), Greenwood (1987) and Pierik (1990) all conclude that the criteria for maturation or rejuvenation must not be confined only to increased regenerative ability or qualitative observa-tions of increased vigor (e.g., Huang et al. 1992). There are many maturational characteristics that appear to change inde-pendently of one another (Poethig 1990). Also, a temporary increase in vigor (reinvigoration) may be a response to changed cultural conditions, rather than true rejuvenation. Consequently, we cannot assume that the apparent rejuvena-tion of one trait, like rooting ability, will be accompanied by long-term rejuvenation of other important traits, like growth rate.
Maturation: changes in gene expression
If the polarization of roots and shoots is a maturational event, then maturation is clearly associated with changes in gene expression. Recently, maturational changes in leaf morphol-ogy (heteroblasty) have been used to examine the role of gene expression in maturation (Poethig 1990). In eastern larch, leaf weight/leaf area and chlorophyll content increase with matu-ration, and net photosynthesis also increases as a result of the increase in chlorophyll content (Greenwood et al. 1989, Hutchison et al. 1990); however, among individual trees, there is no correlation between changes in leaf weight/leaf area and chlorophyll content. In English ivy (Hedera helix L.), leaf weight/leaf area and net photosynthesis increase with matura-tion, but chlorophyll content does not change (Bauer and Bauer 1980). In red spruce (Picea rubens Sarg.), mature nee-dles are much larger than juvenile neenee-dles and exhibit less net photosynthesis per unit area, which is associated with de-creased stomatal conductance (Rebbeck et al. 1992).
In both larch and English ivy, a comparison of cDNA librar-ies made from RNA extracted from juvenile and mature foliage indicates that the types of polyA-RNA (mRNA) produced are mostly similar (Hutchison et al. 1988, Hackett et al. 1991); however, there are some differences in gene expression be-tween juvenile and mature shoots. In both larch and English ivy, the expression of sequences for the chlorophyll a/b binding protein (cab) gene decreases with maturation (Hutchison et al. 1990, Woo et al. 1994). Some other exceptions include the gene for dihydroflavanol reductase (DFR) in English ivy, where anthocyanin is only produced in the petioles of juvenile leaves. The lack of anthocyanin in mature petioles is due to the absence of DFR in the petioles, which in turn is due to lack of
expression of the DFR gene (Murray and Hackett 1991, Mur-ray et al. 1994b). In addition, the proline-rich protein (PRP) gene is expressed more strongly in mature petioles than in juvenile petioles, as indicated by a five- to tenfold increase in the concentrations of PRP mRNA (Woo et al. 1994). Because the expression of the PRP gene occurs in cells associated with root regeneration in the petiole, Murray et al. (1994a) propose that elevated expression of this gene may inhibit root meristem regeneration. Thus, the available evidence (see also studies reviewed in Poethig 1990 and Haffner et al. 1991) indicates that maturation is associated with limited but perhaps signifi-cant changes in gene expression.
Maturation: origin of the mature state
Although the time course and stability of maturation have been characterized in several species, it is very difficult to separate cause from effect during maturation. Hence, we can only speculate about the primary causes of maturation, and how they affect development. Haffner et al. (1991) conclude that phytohormones regulate maturation and suggest that the ratios of abscisic acid (ABA), gibberellins (GAs), auxins and cyto-kinins, rather than their absolute concentrations play a primary role in causing maturational change. Although phytohormones probably do affect changes in the maturation state, changes in their relative concentrations may, like morphological charac-teristics, be symptoms of a more fundamental driving force. The finding that exogenously applied GA3 can cause reversion
of mature English ivy to the juvenile state (Robbins 1957) led to the hypothesis that GA3 is important in the maturation
process. The observation that GA4/7 promotes flowering in
most genera of the Pinaceae suggests that GA promotes matu-ration in conifers (Zimmerman et al. 1985). However, a good argument can be made that the opposite is true. The first flowering observed in young spruces and pines is usually exclusively female, with the ratio of males to females increas-ing steadily with age and size, and GA4/7 usually is much more
effective in promoting the production of female strobili than the production of male strobili (Greenwood and Hutchison 1993). Also, in eastern larch, juvenile scions produce a larger total number of strobili than mature scions, and most of the strobili are female. Mature scions of eastern larch are more responsive to GA4/7 than juvenile scions, but only female
flowering is significantly promoted (Eysteinsson and Green-wood 1993). Therefore, if the production of predominantly female flowers is associated with an immature habit, then any treatment that increases the ratio of female to male flowers must also promote some degree of rejuvenation.
the regulation of hormone metabolism or the mediation of tissue responses to them, and changes in gene expression during maturation have been recently demonstrated, they too may be only symptoms, rather than causes, of maturation itself. At present, we cannot rule out epigenetic factors as the primary cause of maturation (Bonga 1982, von Aderkas and Bonga 1993).
Maturation models
Models developed to account for maturation must consider a variety of causal mechanisms and must account for the matu-rational phenomena that have been demonstrated experimen-tally for several woody plant species. Five major considera-tions are discussed.
(1) The onset of the mature state is usually gradual, but can be abrupt. For example, grafting studies on loblolly pine and eastern larch have shown that maturational change is gradual for most traits, except for plagiotropism and branch frequency (Greenwood 1984, Greenwood et al. 1989). However, in sev-eral conifers, abrupt maturation has been observed immedi-ately following tissue culture propagation from adventitious buds induced on the cotyledons of mature embryos. The best example of this so-called accelerated maturation has been documented in field tests of plantlets and seedlings from the same half-sib families of loblolly pine, where the cotyledon-derived plantlets exhibit slower growth, earlier flowering and reduced branch number after four growing seasons than the seedlings (McKeand 1985, Frampton and Isik 1987). Reduced growth rate by cotyledon-derived plantlets of Virginia pine (Pinus virginiana Mill.) relative to seedlings has also been reported (Aimers-Halliday et al. 1991). In addition, Gronroos et al. (1993) reported that rooted adventitious shoots from zygotic embryos of lodgepole pine (Pinus contorta Dougl. ex Loud.) did not grow as fast as seedlings.
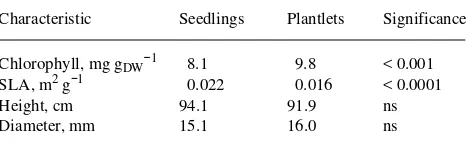
Isogenetic plantlets and seedlings have been prepared from both Douglas-fir (Ritchie et al. 1994) and hybrid larch (Smith, Weber, Hutchison and Greenwood, unpublished data) by re-moving a few cotyledons from a new germinant and inducing buds on them by treatment with the cytokinin benzylamino-purine (BAP). After elongation, the buds were rooted and the resultant plantlets were then compared with the donor seed-lings or cuttings rooted from them. In Douglas-fir, plantlets and rooted cuttings were obtained from the same clones within each of six full-sib families and grown for two years in the field. The plantlets grew significantly less than their rooted cutting ramets. In a similar study, a comparison of the foliage of plantlets and seedlings from four genotypes of a Larix × eurolepis A. Henry hybrid indicated that plantlet foliage exhib-ited significantly reduced specific leaf area compared with seedling foliage (Table 1). In addition, the plantlet foliage exhibited reduced expression of the cab gene relative to seed-ling foliage, corresponding to the differences between juvenile and mature foliage of eastern larch reported by Hutchison et al. (1990). There were too few plantlets or seedlings to assess the significance of differences in height or diameter growth.
Whether the accelerated maturation described in these five
species is due to the origin of the tissue (cotyledons) or the result of exposure to cytokinin or some other stress during bud induction is not known. Cotyledons are determinate organs in that they only persist for a few weeks and then die. They appear to retain regenerative potential while alive, but their determi-nate growth may be associated with the premature onset of mature characteristics in plants regenerated from them. Mature foliar characteristics of somatic seedlings of red spruce have been reported (Nsangou 1994). In addition, Bonga and von Aderkas (1993) report plagiotropic growth by a European larch (Larix decidua Mill.) somatic seedling and several of its ramets that had been regenerated from haploid tissue derived from the megagametophyte. Although cytokinin and 2,4-D were used to produce the embryogenic tissue in red spruce, only 2,4-D was used to induce the embryogenic tissue in European larch. Although these last two observations are pre-liminary, they suggest the need to consider the role of tissue culture stress in the induction of mature growth behavior.
(2) Maturation affects a wide variety of morphological, physiological and biochemical traits, but these traits appear to vary independently of one another (examples discussed above).
(3) Maturational traits are often persistent, and their mainte-nance is not always a function of tree size or proximity to roots. Differences in chlorophyll content, leaf weight/leaf area and xylem morphology among eastern larch scions from trees of different ages grafted at the same time persist for several years (Greenwood 1984, Takemoto, Greenwood and Hutchinson, unpublished data). On the other hand, there is anecdotal evi-dence that mature morphological characteristics disappear quickly after cuttings of Populus and Eucalyptus species are grafted or rooted.
(4) There is evidence that the cells of the apical meristem itself become determined in some woody plants. Grafted api-ces from mature plants of Citrus or Sequoiadendron consisting Table 1. Comparison of maturation characteristics of four pairs of isogenetic plantlets and seedlings of hybrid larch (Larix × eurolepis). Cotyledons were excised from 2--3-week-old seedlings without sacri-ficing the seedlings in January 1990. Shoots were induced on excised cotyledons cultured on BL medium (McLaughlin and Karnovsky 1989) with either 2.4 g l−1 Ca(NO3)2 or 1.5 g l−1 glutamine as N source
and exposed to 10 mg l−1 benzyladenine for 14--21 days. Shoots were transferred to hormone-free WPM medium for elongation and were rooted on 1/2 strength WPM + 5 mg l−1 indole-3-butyric acid, then transferred to soil in the same greenhouse where the original seedlings were growing in March 1990. The isogenetic seedlings and plantlets were overwintered in an unheated greenhouse, and specific leaf area and chlorophyll concentration were measured in July 1991, and height and diameter were measured in November 1991 as described in Hutchison et al. 1990.
Characteristic Seedlings Plantlets Significance
Chlorophyll, mg gDW−1 8.1 9.8 < 0.001 SLA, m2 g−1 0.022 0.016 < 0.0001
Height, cm 94.1 91.9 ns
of only the apical meristem and one to two leaf primordia grow out into plants that have mature characteristics (Navarro et al. 1975, Monteuuis 1991).
(5) Rejuvenation methods bring about gradual reversion to the juvenile condition. For example, Huang et al. (1992) report that a gradual increase in rooting ability occurs during serial regrafting of shoots from mature trees of Sequoia sempivirens (D. Don) Endl. However, Monteuuis (1991) reports rejuvena-tion of a single grafted apex (from a total of 300 attempts) after only one grafting in Sequoiadendron giganteum (Lindl.) Buchh.
Both Greenwood and Hutchinson (1993) and Hackett (1985) contend that maturation involves cellular determination at some point. But whether the cells within the dome of the apical meristem themselves are determined is not certain. Poethig (1990) argues that the transition to the mature state is ‘‘specified by a series of independently regulated, overlapping programs that modify the expression of a common set of processes required for shoot growth.’’ He contends that matu-ration at the level of cell determination is regulated by factors both intrinsic and extrinsic to the cells of the apical meristem itself, but the onset of maturational characteristics is initiated by factors extrinsic to the apical meristem. Thus, the expres-sion of the mature state is a function of both changes in the apical meristem and changed inputs to that meristem as a result of its increasing size and complexity. However, serial propaga-tion of Norway spruce (Picea abies (L.) Karst.) prolongs the juvenile state, but maturation occurs slowly, even though plant size and complexity have been limited (St. Clair et al. 1985). Poethig’s model accounts well for the seeming independence of the expression of different maturation phenomena in the same plant. But his conclusions are mainly based on evidence from herbaceous annual plants, and thus may not account for the persistence of the mature state observed in woody plants. The possibility that the onset of maturation also requires intrin-sic changes in the meristem itself, perhaps driven by the ability to count cell divisions, must also be considered (e.g., Green-wood and Hutchison 1993). Whatever the nature of the intrin-sic change, extrinintrin-sic factors, like the physiological consequences of reaching a critical size, are usually also re-quired for the expression of mature characteristics in normal plants. In the case of accelerated maturation, very young plan-tlets appear juvenile, and mature characteristics are not evident until some vegetative growth has occurred.
Hackett et al. (1992) propose that there are a number of ‘‘switches,’’ either in series or in parallel, or both, that must be activated for the mature state to occur. Several switches oper-ating in parallel would also account for independent onset of mature traits. I contend that a simple model involving only one switch can account for the complex phenomenon of maturation (Greenwood 1987). The switch could reside within individual cells in the apical meristem, and once activated would make that cell mature, so that over time the apex would become a mosaic with an ever increasing percentage of mature cells. Expression of mature characteristics would, therefore, be a function of the ratio of juvenile to mature cells in the apex at a given time. Rejuvenation would result from conditions that favor more rapid multiplication of juvenile cells, which would account for the gradual rejuvenation described by Huang et al. (1992) and others (see Greenwood 1987). Monteuuis’ (1991) report of abrupt rejuvenation of a single sequoiadendron shoot may reflect the presence of an abnormally high number of juvenile cells in one of the meristems (of 300) that he micro-grafted. A comparison of three different types of maturation models in terms of the five characteristics of woody plant maturation described above is shown in Table 2.
A model that successfully explains the primary cause(s) of maturation must also account for natural rejuvenation, which accompanies sexual reproduction. Because plants do not have germ lines specialized for the production of gametes, sexual reproduction begins with cells from the somatic plant body. Rejuvenation may be associated with the formation of gam-etes, but the finding that adventitious embryos in Citrus arise from diploid cells of the nucellus suggests that either somatic cells in the ovule have themselves become rejuvenated, or some of them have never become mature (see discussions in Zimmerman et al. 1985, Pierik 1990). Jorgensen (1991) re-ported that somatic embryos derived from callus originating from the anthers of Aesculus and Quercus were both diploid and haploid, suggesting that some of the embryos came from maternal diploid cells or are the result of subsequent deploidi-zation as described by von Aderkas and Bonga (1993). If the ability to form somatic embryos is a characteristic of truly juvenile cells (as suggested by Greenwood 1987), then these observations provide evidence for the presence of juvenile cells of unknown origin in mature plant parts. In conifers, attempts to obtain explants from reproductive tissue that will regenerate plants have to date been unsuccessful (e.g., Wang et
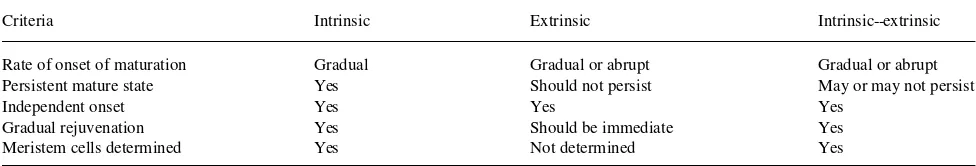
Table 2. Evaluation of three maturation models in terms of five criteria based on experimental studies of woody plant maturation. The intrinsic model assumes that cells in the apex become determined in a mature state. The extrinsic model assumes that meristem cells are not determined, but differentiate into the mature state in the subapical zone in response to inputs external to the apex. The intrinsic--extrinsic model assumes an interaction between partly determined meristematic cells and external inputs.
Criteria Intrinsic Extrinsic Intrinsic--extrinsic
Rate of onset of maturation Gradual Gradual or abrupt Gradual or abrupt
Persistent mature state Yes Should not persist May or may not persist
Independent onset Yes Yes Yes
Gradual rejuvenation Yes Should be immediate Yes
al. 1991). The existence of juvenile cells in mature plant parts may be due to in situ rejuvenation or the presence of a mecha-nism for selectively activating vestigial juvenile cells during the formation of reproductive structures.
If mature cells, such as those in the petioles of English ivy, which do not express DFR, possess DNA sequences (perhaps altered regulatory sequences) that differ from those in corre-sponding juvenile cells, then the creation of a maturation marker that can identify individual mature cells is possible. However, if the differential expression of the DFR gene is due to an epigenetic factor such as DNA methylation, then the identification of a marker for mature cells remains difficult.
Acknowledgment
This manuscript is Paper No. 1923 of the Maine Agricultural Experi-ment Staton.
References
Aimers-Halliday, J., C.R. McKinley and R.J. Newton. 1991. Clonal propagation and genetic testing of Virginia pine. In Proc. 21st South. Tree Improve. Conf. National Tech. Info. Service, Spring-field, VA, pp 161--168.
Bauer, H. and U. Bauer. 1980. Photosynthesis in leaves of the juvenile and adult phase of ivy (Hedera helix). Physiol. Plant. 49:366--372. Bonga, J.M. 1982. Vegetative propagation in relation to juvenility, maturity, and rejuvenation. In Tissue Culture in Forestry. Eds. J.M. Bonga and D.J. Durzan. Martinus Nijhoff/Dr. W. Junk, The Hague, pp 387--412.
Bonga, J.M. and P. von Aderkas. 1993. Rejuvenation of tissues from mature conifers and its implications for clonal propagation in vitro.
In Clonal Forestry: Genetics, Biotechnology and Application. Eds. M.R. Ahuja and W.J. Libby. Springer Verlag, New York, pp 182--199.
Eysteinsson, T. and M.S. Greenwood. 1993. Effects of maturation and gibberellin A4/7 on flowering and branching characteristics of Larix
laricina. Can. J. For. Res. 23:14--20.
Frampton, L.J. and K. Isik. 1987. Comparison of field growth among loblolly pine seedlings and three types produced in vitro. Tappi J. 70:119--123.
Greenwood, M.S. 1984. Phase change in loblolly pine: shoot develop-ments as a function of age. Physiol. Plant. 61:518--522.
Greenwood, M.S. 1987. Rejuvenation of forest trees. Plant Growth Regul. 6:1--12.
Greenwood, M.S. and K.W. Hutchison. 1993. Maturation as develop-mental process. In Clonal Forestry: Genetics, Biotechnology and Application. Eds. M.R. Ahuja and W.J. Libby. Springer-Verlag, New York, pp 14--33.
Greenwood, M.S., C.A. Hopper and K.W. Hutchison. 1989. Matura-tion in larch. I. Effect of age on shoot growth, foliar characteristics, and DNA methylation. Plant Physiol. 90:406--412.
Gronroos, R., G. Flygh, M. Kahr and S. von Arnold. 1993. Growth analyses on in vitro, ex vitro and auxin-rooted hypocotyl cuttings of
Pinus contorta Dougl. ex Loud. New Phytol. 125:829--836. Hackett, W.P. 1985. Juvenility, maturation, and rejuvenation in woody
plants. Hortic. Rev. 7:109--155.
Hackett, W.P., J.R. Murray and A. Smith. 1992. Control of maturation in woody species. In Mass Production Technology for Genetically Improved Fast Growing Forest Tree Species, Syntheses. AFOCEL, Paris, France, pp 45--50.
Hackett, W.P., J. Murray and H. Woo. 1991. Cellular, molecular and biochemical analysis of maturation related characteristics in Hed-era helix. In Woody Plant Biotechnology. Ed. M.R. Ahuja. Plenum Press, New York, London, pp 77--82.
Haffner, V., F. Enjalric, L. Lardet and M.P. Carron. 1991. Maturation of woody plants: a review of metabolic and genomic aspects. Ann. Sci. For. 48:615--630.
Huang, L., L. Suwenza, B. Huang, T. Murashige, E.F.M. Mahdi and R.V. Gundy. 1992. Rejuvenation of Seqouia sempivirens by re-peated grafting of shoot tips onto juvenile rootstocks in vitro. Plant Physiol. 98:166--173.
Hutchison, K.W., P.B. Singer and M.S. Greenwood. 1988. Molecular genetic analysis of development and maturation in larch. In Mo-lecular Genetics of Forest Trees. Petawawa National For. Res. Inst., Chalk River, Ontario, Canada, No. P1-X-80, pp 26--33.
Hutchison, K.W., C.B. Sherman, M.S. Greenwood, J. Weber, S.S. Smith, P.B. Singer and M.S. Greenwood. 1990. Maturation in larch. II. Effects of age on photosynthesis and gene expression in devel-oping foliage. Plant Physiol. 94:1308--1315.
Jorgensen, J. 1991. Somatic embryogenesis in Aesculus hippocas-tanum and Quercus petraea from old trees (10 to 140 years). In
Woody Plant Biotechnology. Ed. M.R. Ahuja. Plenum Press, New York, pp 351--352.
McKeand, S.E. 1985. Expression of mature characteristics by tissue culture plantlets derived from embryos of loblolly pine. J. Am. Soc. Hortic. Sci. 110:619--623.
McLaughlin, J. and D.F. Karnovsky. 1989. Controlling vitrification in
Larix decidua via culture media manipulation. Can. J. For. Res. 19:1334--1337.
Monteuuis, O. 1991. Rejuvenation of a 100-year-old Sequoiadendron giganteum through in vitro meristem culture. I. Organogenic and morphological arguments. Physiol. Plant. 81:111--115.
Murray, J.R. and W.P. Hackett. 1991. Dihydroflavanol reductase activ-ity in relation to differential anthocyanin accumulation in juvenile and mature Hedera helix L. Plant Physiol. 97:343--351.
Murray, J.R., M. Concepcion Sanchez, A.G. Smith and W.P. Hackett. 1994a. Differential competence for adventitious root formation in histologically similar cell types. In Biology of Adventitious Root Formation. Eds. T.D. Davis and B.E. Haissig. Plenum Press, New York, pp 99--110.
Murray, J.R., A.G. Smith and W.P. Hackett. 1994b. Differential dihy-droflavanol reductase transcription and anthocyanin pigmentation in the juvenile and mature phases of ivy (Hedera helix L.). Planta 194:102--109.
Navarro, L., C.N. Roistacher and T. Murashige. 1975. Improvement of shoot tip grafting in vitro for virus-free citrus. J. Am. Soc. Hortic. Sci. 100:471--479.
Nsangou, M. 1994. A comparative study of the developmental mor-phology of propagules regenerated via somatic embryogenesis of hybrid larch (Larix × eurolepis A. Henry) and red spruce (Picea rubens Sarg.). Ph.D. Thesis. Department of Forest Ecosystem Sci-ence, University of Maine, Orono, ME, 150 p.
Pierik, R.L.M. 1990. Rejuvenation and micropropagation. In Progress in Plant Cellular and Molecular Biology. Proc. of the VIIth Int. Congress on Plant Tissue and Cell Culture. Eds. H.J.J. Nikkamp, L.H.W. Van Der Plas and J. Van Aatrijk. Kluwer Academic Publish-ers, Amersterdam, Netherlands, pp 91--101.
Poethig, R.S. 1990. Phase change and the regulation of shoot morpho-genesis in plants. Science 250:923--930.
Ritchie, G.A. and J.W. Keeley. 1994. Maturation in Douglas fir. I. Changes in stem, branch and foliage characteristics associated with ontogenetic aging. Tree Physiol. 14:1245--1259.
Ritchie, G.A., S.D. Duke and R. Timmis. 1994. Maturation in Douglas-fir. II. Maturation characteristics of genetically matched Douglas-fir seedlings, rooted cuttings and tissue culture plantlets during and after 5 years of field growth. Tree Physiol. 14:1261--1275.
Robbins, W.J. 1957. Gibberellic acid and the reversal of adult Hedera
to a juvenile state. Am. J. Bot. 44:743--746.
St. Clair, J.B., J. Kleinschmidt and J. Svolba. 1985. Juvenility and serial vegetative propagation of Norway spruce clones (Picea abies
(L.) Karst.). Silvae Genet. 34:42--48.
Sweet, G.B. 1973. The effect of maturation on the growth and form of vegetative propagules of radiata pine. N.Z. J. For. Sci. 3:191--210.
Trewavas, A.J. 1983. Plant growth substances----metabolic flywheels for plant development. Cell Biol. Int. Rep. 7:569--575.
von Aderkas, P. and J.M. Bonga. 1993. Plants from haploid tissue culture of Larix decidua. Theor. Appl. Genet. 87:225--228. Wang, K.X., D.F. Karnosky and R. Timmis. 1991. Adventitious bud
production from mature Picea abies: rejuvenation associated with female strobili formation. In Woody Plant Biotechnology. Ed. M.R. Ahuja. Plenum Press, New York, pp 83--90.
Woo, H.H, W.P. Hackett and A. Das. 1994. Differential expression of a chlorophyll a/b binding protein gene and a proline rich protein gene in juvenile and mature phase English ivy (Hedera helix). Physiol. Plant. 92:69--78.