Low temperature-induced modifications of cell wall content and
polysaccharide composition in leaves of winter oilseed rape
(
Brassica napus
L. var.
oleifera
L.)
Maria Kubacka-Ze˛balska, Alina Kacperska *
Institute of Experimental Plant Biology,Warsaw Uni6ersity,Pawin´skiego5A,PL-02-106Warsaw,Poland Received 28 December 1998; received in revised form 4 May 1999; accepted 24 June 1999
Abstract
Pronounced modifications in cell wall content and polysaccharide composition were observed in the young leaves of winter oilseed rape plants (Brassica napus L. var. oleifera L. cv Jantar) grown for 3 weeks in cold (2°C) and then exposed to a brief freezing and thawing treatment (−5°C for 18 h). In the cold-grown leaf blades, the increased cell wall content was associated with higher levels of non-covalently bound pectins. The content of galactose, arabinose and glucose in pectins and of galactose and arabinose in hemicelluloses also increased in these leaves. Exposure of cold-acclimated plants to a freeze-thaw treatment resulted in decreased cell wall content, reduced levels of non-covalently bound pectins and decreased contents of xylose and glucose in the hemicellulose fraction. Our findings indicate that the pattern of modifications of cell wall content and polysaccharide composition depends on the range of temperatures the plant has been exposed to. The role of the cell wall in cold-acclimated and freezing treated plants is discussed. © 1999 Elsevier Science Ireland Ltd. All rights reserved.
Keywords:Brassica napusvar.oleifera; Cell wall; Cold; Freezing; Polysaccharides; Winter oilseed rape leaves
www.elsevier.com/locate/plantsci
1. Introduction
Prolonged growth of chilling-resistant herba-ceous plants at temperatures just above 0°C results in the modification of their growth pattern [1] and the adjustment of cellular metabolism to low tem-perature conditions, i.e. in acclimation of plants to the cold [2]. Another attribute of plant acclimation in cold is increased resistance of cells to extracellu-lar freezing [3]. This property is further increased
by a short exposure of plants to sub-zero tempera-tures [3 – 5].
Aside from other factors, resistance of plant cells to freezing depends on the presence of cell walls [6,7]. Rajashekar and Burke [8] showed that the movement of water from cells during extracel-lular freezing and its consequences, cell dehydra-tion and cell collapse, depend on cell wall mechanical properties. It has been proposed that cell wall rigidity may be an important factor in cell resistance to freeze-induced dehydration [9].
The composition of cell wall polysaccharides has been shown to be an important component of plant adaptive responses to factors inducing cell dehydration [10 – 13]. However, information on the low temperature-induced modifications in cell wall composition is very limited. In the cold-grown plants or suspension cultures, cell wall thickening [14 – 16], deposition of extracellular callose [17], accumulation of lipid polymers in cell walls [18] or Abbre6iations: CA, cold-acclimated leaves; CAF1, cold-acclimated
leaves, subjected to a transient freezing and recovered at 2°C in the dark for 6 h; CAF2, cold-acclimated, prefrozen leaves, subjected to
20-h recovery at 2°C; CDTA, trans-1,2-diaminocyclohexane-N,N,N%,N-tetraacetic acid; DMSO, dimethyl sulfoxide; EDTA, ethylenediaminetetraacetic acid; GC, gas chromatography; NA, non-acclimated leaves, sampled at the start of acclimation treatment; TFA, trifluoroacetic acid.
* Corresponding author. Tel.: +48-22-6596072, ext. 5716/5717; tel./fax: +48-22-6584804.
E-mail address:[email protected] (A. Kacperska)
a deposition of silica [19], were observed. Such modifications are thought to affect water balance and the pattern of ice propagation in plant tis-sue rather than the ability of cell walls to un-dergo deformations.
Our previous experiments performed on cold-grown or on frost pretreated winter oilseed rape leaves indicated that the activity of cell wall-as-sociated b-galactosidase decreases in cold-accli-mated winter oilseed rape leaves, whereas it increases rapidly in response to a brief freezing treatment [20]. The enzyme is responsible for the breakdown of b-galactosyl linkage in pectin and hemicellulosic polysaccharides [21]. It is involved in the breakage of bonds between cell wall polysaccharides during cell wall loosening [21,22] and in the degradation of pectic polymers of galactose during cell growth [23]. The freezing-induced increase in cell capability for the turgor-dependent extension growth was actually observed in our previous work [5]. Therefore, it may be anticipated that cell wall properties will differ between cold-acclimated and freeze-af-fected tissues. The major objective of the present experiments was to verify this supposition by ex-amination of cell wall content and polysaccha-ride composition in winter rape oilseed rape plants subjected to cold (\0°C) and freezing
(B0°C) treatments.
2. Materials and methods
2.1. Plant material
Plants of winter oilseed rape (Brassica napus L. var. oleifera L. cv Jantar) were grown in a mixture of sand and peat (1:1, v/v), under a 16-h photoperiod, as described earlier [24] at 20/15°C day/night temperature for 3 weeks (non-accli-mated (NA) plants). Light was provided by cool white and daylight fluorescence tubes ( Pil*a, Poland and Tungsram, Hungary, respectively) in proportion 1:1, the level of photosynthetic active radiation at the top of a plant being 200 mmol m−2 s−1. After 3 weeks of growth, half of the plants were transferred to an acclimation cham-ber at 2°C for 21 days, the light conditions be-ing unchanged (cold-acclimated (CA) plants). Then, CA plants were transferred for 18 h to a freezing chamber (Dual Program Illuminated
In-cubator 818, Precision Scientific, USA) set at −
5°C. Freezing treatment was followed by plant thawing and a recovery at 2°C for 6 or 24 h in darkness (CAF1 and CAF2 plants, respectively). Such a treatment was found previously to in-crease freezing tolerance of the cold-grown win-ter oilseed rape leaves for 3 – 5 K and to increase the growth capability of leaf blades [5]. Samples for analyses were cut from the blades of the fourth and fifth leaves, the expansion of which took place during the experiment and resulted in eight-fold or two-fold increase of blade surface in NA or CA plants, respectively. All analyses were performed on leaf discs (2 cm in diameter) cut from leaf areas located between the lateral veins.
2.2. Cell wall isolation
The samples of leaf discs (in three replicates, 5 g fresh weight each) were frozen in liquid nitro-gen. Cell wall isolation and purification was per-formed according to Waldron and Selvendran [25], using boiling 85% ethanol as the extraction medium. In preliminary experiments, the cell wall yield from the ethanol-extracted material was compared with that obtained from benzene-or K-phosphate buffer extracted tissues. The ethanol-extracted cell wall preparations were sub-jected to 90% (v/v) dimethyl sulfoxide (DMSO) treatment for 24 h at 20°C to remove starch [26]. The starch-free (as checked with an iodine test) cell wall material was washed free of resid-ual DMSO by several washings with 95% ethanol. Samples were evaporated to dryness and weighed.
2.3. Fractionation of cell wall polysaccharides
2.3.1. Pectic substances
pres-sure and weighed. The CDTA-insoluble residues were used for extraction of hemicelluloses.
In an independent set of experiments, pectic substances of the cell walls were fractionated into three subfractions, according to Iraki et al. [11]. Cell wall preparations (100 mg) were extracted sequentially as follows: once with 10 ml ice-cold 5 mM ethylenediaminetetraacetic acid (EDTA) for 12 h with constant stirring, twice with 10 ml of 0.5% ammonium oxalate (pH 6.5) at 100°C for 1 h each, and once with 3.5 ml of 0.1 M KOH for 4 h. The EDTA and ammonium oxalate solutions each extracted pectic substances by chelation of Ca2+
crosslinking the galacturonic acid units, and the 0.1 M KOH removed additional pectic substances by hydrolysis of ester linkages or other weak alkali labile bonds [28].
2.3.2. Hemicelluloses
The cell wall residues, left after CDTA extrac-tion, were treated with sodium chlorite (1%) to remove lignins which interfere with the extraction of hemicellulosic substances. Then, they were ex-tracted three times with 4 M KOH (100 ml g−1 cell wall preparation), supplemented with NaBH4 (3 mg l−1), at 25°C [27]. The combined KOH extracts, acidified to pH 5.0 with glacial acetic acid, were dialysed against deionised water overnight and evaporated under reduced pressure. The mass of air-dry samples were determined by weighing. No uronic acid was left in the hemicellu-lose fractions as checked with the uronic acid assay (see below).
2.3.3. Cellulose content
The KOH-insoluble residues were centrifuged at 2000×g for 15 min. The pellets were washed with deionised water, weighed and hydrolysed in 14 M sulphuric acid for 1 h at room temperature and then in 1 M sulphuric acid for 2 h at 100°C [27]. The glucose content in the hydrolysate was taken as a measure of cellulose content in the sample. It was determined with the anthrone method [29] using b-D-glucose as a standard.
2.4. Determination of sugar content and composition in the pectic and hemicellulose polysaccharides
Pectin and hemicellulose samples were hy-drolysed in 2 M trifluoroacetic acid (TFA) at
125°C for 1 h. The liberated monosaccharides were reduced to their respective alditol acetates and analysed by gas chromatography (GC) with erythritol (0.5 mg (0.1 ml)−1) added as an internal standard [30]. GC analyses was performed using a Hewlett Packard 5890 gas chromatograph equipped with the flame ionisation detector, a splitless injection port, an HP 7673 autosampler and fused silica Wide Bore Open Column (WBOTC), 30 m×0.53 mm i.d. (J & W Scientific, USA), coated with 1 mm DB-Wax. The column temperature program was: 5 min at 195°C, fol-lowed by a rise to 220°C, 5°C per min. Helium was used as a carrier gas at a flow rate of 20 ml min−1.
2.5. Uronic acid content estimation
The studied fractions were hydrolysed in 2 ml of 12 M sulphuric acid at 35°C for 1 h and then in 22 ml of boiling water for 2 h. In cool hydrolysates, uronic acid contents were determined colorimetri-cally [31], with glucuronic acid as a standard. Calculation of the uronic acid concentration was based upon the difference in absorbances between 450 and 400 nm.
2.6. Statistics
All determinations were performed on three replicates, in three independently run experiments. The effects of the temperature treatment were tested by one-way or two-way analysis of variance (ANOVA). Means were compared between the treatments by the least significant difference (L.S.D.) at the 0.05 probability level using Tukey’s test.
3. Results
Fig. 1. Effects of low temperature on cell wall content in winter oilseed rape leaves. Significant differences atPB0.05
are marked with different letters, shown in diagram.
polysaccharide fractions in the studied leaves, whereas hemicellulose content was relatively low (Table 1). The share of these polysaccharide frac-tions in the total cell wall polysaccharide pool in NA leaves (taken as 100%) was 46.7, 37.1 and 16%, respectively.
Cold acclimation of leaves resulted in the pro-nounced, statistically significant increase of the pectin content in cell walls, as compared with the NA samples (Table 1). The content of this fraction decreased by 30% in response to the freeze/thaw
treatment in comparison with that in CA tissues (see data for CAF1 and CAF2 discs). The differ-ence was statistically significant. The contents of hemicellulose and cellulose fractions were not af-fected by low temperature treatments (Table 1). Sequential fractionation of pectic substances into three subfractions of different solubility in calcium/magnesium-chelating or diluted alkaline solutions yielded a total amount of pectins ex-tracted from cell walls similar to that obtained by the CDTA extraction (compare the respective data in Tables 1 and 2). The most abundant fraction consisted of the ammonium-extractable pectins (67%), whereas pectins extracted with diluted KOH showed the lowest share (7%) in the total pectin pool. Acclimation of leaves in the cold resulted in statistically significant increases in pectins extractable by ammonium oxalate or di-luted KOH. The freezing treatment brought about a pronounced decrease of these pectin subfractions to levels similar to those observed in NA leaves. The content of the EDTA-soluble subfraction in the frost-thawed cells increased about twice.
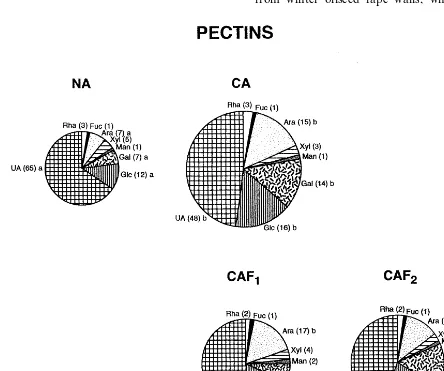
Determination of uronic acid (UA) and neutral monosaccharide contents in pectins indicated that uronic acid was the most abundant component of these polysaccharides (Fig. 2). Its content in pectins from NA leaves was 65% (i.e. 65 mg of
UA in 100 mg of pectins). Although the total content of UA in CA walls increased about twice The pilot experiments showed that there were
no differences in the cell wall content and polysac-charide composition between the non-acclimated leaf blades sampled from the plants just before the start of cold treatment and after the next 3 weeks of plant growth at warm temperature (20/15°C). Therefore, only the blades sampled just before the cold treatment were analysed in the further studies and taken as a reference to the cold-acclimated ones.
3.1. Changes in cell wall content
The content of cell wall in the cold-acclimated (CA) leaf discs was about two-fold higher than that in the non-acclimated (NA) ones (Fig. 1). The exposure of CA plants to the freeze-thaw treat-ment resulted in a statistically significant decrease of cell wall content observed after tissue recovery at 2°C, which resulted in a lower (by 23%) cell wall content in CAF2compared with the CA discs.
3.2. Changes in cell wall polysaccharide contents and composition
Pectins and cellulose were the most abundant
Table 1
Low temperature-induced changes in polysaccharide contents in leaf cell walls (mg [100 mg cell wall]−1)a
Pectins (CDTA extraction)
Treatment Hemicelluloses Cellulose Total polysaccharide pool
24.9a 8.6a
NA 19.8a 53.3
20.9a
9.2a
39.1b
CA 69.2
32.3c 8.9a
CAF1 20.1a 61.3
30.1c 9.0a
CAF2 21.3a 60.4
Table 2
Low temperature-induced modifications in pectin fractions (mg [100 mg cell wall]−1)a
Fraction
Treatment Total polysaccharide pool
Ammonium oxalate KOH (0.1 M) EDTA
aMeans marked with the same letter within the column do not differ significantly at the 0.05% probability level.
in comparison with the NA walls (Fig. 2, compare the surface areas occupied by UA in NA and CA specimens), its share in the CA pectin pool de-creased to 48%. This was due to the inde-creased concentrations of neutral sugars such as arabinose and galactose or glucose in CA pectins, as com-pared with NA pectins (Fig. 2). The high contents of arabinose and glucose were also noted in pectins in CAF1tissues, whereas galactose content markedly decreased. In consequence, the ara-binose to galactose ratio in CAF1 samples in-creased markedly (from 1:1 in NA and CA pectins to 2.5:1 in CAF1 pectins). In pectins from CAF2 discs, galactose and glucose contents increased whereas arabinose share decreased. Rhamnose, xy-lose, fucose and mannose contents were low (equal to or below 5 mg 100 mg −1 pectins) in all the studied leaves and their contents were not modified by the temperature treatments.
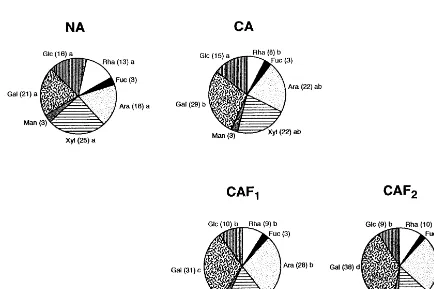
The sugar composition of hemicelluloses (Fig. 3) was less affected by cold acclimation and freezing treatment than that of pectins. Xylose, galactose, arabinose and glucose (in order of decreasing con-centration in NA samples) were the main compo-nents of hemicelluloses in winter oilseed rape leaves. They made up 80 or 85% of the total
hemicellulose sugars in NA or CA leaves, respec-tively. Low temperature (cold and freezing) treat-ments changed the relative proportions of these sugars. The contents of galactose and arabinose increased in the cold- and frost-treated leaves, changes in the arabinose content becoming statisti-cally significant in the frost-pretreated discs. The contents of xylose and glucose decreased in re-sponse to low temperature treatments, the effects of transient freezing resulting in statistically sig-nificant differences between NA and CAF1 and CAF2 discs. The content of rhamnose, relatively high in hemicelluloses from NA discs, decreased in the CA leaves; the freezing treatment was practi-cally of no further effect.
4. Discussion
Our experiments showed that the polysaccharide content in winter oilseed rape leaves amounts to
60% of the total cell wall matter (Table 1).
Therefore, it is lower than that reported for other growing cells of dicotyledons [32]. The difference is unlikely to be caused by an improper procedure of polysaccharide extraction because the prelimi-nary experiments showed that there was no differ-ence in cell wall content obtained with the use of different extraction media (ethanol, benzene or buffer). This difference may be accounted for by the presence of components other than polysac-charides, such as proteins, lipids and phenolics. Identification of these components was beyond the scope of the present studies.
In accordance with other studies [33], the dry weight of leaf cell walls increased dramatically during cold acclimation of B. napus plants. The effect was due to an increased amount of pectins in cell walls of CA leaves (Tables 1 and 2).
osmotic stresses [10]. It seems that the fraction of the Ca2+-insolubilised pectins became more
loosely bound and, hence, more easily extracted from the walls of the frost-pretreated CA leaves. The level of the pectin fraction that was bound to the cellulose-hemicellulose framework by ester linkages (broken down by a diluted KOH) was very low in the studied cell walls of NA leaves (Table 2) and similar to that found in the acetone-powdered cell walls of tobacco [10]. The opposite effects of cold and freezing treat-ments on the amount of this pectin fraction, i.e. its increase in cell walls of CA leaves but its decrease in the frost-treated tissues (Table 2), may indicate that cold and freezing temperature affect cell wall rheological properties in different ways.
In the pectins in CA plants the neutral/uronic acid sugar ratio increased, with arabinose and galactose contents increasing the most (Fig. 2). It seems therefore, that plant growth in the cold resulted in modified proportions of polygalactur-onans to rhamnogalacturpolygalactur-onans. The side-chains of rhamnogalacturonans, composed of arabinans, galactans and arabinogalactans, interact with other components of the cell wall, which rein-forces the cellulose-hemicellulose framework in cell walls of dicotyledons [34]. The marked in-crease in arabinose:galactose ratio in pectins from CAF1 discs (to 2:5) may indicate the freez-ing treatment resulted in the shortenfreez-ing of the galactan side chain in the pectins
A relatively high amount of glucose in pectins from winter oilseed rape walls, which increased
Fig. 2. Sugar composition of pectins in cell wall preparations from leaves subjected to different temperature treatments. Different circle sizes reflect the low temperature-induced changes in the total amount of pectins, in comparison with that determined in non-acclimated (NA) leaves (see Table 1 for details). The section sizes describe the participation of a given sugar in the total pool of pectins in the studied leaf. Sugar contents, in mg 100 mg−1pectins, are indicated by numbers in parentheses. Means marked
Fig. 3. Sugar composition of hemicelluloses in cell wall preparations from leaves subjected to different temperature treatments. Sugar contents, in mg 100 mg−1 hemicelluloses, are indicated by numbers in parentheses. The other descriptions are as in Fig.
2.
further in response to cold and freezing treatments (Fig. 2), might result from degradation of starch polymers, left in walls after DMSO treatment. However, such a possibility seems probable since iodine test performed on cell wall preparations (Section 2) gave negative results. It is also known that cold-acclimated and frost pretreated biennial plants accumulate monosaccharide rather than starch [35]. High contents of glucose in pectins were also found in epicotyls of Cicer arietinum [13], seedlings of Picea glauca [36,37] and melon fruits [38]. Obviously, further studies concerning the mode of linkage of glucose in pectins are required to learn what is the origin of this com-pound in pectins from different plants and tissues. The enrichment of hemicelluloses from CA and CAF leaves in galactose and arabinose (Fig. 2) may indicate that the cold and freezing treatments modify the hemicellulose structure as well. It is possible that -D-galactose and -L-arabinose are
added to -D-xylosyl units of xyloglucans [34]. A
relatively high content (13%) of rhamnose in
hemi-cellulose fractions, may also point to the structural role of rhamnoarabinogalactan in the studied cells walls, as was proposed for hemicellulose fractions of Pisum sati6um epicotyls [39].
adjust-ment to water or to cold stresses. In the osmotic stress-affected cells, the increased hemicellulose contents may prevent a cell collapse caused by dehydration [10,11,38]. In cold-acclimated plants, the increased wall capacity to bind water in pectins seems to be an important element in the preven-tion of cell dehydrapreven-tion. Modificapreven-tion of the wall porosity by the increased amount of pectic sub-stances [40] may also facilitate supercooling since water in small pores freezes at lower temperature than bulk water [41].
The results presented indicate that not only cold (\0°C), but also transient, sublethal frost brings
about pronounced modifications in cell wall con-tent and composition in leaves. It seems that the decreased cell wall content (Fig. 1), the reduced content of more strictly-bound pectins (Table 2), and lower xylose and glucose contents in hemicel-luloses (Fig. 3) may increase cell wall extensibility and allow for wall stress relaxation [32] in the frost-recovered, cold-acclimated leaf cells. This will result in reduction of turgor pressure, thereby enabling cells to take up water and expand physi-cally [32]. A higher expansion capability of winter oilseed rape leaf discs, pretreated with a transient frost, was actually observed in our previous work [5]. It is also possible that a change in tensile properties of cell wall will alleviate the mechanical stress experienced during thawing by protoplasts attached to cell walls [6]. Obviously, the effects of cold and freezing treatments on cell wall mechani-cal properties deserve further studies.
In summary, the results presented here indicate collectively that low temperature brings about im-portant modifications in cell wall content and composition and that the pattern of these modifi-cations depends on whether a plant has been exposed to temperatures above or below 0°C, i.e. it depends on the stage of plant acclimation to low temperature. The finding ought to be taken into consideration in further studies on cell wall in-volvement in plant acclimation to low temperature.
Acknowledgements
The authors thank Barbara Dobrska for her skilful technical assistance. This work was finan-cially supported by grant 6P20400807 from the State Committee for Scientific Research of Poland.
References
[1] A. Kacperska, R.K. Szaniawski, Frost resistance and water status of winter rape leaves as affected by differen-tial shoot/root temperature, Physiol. Plant. 89 (1993) 775 – 782.
[2] A. Kacperska, Metabolic consequences of low tempera-ture stress in chilling-insensitive plants, in: P.H. Li (Ed.), Low Temperature Stress Physiology in Crops, CRC Press, Boca Raton, FL, 1989, pp. 27 – 40 ISBN 0-8493-6567-8.
[3] A. Kacperska-Palacz, Mechanisms of cold acclimation in herbaceous plants, in: P.H. Li, A. Sakai (Eds.), Plant Cold Hardiness and Freezing Stress, Academic Press, New York, 1978, pp. 139 – 152 ISBN 0-12-447650-3. [4] E. Sikorska, A. Kacperska-Palacz, Phospholipid
involve-ment in frost tolerance, Plant Physiol. 47 (1979) 144 – 150.
[5] A. Kacperska, L. Kulesza, Frost resistance of winter rape leaves as related to changes in water potential and growth capability, Physiol. Plant. 71 (1987) 483 – 488. [6] D. Tao, P.H. Li, J.V. Carter, Role of cell wall in freezing
tolerance of cultured potato cells and their protoplasts, Physiol. Plant. 58 (1983) 527 – 532.
[7] A.M. Johnson-Flanagan, J. Singh, Membrane deletion during plasmolysis in hardened and non-hardened plant cells, Plant Cell Environ. 9 (1986) 299 – 305.
[8] C.B. Rajashekar, M.J. Burke, Liquid water during slow freezing based on cell water relations and limited experi-mental testing, in: P.H. Li, A. Sakai (Eds.), Plant Cold Hardiness and Freezing Stress, vol. 2, Academic Press, New York, 1982, pp. 211 – 220 ISBN 0-12-447602-3. [9] C.B. Rajashekar, A. Lafta, Cell-wall changes and cell
tension in response to cold acclimation and exogenous abscisic acid in leaves and cell cultures, Plant Physiol. 111 (1996) 605 – 612.
[10] N.M. Iraki, R.A. Bressan, P.M. Hasegawa, N.C. Carpita, Alteration of the physical and chemical struc-ture of the primary cell wall of growth-limited plant cells adapted to osmotic stress, Plant Physiol. 91 (1989) 39 – 47.
[11] N.M. Iraki, N. Singh, R.A. Bressan, N.C. Carpita, Cell walls of tobacco and changes in composition associated with reduced growth upon adaptation to water and saline stress, Plant Physiol. 91 (1989) 48 – 53.
[12] W.L. Sweet, J.C. Morrison, J.M. Labavitch, M.A. Matthews, Altered synthesis and composition of cell wall of grape (Vitis 6inifera L.) leaves during expansion and
growth-inhibiting water deficits, Plant Cell. Physiol. 31 (1990) 407 – 414.
[13] F.J. Munoz, B. Dopico, E. Labrador, Effect of osmotic stress on the growth of epicotyl of Cicer arietinum in relation to changes in cell wall composition, Physiol. Plant. 87 (1993) 552 – 560.
[14] P.M. Chen, P.H. Li, W.P. Cunningham, Utrastructure differences in leaf cell ofSolanum species in relation to their frost resistance, Bot. Gaz. 138 (1977) 267 – 285. [15] N.P.A. Huner, J.P. Palta, P.H. Li, J.V. Carter,
[16] M. Griffith, G.N. Brown, Cell wall deposits in winter rye
Secale cereale L. ‘Puma’ during cold acclimation, Bot. Gaz. 143 (1982) 486 – 490.
[17] S.J. Wallner, M. Wu, S.J. Anderson-Krengel, Changes in extracellular polysaccharides during cold acclimation of cultured pear cells, J. Am. Soc. Hortic. Sci. 11 (1986) 636 – 639.
[18] M. Griffith, N.P.A. Huner, K.E. Espelle, P.E. Kolat-tukudy, Lipid polymeres accumulate in the epidermis and mestome sheath cell walls during low temperature development of winter rye leaves, Protoplasma 125 (1985) 53 – 64.
[19] W. Larcher, U. Meindl, E. Ralser, M. Ishikawa, Persis-tent supercooling and silica deposition in cell walls of palm leaves, J. Plant Physiol. 139 (1991) 146 – 154. [20] T. Sawicka, A. Kacperska, Soluble and cell
wall-associ-atedb-galactosidases from cold-grown winter rape (Bras
-sica napus L., var. oleifera L.) leaves, J. Plant Physiol. 145 (1995) 357 – 362.
[21] A.K. Murray, S. Bandurski, Correlative studies of cell wall enzymes and growth, Plant Physiol. 56 (1975) 143 – 147.
[22] K. Keegstra, P. Albersheim, The involvement of glycosi-dases in the cell wall metabolism of suspension-cultured
Acer psueudoplatanuscells, Plant Physiol. 45 (1970) 675 – 678.
[23] H. Konno, K. Katoh, An extracellular b-galactosidase secreted from cell suspension cultures of carrot. Its purification and involvement in cell wall polysaccharide hydrolysis, Physiol. Plant. 85 (1992) 507 – 514.
[24] U. Maciejewska, A. Kacperska, Changes in the level of oxidized and reduced pyridine nucleotides during cold acclimation of winter rape plants, Physiol. Plant. 69 (1987) 687 – 691.
[25] K.W. Waldron, R.R. Selvendran, Effect of maturation and storage on asparagus (Asparagus officinalis) cell wall composition, Physiol. Plant. 80 (1990) 576 – 583. [26] R.R. Selvendran, M.A. O’Neil, Isolation and analysis of
cell walls from plant material, in: D. Glick (Ed.), Meth-ods of Biochemical Analysis, vol. 32, Wiley, New York, 1987, pp. 25 – 153 ISBN 0-471-82195-0.
[27] L.C. Greve, J.M. Labavitch, Cell wall metabolism in ripening fruit, Plant Physiol. 97 (1991) 1456 – 1461. [28] M.C. Jarvis, M.A. Hall, D.R. Threlfall, J. Friend, The
polysaccharide structure of potato cell walls: chemical fractionation, Planta 152 (1981) 93 – 100.
[29] E. Van Handel, Direct microdetermination of sucrose, Anal. Biochem. 22 (1968) 280 – 283.
[30] N.C. Carpita, E.M. Shea, Linkage structure of carbohy-drates by gas chromatography-mass spectrophotometry (GC-MS) of partially methylated alditol acetates, in: C.J. Biermann, G.D. McGinnis (Eds.), Analysis of Carbohy-drates by GLC and MS, CRC Press, Boca Raton, FL, 1989, pp. 157 – 216.
[31] H.N. Englyst, G.J. Hudson, Colorimetric method for routine measurement of dietary fibre as non-starch polysaccharides. A comparison with gas-liquid chro-matography, Food Chem. 24 (1987) 63 – 76.
[32] D.J. Cosgrove, Relaxation in a high-stress environmen-tal: the molecular bases of extensible cell wall and cell enlargement, Plant Cell 9 (1997) 1031 – 1041.
[33] R.L. Weiser, J.S. Wallner, J.W. Waddell, Cell wall and extensin mRNA changes during cold acclimation of pea seedlings, Plant Physiol. 93 (1990) 1021 – 1026.
[34] N.C. Carpita, D.M. Gibeaut, Structural models of pri-mary cell walls in flowering plants: consistency of molec-ular structure with the physical properties of the walls during growth, Plant J. 3 (1993) 1 – 30.
[35] A. Sakai, W. Larcher, Frost Survival of Plants. Re-sponses and Adaptation to Freezing Stress, Springer, Berlin, 1987, pp. 102 – 107.
[36] J.J. Zwiazek, Cell wall changes in white spruce (Picea glauca) needles subjected to repeated drought stress, Physiol. Plant. 82 (1991) 513 – 518.
[37] S. Renault, J.J. Zwiazek, Cell wall composition and elasticity of dormant and growing white spruce (Picea glauca) seedlings, Physiol. Plant. 101 (1997) 323 – 327. [38] J.K.C. Rose, K.A. Hadfield, J.M. Labavitch, A.B.
Ben-nett, Temporal sequence of cell wall disassembly in rapidly ripening melon fruits, Plant Physiol. 117 (1998) 345 – 361.
[39] K. Miyamoto, Y. Mitani, K. Soga, J. Ueda, K. Waka-bayashi, T. Hoson, S. Kamisaka, Y. Masuda, Modifica-tion of chemical properties of cell wall polysaccharide in the inner tissues by white light in relation to the decrease in tissue tension in Pisum sati6um epicotyls, Physiol. Plant. 101 (1997) 38 – 44.
[40] O. Baron-Epel, P.K. Gharyal, M. Schindler, Pectins as mediators of wall porosity in soybean cells, Planta 175 (1988) 389 – 395.
[41] E.N. Ashworth, F.B. Abeles, Freezing behaviour of wa-ter in small pores and the possible role in the freezing of plant tissues, Plant Physiol. 76 (1984) 201 – 204.

![Table 2Low temperature-induced modifications in pectin fractions (mg [100 mg cell wall]−1)a](https://thumb-ap.123doks.com/thumbv2/123dok/1036484.929292/5.612.21.527.59.138/table-low-temperature-induced-modications-pectin-fractions-cell.webp)