Naringenin 7-
O
-methyltransferase involved in the biosynthesis of
the flavanone phytoalexin sakuranetin from rice (
Oryza sati
6
a
L.)
Randeep Rakwal
a,*, Ganesh Kumar Agrawal
a, Masami Yonekura
b, Osamu Kodama
caUnited Graduate School,Tokyo Uni6ersity of Agriculture and Technology,Tokyo,Japan bFood Function Laboratory,School of Agriculture,Ibaraki Uni6ersity,Ibaraki,Japan cLaboratory of Phytochemical Ecology,School of Agriculture,Ibaraki Uni6ersity,Ibaraki,Japan
Received 23 August 1999; received in revised form 8 February 2000; accepted 10 February 2000
Abstract
An inducibleS-adenosyl-L-methionine:naringenin 7-O-methyltransferase (NOMT) catalyzing the methylation of naringenin to sakuranetin, a major rice phytoalexin was purified approximately 985-fold from ultraviolet (UV)-irradiated rice leaves. The enzyme is not found in healthy tissues and was purified to a nearly homogeneous preparation in one step using adenosine-agarose affinity chromatography, with 1 g rice leaves (UV-irradiated) as starting material. Gel filtration chromatography resulted in an almost pure enzyme, as evidenced by a major band migrating to a position corresponding to a molecular mass of approximately 41 kDa by sodium dodecyl sulfate polyacrylamide gel electrophoresis. The purified NOMT was strongly inhibited by Mn2+,
Ni2+, Cu2+, Zn2+, Hg2+, and Cd2+, and to a low degree by Co2+, Mg2+, Ba2+, Ca2+ and ethylenediamine tetraacetic acid.
The amino acid sequence of a NOMT cyanogen bromide (CNBr)-cleavage peptide was highly homologous to that of a caffeic acid 3-O-methyltransferase from maize, and about 70% of the amino acid sequence was obtained after sequencing the peptides generated by CNBr and/or formic acid hydrolysis. NOMT was also shown to be induced in a time-dependent manner, and purified from rice leaves treated with jasmonic acid and copper chloride. © 2000 Elsevier Science Ireland Ltd. All rights reserved.
Keywords:Naringenin 7-O-methyltransferase; Oryza sati6a; Sakuranetin; Phytoalexin; Affinity chromatography; Jasmonic acid
www.elsevier.com/locate/plantsci
1. Introduction
Plants possess inducible defence mechanisms against pathogen attack that include the produc-tion of phytoalexins. These are low molecular weight, antimicrobial compounds that are both synthesized by, and accumulate in, plants after
their exposure to microorganisms, as well as to chemical and physical stress [1 – 4]. Sakuranetin (5,4%-dihydroxy-7-methoxyflavanone) is a major rice phytoalexin accumulating both in ultraviolet (UV) irradiated [5] and blast infected [5], as well as in copper chloride (CuCl2) or jasmonic acid (JA), treated rice leaves [6]. It has been suggested that flavonoids, in general, have a function in the pro-tection of plants against UV light and other stresses [7]. Numerous flavonoids possess methoxy groups at different positions of the A and B rings, as well as at position 3 of the heterocycle. O-Methyltransferases that are involved in the biosyn-thesis of flavonoids have been described previously [8,9]. Recently, it was reported that a methyltrans-ferase (naringenin 7-O-methyltransferase (NOMT)) catalyzing the methylation of narin-Abbre6iations: CuCl2, copper chloride; CNBr, cyanogen bromide;
JA, jasmonic acid; NOMT, naringenin 7-O-methyltransferase; SDS-PAGE, sodium dodecyl sulfate polyacrylamide gel electrophoresis; UV, ultraviolet.
* Corresponding author. Present address: Department of Molecu-lar Genetics, National Institute of Agrobiological Resources, Kan-nondai 2-1-2, Tsukuba 305-8602, Ibaraki, Japan. Tel.:
+81-298-387490; fax: +81-298-387032.
E-mail address:[email protected] (R. Rakwal)
genin (5,7,4%-trihydroxyflavanone) to sakuranetin is induced in UV-irradiated rice leaves (Fig. 1) [10]. Furthermore, it has been shown that not only JA, but also some JA amino acid conjugates cause the induction of NOMT and subsequent accumu-lation of sakuranetin [11].
Numerous O-methyltransferases have been purified from plant tissues and cell suspension cultures [12,13]. However, most of the purification protocols are time consuming and require large amounts of starting materials. In this study, the purification and partial characterization of NOMT from UV-irradiated rice leaves is reported. More-over, a rapid microscale purification procedure is used to obtain the purified enzyme efficiently, us-ing affinity and gel filtration chromatography. The enzyme was also purified from JA- and CuCl2 -treated rice leaves, and the induction of NOMT shown to be time dependent, providing a good correlation with sakuranetin accumulation. This is the first report of the purification of a phytoalexin-specificO-methyltransferase from rice, and may be of particular interest in the context of elucidating the mechanism of resistance against the devastat-ing rice blast disease caused by the blast fungus Magnaporthe grisea (anamorph, Pyricularia oryzae).
2. Materials and methods
2.1. Plant material
Rice plants (Oryza sati6a L. Hitomebore) were cultivated and UV-irradiated as previously de-scribed [5]. Leaves were stored at −80°C until analysis for NOMT activity and purification. For treatment with JA and CuCl2, rice leaves were wounded as described previously [6] and floated in sterile Petri dishes on solutions of JA and CuCl2 (0.5 mM), respectively.
2.2. Chemicals
Sakuranetin was purchased from Roth (Karl-sruhe, Germany). Naringenin, apigenin, kaempferol, caffeic acid and polyvinyl-polypyrrolidone (PVPP) (high molecular weight, Product Number P 6755) were obtained from Sigma (St. Louis, USA). Daidzein, luteolin and genistein were from Extrasynthe`se (Genay, France). S-[14
C]adenosyl-L-methionine (48 mCi/
mmol) was purchased from Moravek Biochemicals Inc. (Brea, USA). Adenosine 5%-monophosphate (AMP) agarose (cross-linked 4% beaded agarose with C-8 attachment and a nine-atom spacer, Product Number A 1271) and S-adenosyl-L
-me-thionine (SAM) were from Sigma, and Superdex 75 from Pharmacia Biotech (Uppsala, Sweden). Calf intestinal alkaline phosphatase was purchased from Takara Shuzo Co. Ltd. (Tokyo, Japan). The protein dye reagent, TEMED, ammonium persul-fate, and bisacrylamide were all from Bio-Rad (Richmond, USA). All chemicals were of reagent grade.
2.3. Preparation of adenosine-agarose affinity gel
Essentially, the protocol of Attieh et al. [14] was followed for preparing the affinity matrix with the following modifications. 5%-AMP-agarose (5 ml) was washed with distilled water and incubated with 800 U calf intestinal alkaline phosphatase and 1 ml calf intestinal alkaline phosphatase buffer (10×) (pH 9.0) in a total volume of 10 ml. The gel was dephosphorylated for 24 h at 37°C in a continuously rotating reaction vial, transferred to a column, and washed first with distilled water and then with 10 ml buffer B (0.02 M Tris – HCl (pH 7.8), 10% glycerol, 1 mM ethylenediamine tetraacetic acid (EDTA), and 14 mM 2-mercap-toethanol) containing 2 M NaCl, finally being equilibrated with 100 ml buffer B only.
2.4. Extraction of plant material
All steps were carried out at 4°C unless other-wise stated. UV-Irradiated rice leaves (1 g) were homogenized in a mortar and pestle with 4 ml buffer A (0.2 M Tris – HCl buffer (pH 7.8), con-taining 14 mM 2-mercaptoethanol, 5 mM EDTA, 10% (w/v) glycerol and 10% (w/v) PVPP) in the presence of 0.1 g sea sand. The homogenates were centrifuged at 18 500×g for 5 min, and the result-ing supernatant filtered through a 50 mM nylon mesh. The filtrate was assayed for NOMT enzyme activity and protein concentration, and subse-quently used for NOMT purification.
2.5. Affinity chromatography on adenosine-agarose
The filtrate was directly applied onto an adenosine-agarose column (0.7×15 cm) that had been previously equilibrated with buffer B. The column was washed with 50 ml buffer B at a constant flow rate of 18 ml/h, then with 50 ml of the same buffer containing 0.2 M KCl. The NOMT was selectively eluted with 25 ml of 4 mM SAM in buffer B (0.2 M KCl). Fractions (1.75 ml) were collected and assayed for NOMT activity and protein content.
2.6. Gel filtration on Superdex 75
The fraction from the affinity column contain-ing most of the NOMT activity was loaded on a Superdex 75 gel filtration column (3×30 cm) pre-viously equilibrated with buffer B. Proteins were eluted in the same buffer at a flow rate of 18 ml/h, and 3.5 ml fractions were collected, assayed for NOMT enzyme activity and protein content. The purified enzyme from this step was used for partial characterization and amino acid sequencing.
2.7. Molecular mass estimation
The native molecular mass of NOMT was esti-mated by gel filtration chromatography by high-performance liquid chromatography (HPLC) on a TSKGEL SW G 3000 XL (TOSOH) gel filtration column (7.8×30 cm) calibrated with the following markers: glutamate dehydrogenase (290 000), lac-tate dehydrogenase (142 000), enolase (67 000), adenylate kinase (32 000), and cytochrome C (12 400), and by 13.5% native-polyacrylamide gel
electrophoresis (PAGE) under nondenaturing con-ditions. The protein was eluted with the following buffer: 0.1 M sodium phosphate containing 0.1 M sodium sulphate (pH 7.0) at a flow rate of 1.0/ml. The molecular mass was estimated by sodium dodecyl sulfate (SDS)-PAGE under denaturing conditions [15] using 13.5% polyacrylamide gels calibrated with molecular mass standards in the range 14.4 – 94 kDa (LMW Electrophoresis cali-bration kit; Pharmacia Biotech). Gels were fixed in 20% TCA for 1 h and stained with Coomassie Brilliant Blue R 250 for 1 h. Destaining occurred in acetic acid:methanol:distilled water (1:1.5:2) overnight or until background staining was no longer visible.
2.8. N-Terminal sequencing and microsequencing
Table 1
Purification of naringenin 7-O-methyltransferase (NOMT) from UV-irradiated rice leaves
Purification steps Total activity Total protein Specific activity Recovery Purification (mkat/mg) (%) (x-fold) (mkat) (mg)
5.03 8.9 0.56 100
Crude 1
homogenate
1.84 0.011
Adenosine- 167.3 36.6 296.1
agarose
0.006 556.7
Gel filtration 3.34 66.4 985.2
2.9. Other assays
Methyltransferase activity was assayed as previ-ously described [10]. Protein was determined ac-cording to the method of Bradford [18] using Bio-Rad protein assay dye reagent and bovine serum albumin as standard.
3. Results and discussion
3.1. Purification of naringenin 7-O-methyltransferase
UV-Irradiated rice leaves accumulated the flavanone phytoalexin sakuranetin, and the pres-ence of a methyltransferase catalyzing the methy-lation of the hydroxyl moiety at position 7 of naringenin to yield sakuranetin has been demon-strated in UV-irradiated rice leaves (Fig. 1) [10]. This enzyme, an S-adenosyl-L
-methionine-depen-dent naringenin 7-O-methyltransferase, was purified to near homogeneity by affinity chro-matography and gel filtration chrochro-matography.
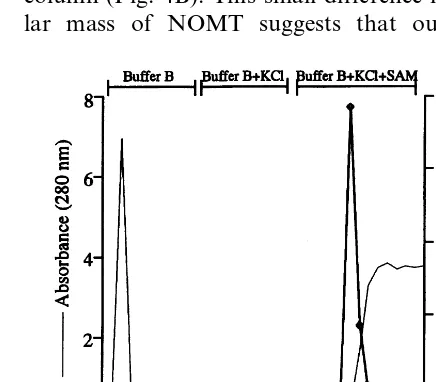
The purification procedure is summarized in Table 1. The key step in the purification procedure is affinity chromatography on adenosine-agarose, leading to a more than 290-fold enrichment of the enzyme (Fig. 2). Another 3.3-fold increase in spe-cific enzyme activity was obtained by gel filtration. Although most of the contaminating proteins were efficiently removed during sample loading and washing with buffer B and buffer B containing high salt, SDS-PAGE showed the presence of two additional bands: one of approximately 70 kDa, and another just below the NOMT band at 41 kDa, which may be a NOMT degradation product [19]. Adenosine 5%-monophosphate-agarose is used
in our experiments to purify a methyltransferase from UV-irradiated rice leaves. The matrix is such
that it can be used repeatedly with negligible loss of binding or eluting efficiencies [14]. The use of this affinity matrix enabled an efficient and rapid purification of NOMT in sufficient amounts from a minimum amount of starting material.
3.2. Molecular mass of naringenin 7-O-methyltransferase
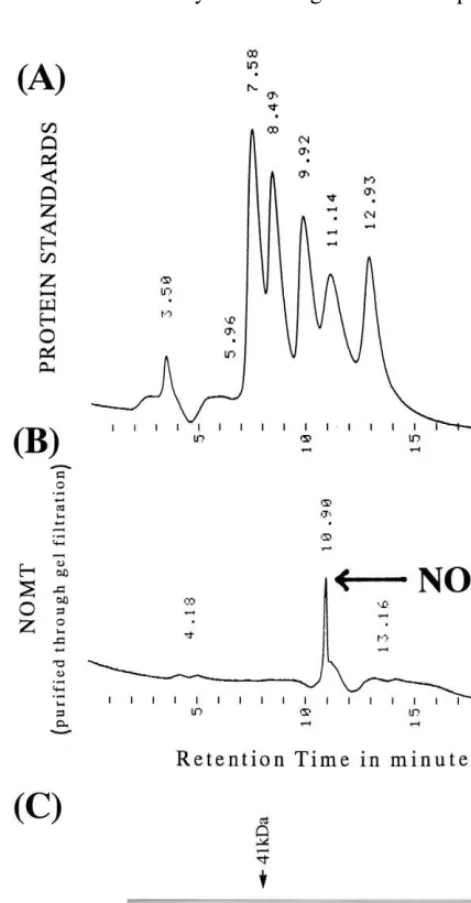
SDS-PAGE revealed the presence of an almost pure protein of 41 kDa, although one extremely faint band of approximately 70 kDa can also be seen in the purified gel filtration fraction (Fig. 3, lane H). The native molecular weight was esti-mated to be ca. 38 kDa (where NOMT eluted as a single peak) employing a TSK gel filtration HPLC column (Fig. 4B). This small difference in molecu-lar mass of NOMT suggests that our enzyme
Fig. 2. Affinity chromatography of NOMT on adenosine-agarose. The enzyme was eluted using the co-substrate SAM in buffer B containing KCl. Eluting fractions were assayed for enzyme activity (2). Protein was monitored continuously at
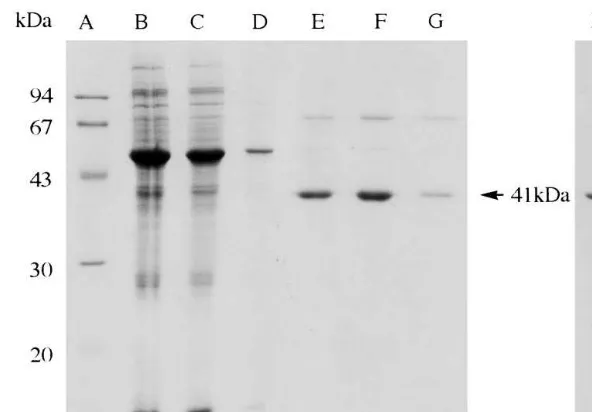
Fig. 3. SDS-PAGE of NOMT activity containing fractions from successive purification steps. Lane A, Low molecular mass markers; lane B, crude extract (approximately 10mg); lane C, buffer B wash; lane D, buffer B+KCl wash; lanes E – G, buffer B+KCl+SAM; lane H, concentrated gel filtration fraction. Arrow on the right indicates purified NOMT. Lanes E – G, protein samples from fractions showing enzyme activity were concentrated before loading. Protein samples were boiled for 1 min with SDS sample buffer containing Bromophenol blue, and separated by 13.5% polyacrylamide gel. The gel was stained with Coomassie Brilliant Blue.
shows slightly anomalous behavior in native and denatured states, and that the gel filtration mobili-ties were effected by the size as well as the confor-mation of the protein [20]. However, comparison of the molecular mass of denatured and native NOMT suggests that the enzyme exists as a monomer. This was confirmed by nondenaturing PAGE (at 4°C in a nonSDS buffer system, gel and sample buffer) showing the presence of a single band at molecular weight 41 kDa, as detected by Coomassie Brilliant Blue staining (Fig. 4C). NOMT has a molecular mass similar to that of a caffeic acid 3-O-methyltransferase involved in lignin biosynthesis in maize (39.5 kDa) [21] and a methyltransferase responsible for the biosynthesis of the phytoalexin pisatin in pea (43 kDa) [22].
3.3. Enzyme stability and pH optimum 6alue
The enzyme in crude homogenates (in buffer A) was stable for over 1 month at −20°C. After affinity chromatography, the enzyme became ex-tremely labile, losing almost all activity after 24 h storage in buffer B at 4°C/ −20°C. In contrast, the enzyme preparation (in buffer B) after gel filtra-tion retained more than 50% of its activity after 2 weeks at −20°C. The pH dependence of NOMT activity was examined over the pH range 6.0 – 10.0
using Tris – HCl and glycine – NaOH buffers. NOMT exhibited a broad pH optimum between pH 7.5 and 9.0 for Tris – HCl and between 8.0 and 10.0 for glycine – NaOH. Activity at pH 7.8 ranged from 2.35 to 2.80mkat/ml for the two buffers. The pH optimum for NOMT was in the middle of the range reported for plant O-methyltransferases (OMTs) (EC 2.1.1.6): pH 6 – 8 for OMTs for inter-mediates in the lignin biosynthetic pathway, and pH 8.7 – 9.7 for flavonoid specific OMTs [8].
3.4. Effect of di6alent cations on naringenin 7-O-methyltransferase acti6ity
The requirement for Mg2+ by SAM-dependent
methyltransferases from plants varies [22]. The gel filtration purified NOMT preparation was used for the measurements of divalent cation effects. NOMT-catalyzed methylation decreased on the addition of EDTA, indicating some requirement for cations (Table 2). However, when the effect of several divalent cations on NOMT activity was analyzed, it was observed that the enzyme was strongly inhibited by Mn2+, Ni2+, Cu2+, Zn2+,
Hg2+, and Cd2+, and inhibition to some extent
was obtained by addition of Ca2+, Ba2+, Mg2+
3.5. Substrate specificity
Crude rice leaf extracts catalyzed the conversion of flavonoids and also of caffeic acid to their respective methyl ethers and esters [10]. As other flavonoids acted as methyl acceptors using the crude enzyme preparation, it became necessary to purify NOMT in order to clarify the actual sub-strate of the enzyme. The gel filtration purified
Table 2
Influence of divalent cations on enzymatic activitya
Concentration
Addition Relative activity (mM) (% of control)
None 100
5 37
CaCl2
5
BaCl2 40
MnCl2 5 0
NiCl2 5 0
5
CuCl2 0
5
ZnCl2 0
5
HgCl2 0
CdCl2 5 0
CoCl2 5 61
5
MgCl2 60
5
Na2EDTA 89
10 69
aEnzyme was assayed in 0.1 M glycine–NaOH (pH 9.5)
with the indicated addition. Gel filtration purified NOMT preparation was used for the assays.
Fig. 4. Elution of naringenin 7-O-methyltransferase from a TSK gel filtration HPLC column. (A) Protein standards, (B) fraction containing NOMT (marked by an arrow) was iden-tified by enzyme activity as described previously [10], and (C) Coomassie Brilliant Blue stained, native-PAGE showing purified NOMT (marked by an arrow) after gel filtration (lane B; lane A shows low molecular mass markers).
Table 3
Substrate specificity of gel filtration purified naringenin 7-O -methyltransferase from UV-irradiated rice leaves
Relative Substrate
activitya (%)
Naringenin (4%,5,7-trihydroxyflavanone) 100
Apigenin (4%,5,7-trihydroxyflavone) 81 Luteolin (3%,4%,5,7-tetrahydroxyflavanone) 148
Kaempferol 0
(3,4%,5,7-tetrahydroxyflavanone)
Genistein (4%,5,7-trihydroxyisoflavone) 0 Daidzein (4%,7-dihydroxyisoflavone) 0
Caffeic acid (3,4-dihydroxycinnamic acid) 0
Sakuranetin 0
(5,4%-dihydroxy-7-methoxyflavanone)
aRelative activity was calculated with activity against (9)
naringenin as 100.
Table 4
Amino acid sequence of a CNBr-generated NOMT peptide and comparison with the deduced amino acid sequence of OMTs from maize, aspen, alfalfa, purple nonsulfur photosyn-thetic bacterium and bovine pineal glandsa
aThe protein sequences are shown in single-letter code. The
superscript numbers at the start and end of the sequences indicate the position of amino acid residues compared and which are homologous with NOMT peptide (40 amino acids). The box (dotted line) indicates the conserved region.
was treated with CNBr and/or formic acid to generate peptides through cleavage at the carboxyl side of methionine residues [16] and at aspartic acid residues, respectively. One major peptide gen-erated by CNBr was initially microsequenced and the sequence of 40 amino acids determined. This sequence is highly homologous to that of a caffeic acid 3-O-methyltransferase from maize involved in lignin biosynthesis [21]. It contains a stretch of 17 amino acids, which are conserved in OMTs from aspen, alfalfa, a purple nonsulfur photosynthetic bacterium and bovine pineal glands, as well as maize (Table 4) [21]. This region is one of the five highly conserved regions in OMTs [23]. Then, all the seven CNBr generated peptides and the ten peptides generated by formic acid hydrolysis were sequenced, and the composite partial sequence data of NOMT is presented in Fig. 5. Recently, a putative O-methyltransferase induced by fungal pathogens and UV light has been cloned from barley and suggested to have a function in flavonoid biosynthesis [24]. However, this enzyme does not show any homology with the partial sequence of NOMT reported in this paper. Fur-ther work is necessary to obtain the complete amino acid sequence of NOMT and to study its phylogenetic relationship with other plant OMTs.
3.7. Comparison and purification of naringenin 7-O-methyltransferase acti6ities from JA- and
CuCl2-treated rice lea6es
Sakuranetin also accumulates in rice leaves treated with JA or CuCl2[6]. Therefore, the induc-tion of NOMT by JA and CuCl2 was determined possible explanation for this is that these
flavonoids carry hydroxy moieties at positions ac-cessible for methylation. The fact that NOMT is induced by UV irradiation, which also elicits accu-mulation of sakuranetin, a major rice phytoalexin, suggests that this enzyme is responsible for this phytoalexin response.
3.6. Partial amino acid sequence of NOMT
N-Terminal amino acid sequencing was per-formed; however, no amino acid could be iden-tified in several cycles, suggesting that the protein is N-terminally blocked. Therefore, the enzyme
Fig. 6. (A) Induction of NOMT by JA and CuCl2treatment
of rice leaves. Data are the average of two independent experiments. (B) SDS-PAGE of fractions during NOMT purification from JA- and CuCl2-treated rice leaves. Lane 1,
Low molecular mass markers; lanes 2 – 4 (JA-treated rice leaves) and lanes 5 – 7 (CuCl2-treated rice leaves), crude
ex-tract, affinity column, and gel filtration, respectively. Samples were prepared and resolved on SDS-PAGE as described for Fig. 3.
treated rice leaves. The time 0 values of enzyme activity was measured and found to be substan-tially low, considered as basal activity values, and thus the given NOMT activity in Fig. 6A is after the subtraction. Therefore, time 0 values have not been shown in Fig. 6A. The induction and level of NOMT accumulation correlates with the amounts of sakuranetin obtained in rice leaves treated with JA or CuCl2 [6]. A very high accumulation of sakuranetin was induced by JA compared with comparatively low levels that accumulated after CuCl2 treatment. This induction pattern also cor-relates with the accumulation of sakuranetin upon UV irradiation of rice leaves, where an increase in NOMT levels precedes the increase in sakuranetin levels [10]. Taken together, these findings may indicate that JA is involved in the signal transduc-tion pathway leading to phytoalexin productransduc-tion. NOMT induction was drastically inhibited by cy-cloheximide, an inhibitor of cytoplasmic protein synthesis, in both JA- and CuCl2-treated rice leaves, but not by tetracycline, an inhibitor of protein synthesis in plant chloroplast and mito-chondria, indicating that the enzyme is synthesized de novo in the plant cytoplasm (Table 5). Purifica-tion of NOMT from JA- and CuCl2-treated rice leaves revealed protein bands migrating to a simi-lar position, at approximately 41 kDa on SDS-PAGE (Fig. 6B). This study opens a door for cloning of the gene (using a PCR-based approach) encoding this important enzyme (NOMT) from rice, and to study its role in rice plant defence.
Acknowledgements
We wish to thank Dr H.S. Saini (Universite´ of Montre´al, Canada) for suggesting the use of the
Table 5
Effect of cycloheximide (CHX) and tetracycline (TET) on induction of naringenin 7-O-methyltransferase in JA- and CuCl2-treated rice leavesa
Treatment NOMT activity (mkat/ml crude extract)
Control
+TET
+CHX
1.8090.08
0.1490.02 2.6490.12 JA
0.8090.05
0.2290.03 0.9090.07 Cul2
aNOMT activity was assayed 60 h after treatment. Control
does not contain either CHX or TET. Data are means9S.D. of three replicates.
-5%-AMP-agarose matrix and Dr Wolfgang
Knogge, Max-Planck-Institut fu¨r Zu¨chtungs-forschung, Germany for a critical review of the manuscript.
References
[1] B.J. Deverall, Introduction, in: J.A. Bailey, J.W. Mansfield (Eds.), Phytoalexins, Wiley, New York, 1982, pp. 1 – 16.
[2] N.T. Keen, B. Bruegger, Phytoalexins and chemicals that elicit their production in plants, ACS Symp. Ser. 62 (1977) 1 – 26.
[3] I.A.M. Cruikshank, D.R. Perrin, Isolation of a phy-toalexin fromPisum sati6umL, Nature 187 (1960) 799 –
800.
[4] J. Kuc, Phytoalexins, Annu. Rev. Phytopathol. 10 (1972) 207 – 232.
[5] O. Kodama, J. Miyakawa, T. Akatsuka, S. Kiyosawa, Sakuranetin, a flavanone phytoalexin from ultraviolet-ir-radiated rice leaves, Phytochemistry 31 (1992) 3807 – 3809.
[6] R. Rakwal, S. Tamogami, O. Kodama, Role of jasmonic acid as a signal molecule in copper chloride-elicited rice phytoalexin production, Biosci. Biotechnol. Biochem. 60 (1996) 1046 – 1048.
[7] W. Knogge, G. Weissenbock, Purification, characteriza-tion, and kinetic mechanism of S-adenosyl-L -methion-ine:vitexin 2-O-rhamnoside 7-O-methyltransferase of
A6ena sati6aL., Eur. J. Biochem. 140 (1984) 113 – 118. [8] J.E. Poulton, Transmethylation and demethylation
reac-tions in the metabolism of secondary plant products, in: P.K. Stumpf, E.E. Conn (Eds.), The Biochemistry of Plants, Academic Press, New York, 1981, pp. 667 – 723. [9] J.W. McClure, Physiology and function of flavonoids, in:
J.B. Harborne, T.J. Mabry (Eds.), The Flavonoids, Aca-demic Press, New York, 1975, pp. 970 – 1055.
[10] R. Rakwal, M. Hasegawa, O. Kodama, A methyltrans-ferase for the synthesis of the flavanone phytoalexin sakuranetin in rice leaves, Biochem. Biophys. Res. Com-mun. 222 (1996) 732 – 735.
[11] S. Tamogami, R. Rakwal, O. Kodama, Phytoalexin pro-duction by amino acid conjugates of jasmonic acid through induction of naringenin 7-O-methyltransferase, a key enzyme on phytoalexin biosynthesis in rice (Oryza sati6aL.), FEBS Lett. 401 (1997) 239 – 242.
[12] W. Wanek, A. Richter, Purification and characterization ofmyo-inositol 6-O-methyltransferase fromVigna umbel
-lataOhwi et Ohashi, Planta 197 (1995) 427 – 434. [13] A.E. Pakusch, R.E. Kneusel, U. Matern,S-Adenosyl-L
-methionine:trans-caffeoyl-coenzyme A 3-O -methyltrans-ferase from elicitor-treated parsley cell suspension cultures, Arch. Biochem. Biophys. 271 (1989) 488 – 494. [14] J.M. Attieh, A.D. Hanson, H.S. Saini, Purification and
characterization of a novel methyltransferase responsible for biosynthesis of halomethanes and methanethiol in
Brassica oleracea, J. Biol. Chem. 270 (1995) 9250 – 9257. [15] U.K. Laemmli, Cleavage of structural proteins during assembly of the head of bacteriophage T4, Nature 227
(1970) 680 – 685.
[16] E. Steers, G.R. Craven, C.B. Anfinsen, J.L. Bethune, Evidence for nonidentical chains in theb-glactosidase of
Escherichia coliK12, J. Biol. Chem. 240 (1965) 2478 – 2484. [17] H. Schagger, G. vonJagow, Tricine – sodium dodecyl sul-phate-polyacrylamide gel electrophoresis for the separa-tion of proteins in the range from 1 to 100 kDa, Anal. Biochem. 166 (1987) 368 – 379.
[18] M.M. Bradford, A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein – dye binding, Anal. Biochem. 72 (1976) 248 – 254.
[19] J.V. Doorsselaere, B. Dumas, M. Baucher, B. Fritig, M. Legrand, M.V. Montagu, D. Inze, One-step purification and characterization of a lignin-specific O -methyltrans-ferase from poplar, Gene 133 (1993) 213 – 217.
[20] G. Voordouw, G.M. Gaucher, R.S. Roche, Anomalous molecular weights of proteases in gel chromatography, Biochem. Biophys. Res. Commun. 58 (1974) 8 – 12. [21] P. Collazo, L. Montoliu, P. Puigdomenech, J. Rigau,
Structure and expression of the ligninO-methyltransferase gene from Zea mays L., Plant Mol. Biol. 20 (1992) 857 – 867.
[22] C.L. Presig, D.E. Matthews, H.D. VanEtten, Purification and characterization of S-adenosyl-L
-methionine:6a-hy-droxymaackiain 3-O-methyltransferase from Pisum sa
-ti6um, Plant Physiol. 91 (1989) 559 – 566.
[23] R.C. Bugos, V.L. Chiang, W.H. Campbell, cDNA cloning sequence analysis and seasonal expression of lignin-bispe-cific caffeic acid/5-hydroxyferulic acid O-methyltrans-ferase of aspen, Plant Mol. Biol. 17 (1991) 1203 – 1215. [24] P.L. Gregersen, A.B. Christensen, J.S. Knudsen, D.B.
Collinge, A putativeO-methyltransferase from barley is induced by fungal pathogens and UV light, Plant Mol. Biol. 26 (1994) 1797 – 1806.