Plants see light through multiple photoreceptors, including phytochromes and cryptochromes. Cryptochromes are flavoproteins that participate in many blue-light responses, including phototropism in plants and entrainment of circadian rhythms in plants and animals. A novel flavoprotein, NPH1, is also implicated in plant phototropism. Phytochromes function as serine/threonine kinases whose potential interacting partners include cryptochrome (CRY1 and CRY2).
Addresses
LPDP Universite Paris VI, UMR CNRS 7632, Tour 53 E5 Casier 156, 4 Place Jussieu, 75252 Paris Cedex 05, France;
e-mail: [email protected]
Current Opinion in Plant Biology1999, 2:230–235 http://biomednet.com/elecref/1369526600200230 © Elsevier Science Ltd ISSN 1369-5266
Introduction
Light affects virtually all aspects of plant growth and devel-opment, from germination and de-etiolation to aspects of vegetative morphology (stem growth and leaf expansion), the onset of reproductive growth and floral initiation, entrainment of circadian rhythms, gene expression, gravit-ropism and phototgravit-ropism [1]. Plants respond to light through photoreceptors which consist of light-absorbing pigment, or chromophore, bound to a protein effector mol-ecule, or apoprotein. Absorption of light by the chromophore induces a chemical or confomational change in the receptor apoprotein that is transmitted to down-stream signalling molecules. All of the complex and diverse responses to light are mediated by just a few different types of photoreceptors. These include the phytochrome pho-toreceptors which absorb primarily in the red/far-red region of the visible spectrum (600–800 nm wavelength) [2], and specific blue/UV-A light absorbing photoreceptors (350–500 nm) known as the cryptochromes [3]. There is in addition evidence for specific UV-B absorbing photorecep-tors [1], but the nature of these molecules is not known. The structure and expression characteristics of the red-light receptor phytochrome has been known for quite some time; however its biochemical function and molecular tar-gets have remained a mystery. Even less is known concerning the molecular identity and mode of action of the blue-light photoreceptors. In this review I will focus on recent major progress in the characterization of red and blue-light absorbing photoreceptors, their role in light sig-naling and their biochemical mechanisms of action.
CRY1, a plant blue-light photoreceptor related
to blue-light-activated DNA repair enzymes
Since the time of Darwin it has been known that plants respond specifically to blue light and that distinctblue-light absorbing photoreceptors must exist. This fol-lows from the observation that certain plant resources, for instance phototropsim — curvature towards the light — are elicited preferentially or exclusively by blue light (wavelengths less than 500 nm) and not by red light, elim-inating phytochrome as a possible photoreceptor. Recently, one such blue light photoreceptor was identified, by cloning of hy4, a partially blue-light insensitive mutant of
Arabidopsis[4]. The structure of the HY4 protein, deduced from the nucleotide sequence, revealed an amino-terminal domain with significant homology to microbial DNA pho-tolyases, which catalyze the blue-light dependent repair of UV-damaged DNA [5]. In addition, there was a carboxy-terminal extension of 180 amino acids, required for photoreceptor function, which is not found in photolyases (Figure 1). With some functional characteristics known, the HY4 protein was then renamed CRY1 for cryp-tochrome, and proposed to function in plants as the elusive and long sought-after blue-light photoreceptor. Recombinant CRY1 protein purified from an E. coli
expression system bound two blue-light absorbing chro-mophores (a flavin and a pterin), and lacked detectable DNA repair activity in vitro. Overexpression of CRY1 in transgenic tobacco and Arabidopsis plants conferred hyper-sensitivity to blue light, shown particularly through the inhibition of hypocotyl elongation and anthocyanin accu-mulation. It seems likely, therefore, that the CRY1 protein evolved from a blue-light sensing DNA photolyase that acquired a novel function as a photoreceptor in plants [3].
CRY1 is a member of a gene family with
homologues in animal systems
The hy4mutant (lacking CRY1) is not completely blind to blue light, and retains considerable blue-light responsive-ness, arguing for the presence of additional blue-light photoreceptors. It was, therefore, no surprise to find anoth-er sequence in the Arabidopsisgenome with considerable homology to CRY1, which was named CRY2 for cryp-tochrome-2 [3] (Figure 1). CRY2 overexpression studies in transgenic plants, as well as the identification of null mutant alleles in CRY2, revealed that CRY2 function is essentially redundant to that of CRY1. For instance, CRY2 participates in inhibition of hypocotyl elongation, antho-cyanin accumulation, and leaf and cotyledon expansion, although to a reduced extent relative to CRY1 [6•,7]. Unlike CRY1, CRY2 protein is rapidly degraded in seedlings upon irradiation by wavelengths of light (UV, blue, green) that activate this receptor [6•,7]. Thus, CRY2 protein does not accumulate to high levels in bright white light and may, therefore, play less of a role in many high-irradiance responses. CRY2, like CRY1, contributes to blue-light dependent promotion of floral initiation [8,9]. In this response CRY2 contributes relatively more than
Seeing the world in red and blue: insight into plant vision and
photoreceptors
CRY1, and has been shown to be allelic to a previously identified late-flowering mutant, fha. Whether the increased role of CRY2 in floral initiation reflects increased stability of CRY2 protein in floral organs, or differential tis-sue expression relative to CRY1,remains to be determined.
CRY1 and CRY2 proteins are highly homologous within their amino-terminal chromophore-binding domain (Figure 1) and indeed both CRY1 and CRY2 from mustard bind the same chromophores when expressed in E. coli
[10]. The carboxy-terminal domains of CRY1 and CRY2, by contrast, show almost no sequence relatedness. Nevertheless, domain swap experiments show that all of the domains of CRY1 and CRY2 proteins are functionally interchangeable in chimeric photoreceptors. Therefore, although the carboxy termini of the members of the cryp-tochrome gene family differ greatly in sequence, they appear to have similar or idenitical function [6•]. This would indicate that the reaction partners of both CRY1 and CRY2 photoreceptors may also be identical. Additional
cryptochrome gene family members, have been identified in numerous plant species including ferns and algae [3,11]; all sequences have considerable similarity in their chro-mophore-binding domains but divergent carboxy-termini. By analogy with CRY1 and CRY2, it is likely that all these proteins share considerable functional overlap, although higher and lower plant transduction pathways may have diverged somewhat in the course of evolution.
The discovery of cryptochrome in plants greatly stimulat-ed the search for relatstimulat-ed sequences in other organisms that might also function as photoreceptors. Interestingly, such homologous genes were identified in mouse (mCRY1 and mCRY2) and humans (hCRY1 and hCRY2) [12,13]. The encoded proteins lack DNA repair activity in vitro, consis-tent with possible novel functions as blue-light photoreceptors [14,15]. The mouse genes (mCRY1 and mCRY2) are expressed in many tissues including the suprachiasmatic nucleus, where the transcripts oscillate in a circadian manner [16••]. Generation of knockout mice Figure 1
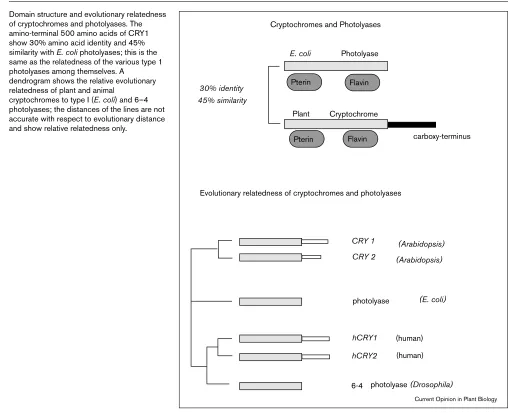
Domain structure and evolutionary relatedness of cryptochromes and photolyases. The amino-terminal 500 amino acids of CRY1 show 30% amino acid identity and 45% similarity with E. coliphotolyases; this is the same as the relatedness of the various type 1 photolyases among themselves. A
dendrogram shows the relative evolutionary relatedness of plant and animal
cryptochromes to type I (E. coli) and 6–4 photolyases; the distances of the lines are not accurate with respect to evolutionary distance and show relative relatedness only.
Cryptochromes and Photolyases
E. coli Photolyase
Pterin
Pterin
Flavin
Flavin 30% identity
45% similarity
carboxy-terminus Plant
Evolutionary relatedness of cryptochromes and photolyases
CRY 1 CRY 2
(Arabidopsis) (Arabidopsis)
(E. coli) photolyase
(human) (human) hCRY1
hCRY2
6-4 photolyase (Drosophila) Cryptochrome
with a deletion of mCRY2 resulted in a one hour length-ening of the free-running period of behavioral rhythms [17], consistent with a biological role in entrainment of cir-cadian rhythms in mammals. Similarly in Drosophila, a cryptochrome-like gene (cry) has been identified whose transcript is regulated in a circadian and clock-gene depen-dent fashion [18••]. Null mutations within this cryptochrome gene abolished light-dependent oscillation in levels of per or timeless clock gene products in mutant flies. Behavioral rhythms were also affected in cry mutant flies, but only in those which had been visually blinded (i.e. lacked functional eyes). Drosophila CRY, therefore, mediates light-dependent entrainment of multiple cellular circadian rhythms, although its role in behavioral rhythms is redundant in visually intact flies [19••].
Interestingly, plant CRY1 also plays a role in entrainment of circadian rhythms in Arabidopsis [20•], albeit a minor one relative to that of phytochrome. It should be noted, howev-er, that the mouse and fly cryptochrome gene sequences are significantly more similar to a distinct class of DNA photolyase repair enzyme than they are to the cryp-tochrome blue-light photoreceptor of plants (Figure 1) [21]. The animal photoreceptors, therefore, seem to have arisen independently in the course of evolution from a different ancestral photolyase enzyme, and do not share any direct relatedness with the plant cryptochrome photoreceptors. It will be interesting to see whether this convergent evolution of animal and plant cryptochromes resulted in comparable mechanisms of action and signaling pathways.
Cryptochrome and phototropism
One of the best-known, yet most elusive plant blue-light responses has been the phenomenon of phototropism — the curvature of plants towards a unilateral light source. Elegant kinetic experiments in Arabidopsis have provided compelling evidence that at least two different specific blue-light photoreceptors are involved in mediating pho-totropic curvature [22]. As the wavelength specificity and phytochrome modulation of phototropic curvature is very similar to that seen for cryptochrome responses [3,22–24], it was surprising that initial experiments with hy4 mutants (lacking CRY1) showed normal phototropic curvature [25]. Double mutants lacking both CRY1 and CRY2, however, showed little detectable curvature in response to single-pulse low fluence blue light (inducing the first positive phototropic curvature) [26••]. Furthermore, transgenic seedlings overexpressing either CRY1or CRY2
genes had a lower fluence threshold for the onset of cur-vature, revealing hypersensitivity to blue light in the phototropic response [26••]. These findings are consis-tent with a redundant role in phototropism for both CRY1 and CRY2, such that only in the absence of both of these receptors is there a significant reduction in responsive-ness. Under continuous blue-light irradiation, however, even cry1 cry2 double mutant seedlings show consider-able phototropic response (second positive phototropic curvature). Cryptochrome, therefore, participates in
mediating phototropic curvature together with at least one other, unrelated blue-light absorbing photoreceptor in addition to CRY1 and CRY2.
NPH1, a novel flavoprotein implicated
in phototropism
The blue-light dependent phosphorylation of an approxi-mately 120 kDa protein can be detected within minutes after irradiation of etiolated seedlings in vivoor microsomal extracts in vitro[27]. This phosphorylation reaction could be induced in vivo by similar light intensities and wave-lengths of blue light as induced phototropic curvature, and was absent in a mutant of Arabidopsis that is null for pho-totropism in etiolated seedlings, designated nph-1 (for non-phototropic hypocotyl) [28]. Thus, blue-light depen-dent autophosphorylation of a 120 kDa protein was proposed as one of the early steps in the signaling pathway mediating phototropism.
The NPH1 gene has been cloned by positional cloning [29••] and encodes a protein (predicted MW 112 kDa) whose carboxy-terminal domain had significant homology to a serine-threonine protein kinase (falling into the PVPK1 family within the protein kinase C group). In addi-tion, the amino-terminus of NPH1encodes two similar 107 amino acid sequences, which appear in a number of pro-teins determined to have a role in light, oxygen or voltage sensing (hence named the LOV domain). Interestingly, at least two of these proteins (prokaryotic NIFL and Aer) function in redox sensing via oxidation or reduction of a flavin, whereas a third (WC-1) is known by genetic evi-dence to be involved in blue-light sensing in Neurospora
(discussed in [29••]). Recombinant NPH1 protein was expressed in a baculovirus system and shown to bind a flavin, FMN, and a blue-light dependent phosphorylation reaction occurred in crude insect cell extracts expressing NPH1 consistent with its possible direct role in blue light sensing. On the basis of these data, NPH1 has been pro-posed as a photoreceptor for phototropism [30••].
So, what could the relationship between the cryptochromes and NPH1 in the phototropic response be? One possibility is that the cryptochromes may function as light harvesting molecules that pass light energy directly to NPH1 via an electron transfer mechanism (NPH1 then presumably transducing the light signal via a phosphorylation reaction to an appropriate substrate). This possibility is supported by the observation that blue-light dependent phosphoryla-tion of NPH1 is impaired under low light intensities in
How does phytochrome work?
Phytochrome — the red/far-red light absorbing photore-ceptor — was the first plant photorephotore-ceptor to be identified and purified although for almost 40 years its mechanism of action has remained a mystery. Phytochrome consists of an amino-terminal chromophore-binding domain and a car-boxy-terminal ‘effector’ domain important for dimerization and protein/protein interactions. In Arabidopsis and other higher plants, phytochrome is encoded by a gene family of multiple members with considerable sequence similarity within the amino-terminal chromophore-binding domain and greater sequence divergence at the carboxy-termini. Different members of the phytochrome gene family have been implicated to different extents in the various responses of the plant to red or far-red light; for instance phyB, the most abundant phytochrome in light-grown plants, has a predominant role in red-light dependent inhi-bition of hypcotyl elongation, stem and leaf expansion, vegetative growth, and floral initiation while other less abundant phytochrome gene family members appear to play a minor and partially redundant role (phyC and phyD). Phys A is photolabile and the active form (Pfr) rapidly degraded in light, but accumulates to high levels in dark-grown seedlings. PhyA participates in high-irradiance responses under far-red light, as well as in shade-avoidance responses and photoperiodic induction of flowering in light-grown plants [2]. Elegant domain swap experiments between phyA and phyB — two phytochrome gene family
members that are considerably diverged in apparent phys-iological function — demonstrate that the carboxy-terminal domains of different phytochromes are fully functionally interchangeable [31]. The biochemical mode of action and immediate reaction partners of the many phytochrome gene family members, therefore, are likely to be identical. This interpretation is supported by identification of muta-tional hotspots in homologous regions of both phyA and phyB carboxy-termini, consistent with similarity in bio-chemical function [2].
The first clue concerning the molecular function of phy-tochrome came from the observation that purified preparations of higher plant phytochrome A displayed ser-ine/threonine kinase activity [32]. This observation was supported by sequence analysis of the carboxy-terminal domains of several higher plant phytochromes, which revealed significant homology to histidine kinase transmit-ter modules found on bactransmit-terial sensor proteins [33]. Moreover, a recently characterized prokaryotic phy-tochrome from Synechocystis PCC6803, when expressed in a heterologous yeast system was demonstrated to have histi-dine kinase activity in vitro. The activity was inducible by far-red light and reversible by red [34•], in contrast to high-er plants whhigh-ere red light is inductive.
The breakthrough in determining the function of the high-er plant phytochromes came from analysis of recombinant
Figure 2
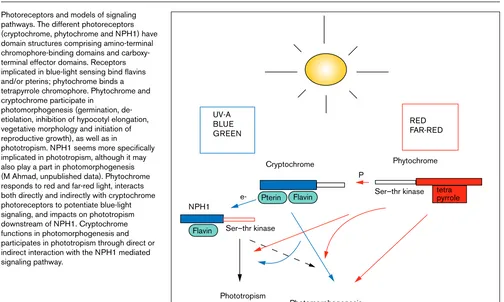
Photoreceptors and models of signaling pathways. The different photoreceptors (cryptochrome, phytochrome and NPH1) have domain structures comprising amino-terminal chromophore-binding domains and carboxy-terminal effector domains. Receptors implicated in blue-light sensing bind flavins and/or pterins; phytochrome binds a tetrapyrrole chromophore. Phytochrome and cryptochrome participate in
photomorphogenesis (germination, de-etiolation, inhibition of hypocotyl elongation, vegetative morphology and initiation of reproductive growth), as well as in
phototropism. NPH1 seems more specifically implicated in phototropism, although it may also play a part in photomorphogenesis (M Ahmad, unpublished data). Phytochrome responds to red and far-red light, interacts both directly and indirectly with cryptochrome photoreceptors to potentiate blue-light signaling, and impacts on phototropism downstream of NPH1. Cryptochrome functions in photomorphogenesis and participates in phototropism through direct or indirect interaction with the NPH1 mediated signaling pathway.
Phytochrome UV-A
BLUE GREEN
RED FAR-RED
NPH1
Cryptochrome
P
e-Ser–thr kinase
Phototropism
Photomorphogenesis
Ser–thr kinase tetra pyrrole Pterin Flavin
Flavin
oat phyA expressed in yeast, which led to the demonstra-tion of the protein’s serine–threonine kinase activity in vitro. Phosphorylation activity was dependent on proper chromophore assembly and stimulated by red light. As the properties of recombinant phyA were identical to those described previously for purified plant phytochromes, the kinase activity must be intrinsic to the photoreceptor. On the basis of sequence homology data and the function of cyanobacterial phytochromes, it is likely that all higher plant phytochromes and the majority of lower plant phy-tochromes evolved from prokaryotic histidine kinase two-component regulators that acquired a novel, serine–threonine specificity during the course of evolu-tion [35••].
Do the different photoreceptors speak to each
other?
Having at least two distinct sets of photoreceptors that respond preferentially to red and blue light enables the plant to maximize its responsivity throughout the visible spectrum. Do these different visual pathways function independently of each other, or is there communication between them? In fact, reports of interactions between blue and red-light sensing systems abound [36], and genet-ic evidence has shown signifgenet-icantly reduced CRY1 activity in the absence of both phyA and phyB [24]. Remaining CRY1 function in phyA phyB mutants [24,37–39] may be potentiated by additional phytochrome gene family mem-bers that are found in Arabidopsis (phyD, E and F). The
cry1 cry2 double mutant (lacking cryptochrome but con-taining wild type levels of phytochrome) shows virtually no blue-light responsiveness in this assay [26••]. The role of phytochrome in blue-light dependent inhibition of hypocotyl elongation is therefore solely to enhance the activity of CRY1 and CRY2, and not to participate directly as has been proposed by some authors [37–39].
Cryptochrome is a substrate for
phytochrome-associated kinase activity
Given the involvement of phytochrome in cryptochrome-mediated signaling pathways, one possibility is that the two different photoreceptors might interact directly. Phosphorylation experiments in vitrowith purified recom-binant photoreceptors indeed showed that CRY1 serves as a substrate for phytochrome-dependent kinase activity [40••]. Phosphorylation occurred at the carboxy-terminus of CRY1, in a serine-rich region that is conserved in all higher plant cryptochromes that have been sequenced [40••]. Thus, cryptochrome is one of the few biological substrates proposed for phytochrome to date (Figure 2).
Given its function as a kinase, phytochrome may have multiple substrates. Indeed, an Arabidopsis protein, PIF3, which interacts with both phyA and phyB in yeast two-hybrid interaction cloning experiments is another possible reaction partner proposed for phytochrome [41••]. PIF3 is a nuclear-localized basic helix-loop-helix protein that appears to be involved in phytochrome responses in phyB
overexpressing and antisense transgenic plants. As phyB in particular appears nuclear-localized, phyB may interact directly with PIF3 in vivo, and possibly also with others of the numerous transcription factors implicated in light responses [42•,43]. It will be interesting to see whether PIF3 serves as substrate for phosphorylation by phy-tochrome-associated kinase activity.
Emerging themes: simplicity
underlying complexity
The many diverse plant light responses are mediated by only a handful of photoreceptors (principally cryptochrome and phytochrome). These occur as families of homologues whose members function similarly and may have identical reaction partners. Complexity of response can result from differential expression levels, localization, and accessibility to signaling intermediates of the different members of a photoreceptor gene family. Additional flexibility is achieved by communication between the different classes of photoreceptors and their transduction pathways. NPH1 is a novel flavoprotein with serine–threonine kinase activity that functions in phototropism, possibly in associa-tion with cryptochrome. Phytochrome funcassocia-tions as a serine–threonine kinase with multiple possible substrates, including cryptochrome and nuclear transcription factors.
In conclusion, the number and types of different photore-ceptors, their mode of action and their inter-relationship in various signaling pathways are beginning to come more sharply into focus.
Acknowledgements
I am indebted to B Sotta, E Miginiac and J-C Kader for helpful comments and critical reading of the manuscript.
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest ••of outstanding interest
1. Kendrick RE, Kronenberg GHM:Photomorphogenesis in Plants, edn 2. Dordrecht: Kluwer Academic Publishers; 1994.
2. Quail PH: The phytochrome family: dissection of functional roles and signalling pathways among family members.Philos Trans R Soc Lond B Biol Sci1998, 353:1399-1403.
3. Ahmad M, Cashmore AR: Seeing blue: the discovery of cryptochrome.Plant Mol Biol1996, 30:851-861.
4. Ahmad M, Cashmore AR: Hy4 gene of A. thaliana encodes a protein with characteristics of a blue-light photoreceptor.Nature 1993, 366:162-166.
5. Sancar A: Structure and function of DNA photolyase.Biochemistry 1994, 33:2-9.
6. Ahmad M, Jarillo JA, Cashmore AR: Chimeric proteins between cry1 • and cry2 Arabidopsisblue light photoreceptors indicate
overlapping functions and varying protein stability.Plant Cell 1998, 10:197-208.
This paper explores the functional relatedness of cryptochrome gene family mem-bers and suggests a similar function and mechanism of action in Arabidopsis.
7. Lin C, Yang H, Guo H, Mockler T, Chen J, Cashmore AR:
8. Guo H, Yang H, Mockler TC, Lin C: Regulation of flowering time by
Arabidopsis photoreceptors.Science1998, 279:1360-1363. 9. Bagnall DJ, King RW, Hangarter RP: Blue-light promotion of
flowering is absent in hy4mutants of Arabidopsis.Planta1996, 200:278-280.
10. Malhotra K, Sang-Tae K, Batschauer A, Dawut L, Sancar A: Putative blue-light photoreceptors from Arabidopsis thaliana and Sinapis alba with a high degree of sequence homology to DNA photolyase contain the two photolyase cofactors but lack DNA repair activity.Biochemistry1995, 34:6892-6899.
11. Kanegae T, Wada M: Isolation and characterization of homologues of plant blue-light photoreceptor (cryptochrome) genes from the fern Adiantum capillus-veneris.Mol Gen Genet1998, 259:345-353. 12. van der Spek PJ, Kobayashi K, Bootsma D, Takao M, Eker APM,
Yasui A: Cloning, tissue expression, and mapping of a human photolyase homolog with similarity to plant blue-light receptors. Genomics1996, 37:177-182.
13. Kobayashi K, Kanno S, Smit B, van der Horst GT, Takao M, Yasui A: Characterization of photolyase/blue-light receptor homologs in mouse and human cells.Nucleic Acids Res1998, 26:5086-5092.
14. Todo T, Tsuji H, Otoshi E, Hitomi K, Kim ST, Ikenaga M:
Characterization of a human homolog of (6-4) photolyase.Mutat Res1997, 384:195-204.
15. Hsu DS, Zhao XD, Zhao SY, Kazantsev A, Wang RP, Todo T, Wei YF, Sancar A: Putative human blue-light photoreceptors hCRY1 and hCRY2 are flavoproteins. Biochemistry1996, 35:13871-13877.
16. Thresher RJ, Vitaterna MH, Miyamoto Y, Kazantsev A, Hsu DS, Petit C,
•• Selby CP, Dawut L, Smithies O, Takahashi JS, Sancar A: Role of mouse cryptochrome blue-light photoreceptor in circadian photoresponses. Science1998, 282:1490-1494.
This paper explores the circadian phenotype resulting from knockout of a mouse cryptochrome gene. This is the first conclusive demonstration of a role for cryptochrome photoreceptors in mammals.
17. Miyamoto Y, Sancar: A vitamin B2-based blue-light photoreceptors in the retinohypothalamic tract as the photoactive pigments for setting the circadian clock in mammals.Proc Natl Acad Sci USA 1998, 95:6097-6102.
18. Emery P, So WV, Kaneko M, Hall JC, Rosbash M: CRY, a Drosophila
•• clock and light-regulated cryptochrome, is a major contributor to circadian rhythm resetting and photosensitivity.Cell1998, 95:669-679.
This paper characterizes a cryptochrome gene from Drosophila that is impli-cated in circadian rhythms.
19. Stanewsky R, Kaneko M, Emery P, Beretta B, Wagner-Smith K,
•• Kay SA, Rosbash M, Hall JC: The cryb mutation identifies cryptochrome as a circadian photoreceptor in Drosophila.Cell 1998 95:681-692.
In this paper the circadian phenotypes of a fly with a mutation in the Drosophila cryptochrome gene is analyzed, and a role in multiple circadian rhythms of the fly established.
20. Somers DE, Devlin PF, Kay SA: Phytochromes and cryptochromes • in the entrainment of the Arabidopsiscircadian clock.Science
1998, 282:1488-1490.
This paper demonstrates a role for CRY1 in entrainment of circadian rhythms in Arabidopsis.
21. Kanai S, Kikuno R, Toh H, Ryo H, Todo T: Molecular evolution of the photolyase-blue-light photoreceptor family.J Mol Evol 1997, 45:535-548.
22. Konjevic RB, Steinitz B, Poff KL: Dependence of the phototropic response of Arabidopsis thalianaon fluence rate and wavelength. Proc Natl Acad Sci USA1989, 86:9876-9880.
23. Janoudi A-K, Konjevic R, Whitelam G, Gordon W, Poff KL: Both phytochrome A and phytochrome B are required for the normal expression of phototropism in Arabidopsis thalianaseedlings. Physiologia Plantarum1997, 101:278-282.
24. Ahmad M, Cashmore AR: The blue-light receptor cryptochrome 1 shows functional dependence on phytochrome A or phytochrome B in Arabidopsis thaliana.Plant J1997, 11:421-427.
25. Liscum E, Young JC, Poff KL, Hangarter RP: Genetic separation of phototropism and blue light inhibition of stem elongation.Plant Physiol1992, 100:267-271.
26. Ahmad M, Jarillo J, Smirnova O, Cashmore AR: Cryptochrome blue •• light photoreceptors of Arabidopsisimplicated in phototropism.
Nature1998, 392:720-723.
This paper establishes the role of the cryptochrome in phototropism, and is there-by the first report of a blue-light receptor implicated in the phototropic response.
27. Short TW, Briggs WR: The transduction of blue-light signals in higher-plants.Annu Rev Plant Physiol Plant Mol Biol1994, 45:143-171.
28. Liscum E, Briggs WR: Mutations in the nph1locus of Arabidopsis
disrupt the perception of phototropic stimuli.Plant Cell1995, 7:473-485.
29. Huala E, Oeller PW, Liscum E, Han I-S, Larsen E, Briggs WR: •• ArabidopsisNPH1: a protein kinase with a putative redox-sensing
domain.Science1997, 278:2120-2123.
This paper describes the cloning of NPH1, a gene that has been implicated in an early event in the phototropism signaling pathway and may thereby define a novel blue-light photoreceptor.
30. Christie JM, Reymond P, Powell GK, Bernasconi P, Raibekas AA,
•• Liscum E, Briggs WR: Arabidopsis NPH1: a flavoprotein with the properties of a photoreceptor for phototropism.Science1998, 282:1698-1701.
This paper is a follow-up of the previous paper on cloning of NPH1, demon-strating that it binds flavin and particpates in blue-light dependent phospho-rylation reaction in a heterologous system. These data are consistent with function as a photoreceptor.
31. Wagner D, Fairchild CD, Kuhn RM, Quail PH: Chromophore-bearing NH2-terminal domains of phytochromes A and B determine their photosensory specificity and differential light lability.Proc Natl Acad Sci USA1996, 93:4011-4015.
32. Wong Y-S, Cheng H-C, Walsh DA, Lagarias JC:Phosphorylation of Avena phytochrome in vitro as a probe of light-induced conformational changes.J Biol Chem 1986, 261:12089-12097.
33. Schneider-Poetsch HAW, Braun B, Marx S, Schaumburg A: Phytochromes and bacterial sensor proteins are related by structural and functional homologies.FEBS Lett1991, 281:245-249.
34. Yeh KC, Wu S-H, Murphy JT, Lagarias JC: A cyanobacterial • phytochrome two-component light sensory system.Science1997,
277:1505-1508.
In this paper, a prokaryotic phytochrome (presumably ancestral to all plant phytochromes) is demonstrated to have histidine kinase activity in vitro.
35. Yeh KC, Lagarias JC: Eukaryotic phytochromes: light-regulated •• serine/threonine protein kinases with histidine kinase ancestry.
Proc Natl Acad Sci USA1998, 95:13976-13981.
This paper provides conclusive evidence for the first time of the mechanism of action of phytochrome, as a serine/threonine dependent protein kinase.
36. Mohr H: Coaction between pigment systems.In Photomorphogenesis in Plants, edn 2. Edited by Kendrick RE, Kronenberg GHM. Kluwer Academic Publishers: Dordrecht; 1994:353-372.
37. Casal JJ, Mazella MA: Conditional synergism between
cryptochrome 1 and phytochrome B is shown by the analysis of
phyA, phyB, and hy4simple, double, and triple mutants in
Arabidopsis.Plant Physiol1998, 118:19-25.
38. Neff MM, Chory J: Genetic Interactions between phytochrome A, phytochrome B, and cryptochrome 1 during Arabidopsis
development.Plant Physiol1998, 118:27-36.
39. Poppe C, Sweere U, Drumm-Herrel H, Schafer E: The blue-light receptor cryptochrome 1 can act independently of phytochrome A and B in Arabidopsis thaliana. Plant J1998, 16:465-471. 40. Ahmad M, Jarillo J, Smirnova O, Cashmore AR: The CRY1 blue light •• photoreceptor of Arabidopsis interacts with phytochrome A
in vitro.Mol Cell1998, 1:939-948.
This paper demonstrates that cryptochrome is a substrate for phosphoryla-tion by phytochrome in vitro. This is the first report of a possible biological substrate for photochrome-associated phosphorylation activity, and also defines a possible means by which these two major photoregulatory systems within the plant may communicate.
41. Ni M, Tepperman JM, Quail PH: PIF3, a phytochrome-interacting •• factor necessary for a normal photoinduced signal transduction,
is a novel basic helix-loop-helix protein.Cell 1998, 95:657-667. In this paper, a possible interacting factor for phytochrome is identified in yeast two-hybrid interaction studies, and implicates a transcription factor directly in the phytochrome response.
42. Fankhauser C, Chory J: Light control of plant development.Annu
• Rev Cell Dev Biol1997, 13:203-229.
This paper demonstrates that a transcription factor implicated in phy-tochrome responses may participate in the entrainment of circadian rhythms.