bean and soybean roots
Marta Albareda1, Marta Susana Dardanelli2, Carolina Sousa2, Manuel Meg´ıas2, Francisco Temprano1& Dulce N. Rodr´ıguez-Navarro1
1CIFA-Las Torres-Tomejil (IFAPA), Alcal ´a del R´ıo, Sevilla, Spain; and2Departamento de Microbiolog´ıa y Parasitolog´ıa, Facultad de Farmacia, Universidad
de Sevilla, Sevilla, Spain
Correspondence:Dulce N. Rodr´ıguez-Navarro, CIFA-Las Torres-Tomejil (IFAPA), 41200 Alcal ´a del R´ıo, Sevilla, Spain. Tel.:134 955 04 55 04; fax:134 955 04 56 25; e-mail: [email protected]
Received 3 January 2006; revised 9 March 2006; accepted 22 March 2006. First published online 21 April 2006.
doi:10.1111/j.1574-6968.2006.00244.x
Editor: Yaacov Okon
Keywords
rhizobacteria; root-attachment; legumes.
Abstract
The plant rhizosphere is an important soil ecological environment for plant– microorganism interactions, which include colonization by a variety of micro-organisms in and around the roots that may result in symbiotic, endophytic, associative, or parasitic relationships within the plant, depending on the type of microorganisms, soil nutrient status, and soil environment. Rhizosphere compe-tence may be attributable to the differences in the extent of bacterial attachment to the root surface. We present results of the effect of various factors on the attachment to bean (Phaseolus vulgaris) and soybean (Glycine max) roots of some bacterial species of agronomic importance, such asRhizobium tropici, Rhizobium etli, Ensifer fredii (homotypic synonym Sinorhizobium fredii), and Azospirillum brasilense; as well as the attachment capability of the plant growth promoting rhizobacteria Pseudomonas fluorescens and Chryseobacterium balustinum. Addi-tionally, we have studied various bacterial traits, such as autoaggregation and flagella movements, which have been postulated to be important properties for bacterial adhesion to surfaces. The lack of mutual incompatibility between rhizobial strains andC. balustinumhas been demonstrated in coinoculation assays.
Introduction
The plant rhizosphere is an important soil ecological environment for plant–microorganism interactions, which involves colonization by a variety of microorganisms in and around the roots. The rhizosphere refers in general to the portion of soil adjacent to the roots of living plants. It supports a diverse and densely populated microbial com-munity, and is subjected to chemical transformations caused by the effect of root exudates and metabolites of microbial degradation. The bacterial communities associated with this microzone are thought to be determined by the quantity and composition of root exudates that serve as substrate for microbial growth. Root exudates can also selectively affect the growth of bacteria and fungi that colonize the rhizo-sphere by serving as selective growth substrates for soil microorganisms. These microbial associations may result in endophytic, symbiotic, associative, or parasitic relationships within the plant, depending on the type of microorganisms, soil nutrient status, and soil environment (Parmar & Da-darwal, 1999). The best-known groups are symbiotic mem-bers of the familyRhizobiaceae, mycorrhizal fungi, and plant growth promoting rhizobacteria (PGPR).
between the symbionts have also been the subject of debate, including culture age, bacterial and root pretreatment con-ditions, bacterial chemotaxis and motility, presence and extension of bacterial fimbriation (Vesper & Bauer, 1986), and bacterial surface polysaccharides (EPS and LPS).
In a framework of sustainable agriculture, microbial soil diversity is promoted by certain practices that lead to better nutrient cycling, disease suppression, and nitrogen fixation. One of these is the introduction of beneficial bacteria into soil, through the use of microbial inoculants (biofertilizers) based on single species (e.g. Rhizobium) inoculants or on several PGPRs species. Biofertilizer is a recently coined term whose exact definition is still unclear, but which most commonly refers to the use of soil microorganisms to increase the availability and uptake of mineral nutrients for plants. The experiments carried out in this work are in the context of a national project whose main goal is to optimize the symbiotic association between soybean/Ensifer frediiand bean/Rhizobiumspp. using mixed inoculants. In this frame-work, it has to be provided that there are no problems of mutual exclusion, displacement, or competence between the inoculant strains. Before these beneficial biofertilizers are formulated and released into the environment, preliminary studies are needed on the first stages of root colonization (attachment) and on absence of mutual incompatibility.
The aim of this work has been the study of some factors that may affect bacterial attachment to legume roots: (i) presence of salt (50 mM of NaCl), (ii) culture age, (iii) size of the inoculum, (iv) pH, and (v) presence of other rhizo-spheric bacteria. Additionally, some bacterial traits related to surface adhesion have been investigated, as well as the ability of coinoculated bacteria to grow in root exudates. Our data suggest that the optimum attachment is dependent of the bacteria added. The presence of moderate salt concentration in the plant rooting medium or acidification of the attach-ment buffer are factors that might affect bacterial adhesion. The culture age of the inoculated bacteria was found to be important for adhesion; thus, attachment of stationary phase cells is greater than that of exponential phase cells. The presence of Chryseobacterium balustinum (up to 105CFU mL 1) affected adhesion ofEnsifer frediistrains to soybean roots. Finally, the degree of rhizobacterial root attachment seems to be independent of certain bacterial surface properties, such as autoaggregation and flagella movement, and of the legume–host species.
Materials and methods
Bacterial strains and growth media
The bacterial strains used in this work were Pseudomonas fluorescensstrain WCS417r (Pieterseet al., 1996), Azospir-illum brasilensestrain Sp7 (Tarrandet al., 1978),
Chryseo-bacterium balustinumstrain Aur9 (Guti´errez-Ma˜neroet al., 2003),Rhizobium tropicistrain CIAT899 (Mart´ınez-Romero
et al., 1991), Rhizobium etli strain ISP42 (Rodr´ıguez-Navarro et al., 2000), E. fredii strain HH103 (Dowdle & Bohlool, 1985) andE. frediistrain SMH12 (Cleyet-Marel, 1987).
All rhizobial and Pseudomonas strains were grown in B medium (Spaink et al., 1992), Azospirillum brasilense
was grown in NFb (D¨obereiner & Baldani, 1979), andC. balustinumwas grown in TY (Beringer, 1974), with shaking at 281C, until stationary (OD600 nm41.0) or late exponen-tial (OD600 nm= 0.5) phase. For studies under saline condi-tions, bacterial media were supplemented with 50 mM of NaCl.
Plant species and growth conditions
We used 7-day-old roots of bean and soybean for attach-ment studies. Surface disinfections of bean (cv. BBL) and soybean (cv. Osumi) seeds, germination, and growth on nitrogen-free medium have been described elsewhere (Ca-macho et al., 2002). Germinated seeds were aseptically transferred to the sterile growth system. This system was designed to allow the growth of seedlings in hydroponic conditions, and consists of a stainless-steel sieve coupled to a glass reservoir ofc. 200 mL (Rigaud & Puppo, 1975). N-free plant nutrient solution (1/2 strength, pH 6.8) was used for plant growth. Plants were maintained in a growth chamber at 281C (14 h) and 181C (10 h) under a photosynthetically active radiation of 124mE m 2s 1 provided by fluorescent
tubes for 7 days.
Roots were then removed; portions ofc. 1 cm were cut off, and collected in 25 mM phosphate buffer (pH 7.5). When saline conditions were imposed, 50 mM of NaCl was added to the nutrient solution. For studies with preinoculated roots, plantlets of bean or soybean were inoculated withC. balustinumstrain Aur9 to a final density of 106CFU mL 1, 15 h or 48 h, respectively, before rhizobial strains were inoculated.
Bacterial attachment assay
Five root pieces (1 cm in length) were aseptically transferred to an Eppendorf tube with 1 mL 25 mM phosphate buffer (pH 7.5) (or pH 5.5 when acidic conditions were imposed). Bacterial cells were harvested by centrifugation at 8064gfor
5 min, washed twice with 25 mM phosphate buffer (pH 7.5), resuspended in 100mL of the same buffer, and then added to
To determine rhizobial attachment based on inoculum size, saturated cultures of CIAT899 and SMH12 strains were obtained (109cells mL 1), and decimal dilutions were in-oculated on bean or soybean roots, respectively. Then the adhesiveness assay was performed as above.
Autoaggregation assay
Autoaggregation assays were performed according to Kos
et al. (2003), with minor modifications. Rhizobial species were grown in yeast-mannitol broth, andPseudomonasand
Chryseobacteriumin liquid TY, at 251C to give cultures ofc. 108CFU mL 1. The cells were harvested by centrifugation at 5000gfor 15 min, washed twice, and resuspended in
phos-phate-buffered saline (PBS). Cell suspensions were mixed by vortexing, and autoaggregation was determined during 24 h of static incubation at room temperatures. At intervals of 4 h, 0.1 mL of the upper layer of the bacterial suspension was transferred to another tube with 3.9 mL of PBS, and the absorbance (A) was measured at 600 nm. Autoaggregation percentage is expressed as 1 (At/A0)100, where At represents the absorbance at different sampling times, and
A0the absorbance att= 0.
Motility assays
Swimming and swarming motilities were analysed according to the methods described by D´eziel et al. (2001). Ensifer frediistrain HH103 was grown on soft yeast-mannitol agar.
Data analysis
All the experiments were carried out in duplicate. One-way ANOVAwas used to analyze the results of the inoculum size assay, using Statistix 7.0. When the analysis of variance showed significant treatment effect, the least significant differences test (LSD,Po0.05) was applied to make com-parisons between the means.
Results
Attachment of different rhizobacteria to legume roots
All the strains used in this work have been tested for their capacity to attach to both bean and soybean roots, under control and saline conditions (Tables 1 and 2). When moderately saline conditions were tested, plantlets and bacteria were grown in the presence of 50 mM NaCl. Attachment of the PGPR strains Pseudomonas fluorescens,
A. brasilense,andC. balustinumto both legume roots was, in general, not affected in the presence of moderate salt concentration, but C. balustinum showed a better attach-ment capacity under saline conditions; moreover,P. fluor-escens WCS417r and C. balustinumAur9 cells did show a significant (Po0.10) preferential adhesion to soybean roots.
Adhesion of rhizobial strains to specific (E. frediistrains/ soybean andR. tropici, R. etli/bean) and nonspecific legume Table 1.Attachment of different plant growth promoting rhizobacteria to legume roots under control and salty conditions
Plant conditions
Rhizobacterial species
Pseudomonas fluorescens(WCS417r) Azospirillum brasilense(Sp7) Chryseobacterium balustinum(Aur9)
Phaseolus vulgaris
Control 5.730.38a 5.020.26a 4.930.40c
Salt 5.670.44a 5.390.33a 5.870.11b
Glycine max
Control 6.220.30a 5.440.43a 6.220.16ab
Salt 6.530.34a 4.820.70a 6.750.17a
Data represent mean log10SD values of attached bacteria, from at least two independent assays. Significant differences (Po0.10) between values
within a column are indicated by different letters. Salt concentration: 50 mM NaCl.
Table 2.Attachment of rhizobial strains to legume roots under control and salty conditions
Plant conditions
Rhizobial strain
Rhizobium tropici(CIAT899) Rhizobium etli(ISP42) Ensifer fredii(SMH12) Ensifer fredii(HH103)
Phaseolus vulgaris Glycine max
Control 6.340.08a 5.430.16a 5.240.07a 5.130.34a
Salt 5.920.27a 4.880.21b 3.640.16b 3.460.06b
Data represent mean log10SD values of attached bacteria, from at least two independent assays. Significant differences (Po0.10) between values
roots was studied (Tables 2 and 3). Ensifer fredii strains SMH12 and HH103 showed a low degree of root adhesion, in comparison with the other rhizobial and PGPR species; moreover, the attachment to soybean roots grown under saline conditions significantly (Po0.10) declined in rela-tion to control roots. Of the rhizobial bean-nodulating species,R. tropicistrain CIAT899 showed a higher degree of root attachment than didR. etlistrain ISP42; however, there was no differences between species in the attachment to bean or soybean roots (nonspecific legume) (data not shown). The presence of salt significantly reduced the adhesion ofR. etlito bean roots (Table 2).
Effect of culture age, inoculum size, and pH on root attachment
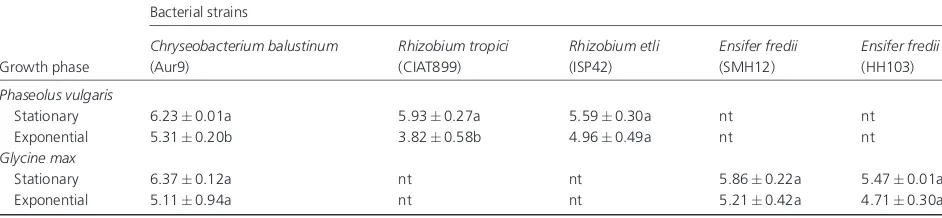
The effect of culture age of the inoculant strains on root attachment was investigated with specific rhizobia/legume combinations and withC. balustinumAur9 on both legume species (Table 3). In general, bacterial cells at stationary phase showed a higher capacity to adhere to roots, but in only two of six cases studied (C. balustinum/PhaseolusandR. tropici/ Phaseolus) was the rate of adhesion significantly higher (Po0.10). The effect of the size of the inoculum ofR. tropici CIAT899 andE. frediiSMH12 strains on the attachment to bean and soybean roots, respectively, was tested (Table 4). In the case of SMH12, a significantly higher number of rhizobia attached to roots when the applied inoculum was in the range of 108–109CFU mL 1 compared with diluted inocula (107CFU mL 1). More-dilute inocula (105–106CFU mL 1) gave levels of attachment below quantifiable limits (BQL). In contrast, saturated cultures of CIAT899 led to significantly fewer attached cells, with 105–106CFU mL 1giving a max-imum level of root adhesion.
The effect of pH on the attachment of three rhizobacteria to the two legumes was studied. Both specific and nonspe-cific rhizobial strains and C. balustinumwere selected for these studies. The pH of the incubation buffer had a slight effect on the bacterial attachment to legume roots. At acidic pH (5.5), there was in general a lower ratio of adhesion to
roots, but with some exceptions (SMH12/Phaseolus and Aur9/Glycine) (data not shown).
Bacteria traits associated with adhesion
In order to elucidate whether some bacterial traits might be responsible for the low degree of rhizobial root attachment, we performed studies of autoaggregation and flagella-asso-ciated movement, as bacterial properties linked to surface adhesion. There was no correlation between root attach-ment and the autoaggregation ability of the studied bacteria. The highest percentage of autoaggregation was shown byE. frediistrains SMH12 (47–62%) and HH103 (65–88%) after 24 h of incubation; on the other hand, these strains had shown the lowest degree of attachment. For the other bacterial species, the percentage of autoaggregation varied from 30% to 40%.
All the studied bacteria showed both modes of flagella-associated motilities. Swimming motility (owing to the presence of polar flagella) was determined on plates (0.3% agar). In the rhizobial strains, the greatest motility was observed with R. etli strain ISP42 (90 mm) and E. fredii
strain HH103 (80 mm) cells. The rhizobacteriaP. fluorescens
showed the greatest movement (75 mm), andC. balustinum
showed a dispersal growth from the point of inoculation without clear growth rings. Swarming motility (owing to the presence of lateral flagella) was determined on 0.5% agar plates. All the strains showed a very uniform swarming ability, with growth rings ranging from 10 to 30 mm.
Attachment of rhizobial strains to preinoculated roots withC. balustinum
The effect of the presence ofC. balustinum(Aur9) in the rooting system of bean and soybean plantlets was investi-gated, before the rhizobial attachment assay using specific rhizobia/legume combinations, under control and saline conditions [Tables 5(a) and (b)]. Strain Aur9 was added to the plant rooting medium 14 h (bean) or 48 h (soybean) before the attachment assay. The presence of Aur9 (ranging
Table 3. Effect of bacterial growth phase on the attachment of rhizobacterial strains
Growth phase
Bacterial strains
Chryseobacterium balustinum (Aur9)
Rhizobium tropici (CIAT899)
Rhizobium etli (ISP42)
Ensifer fredii (SMH12)
Ensifer fredii (HH103)
Phaseolus vulgaris
Stationary 6.230.01a 5.930.27a 5.590.30a nt nt
Exponential 5.310.20b 3.820.58b 4.960.49a nt nt
Glycine max
Stationary 6.370.12a nt nt 5.860.22a 5.470.01a
Exponential 5.110.94a nt nt 5.210.42a 4.710.30a
Data represent mean log10SD values of attached bacteria, from at least two independent assays. Significant differences (Po0.10) between values
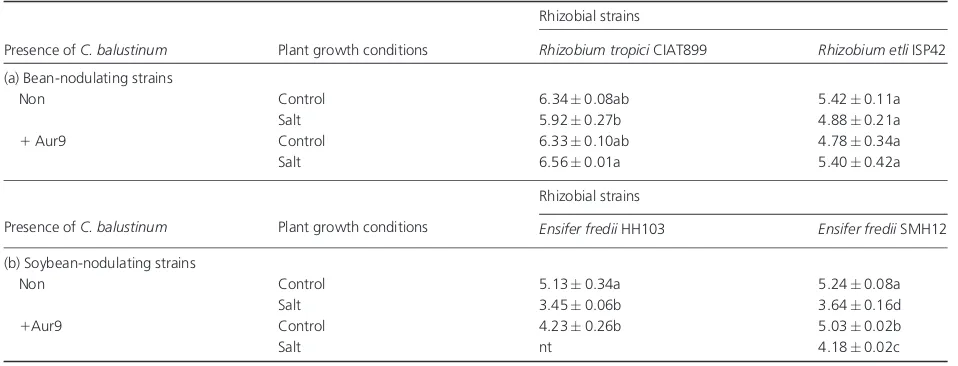
103–104CFU mL 1) did not impair the capacity of bean-nodulating species to attach to bean roots. In the case of
E. frediistrains, the presence of Aur9 (104–106CFU mL 1) lessened their capacity of attachment to soybean roots; however, under saline conditions, the adhesion of the SMH12 strain to preinduced soybean roots was significantly better than to control roots. The adhesion of the CIAT899 strain to preinduced bean roots, under saline conditions, was also better. In the case of theR. etliISP42 strain, there was no variation in its attachment capacity under the different assay conditions.
Discussion
PGPRs have drawn much attention in recent years because of their contribution to the biological control of plant pathogens and the improvement of plant growth. Inocula-tion of plants with ‘dual’ microbial inoculants, or even a consortium of them, is becoming more important in a framework of sustainable agriculture for the advantage their beneficial effects afford, providing there is no competition
between inoculants. However, the practical use of these beneficial bacteria sometimes fails because of their inability to colonize the rhizosphere or the rhizoplane of inoculated plants. Colonization of roots by inoculant strains thus appears to be a critical step in the interaction between beneficial bacteria and host plants. Attachment of rhizobia to host roots seems to be the first requisite step in infection and nodulation (Bohlool & Schmidt, 1974), and is consid-ered one of the physiologically important characteristics in determining competitive ability among rhizobial strains (Smith & Wollum, 1991).
Root colonization is defined as a complex phenomenon depending upon several biotic and abiotic factors. In this work, we have defined a hydroponic system to grow plants under bacteriologically controlled conditions, allowing changes in the rooting media (saline or control conditions), the controlled addition of bacterial inoculants, or pH changes. The attachment assay used in this work is similar to that of Dardanelli et al. (2003), and the values for bacterial attachment presented here can be considered those of tightly attached bacteria. Chabot et al. (1996) have described rhizobial strains that were better colonizers of maize and lettuce roots than were other PGPR bacteria; our results, however, indicated that the studied PGPRs are in general better colonizers of the two legumes than are the specific rhizobial species, except Rhizobium tropici/bean roots. An attempt was made to correlate the low degree of rhizobial attachment with physicochemical characteristics of the cell surface by measuring the autoaggregation of the studied rhizobacteria, as well as the flagella-associated motilities. There is a relationship between the bacterial ability for autoaggregation and that of adhesion to different Table 4.Effect of bacterial inoculum size on the attachment of rhizobial
strains to specific legume–host roots
Strains
Inoculum (Lg CFU mL1)
9 8 7 6 5
SMH12 52.4b 84.3a 11.1c BQL BQL
CIAT899 88.8c 260.8b 245.8b 925.5a 803.8a
Data are means of two replicates. Significant differences (Po0.05)
between values within each row are indicated by different letters. BQL, below quantifiable limits.
Table 5.Attachment of (a) bean-nodulating and (b) soybean-nodulating strains to non- or preinoculated roots withChryseobacterium balustinum strain Aur9
Presence ofC. balustinum Plant growth conditions
Rhizobial strains
Rhizobium tropiciCIAT899 Rhizobium etliISP42
(a) Bean-nodulating strains
Non Control 6.340.08ab 5.420.11a
Salt 5.920.27b 4.880.21a
1Aur9 Control 6.330.10ab 4.780.34a
Salt 6.560.01a 5.400.42a
Presence ofC. balustinum Plant growth conditions
Rhizobial strains
Ensifer frediiHH103 Ensifer frediiSMH12
(b) Soybean-nodulating strains
Non Control 5.130.34a 5.240.08a
Salt 3.450.06b 3.640.16d
1Aur9 Control 4.230.26b 5.030.02b
Salt nt 4.180.02c
Data represent mean log10SD values of attached bacteria, from at least two independent assays. Significant differences (Po0.10) between values
surfaces (Zaady & Okon, 1990; Koset al., 2003). Our data agree with those of Zaady & Okon, as they have shown that
Azospirillumcell treatments leading to strong inhibition of aggregation culminate in the greatest adsorption to maize roots. In our case, the highest autoaggregation percentage was shown by E. fredii strains, which showed the lowest degree of root attachment. However, our results do not agree with those obtained by Koset al. (2003) usingLactobacillus
strains. It has been suggested that anionic repulsions between root and rhizobia may explain why only a small proportion of a rhizobial population attaches to legume roots (Vesper & Bauer, 1985; Caetano-Anolles & Favelukes, 1986). In addition, bacterial cell movements because of flagella have been linked to adherence of some Gram-negative gastrointestinal bacteria to epithelial cellsin vitro
and to other inert surfaces. We have determined the modes of flagella-associated motilities, owing to polar and lateral flagella, and found no defective swimming or swarming motilities that might explain the differences in root attach-ment capacity of the studied bacteria.
The level of rhizobial adhesion to the specific or non-specific host legume roots was similar for all studied species, although bean-nodulating rhizobia are better colonizers than soybean-E. frediimicrosymbionts. Nevertheless, in our study the proportion of attached cells with respect to inoculated levels is much lower than that reported in other studies (Vesper & Bauer, 1985, 1986). These results reinforce the concept of lack of specificity of host legume exudates for their homologous rhizobial microsymbionts, as has been largely demonstrated (Pueppke, 1984; Vesper & Bauer, 1985; Dolhem-Biremon et al., 1993). A nonspecific mechanism, independent of the symbiotic properties, must contribute to root attachment of rhizobia, as the same colonization level has been reported for P. fluorescens and Sinorhizobium melilotiin alfalfa roots (Villacieroset al., 2003). However, a certain specificity of plant exudates to their bacterial isolates have also been proposed (Mandimbaet al., 1986; Bacilio-Jim´enezet al., 2003).
It has been reported that culture age affects the extent of bradyrhizobial attachment to soybean roots, and that it is strain-dependent (Smith & Wollum, 1991). We have also found that culture age has a distinct effect on root attach-ment of certain strains, and there was a general and positive tendency for a greater attachment level in stationary cells. Although bacterial cultures were twice washed and centri-fuged with buffer, so that extracellular polymeric substances should have been removed, when cells enter the stationary phase of growth, other morphological and biochemical changes occur that might modify the degree of attachment. Moreover, acidification (pH 5.5) of the attachment buffer did not greatly affect the adhesion of studied bacteria in comparison with control buffer (pH 7.5). Possible modifica-tions of the bacterial cell surface during 1 h of incubation
might account for the slight differences observed, as changes in root surface are probably negligible; in fact, the final pH of the rooting medium usually decreases after 1 week of root development. Using two organic buffering systems, Smith & Wollum (1993) found an optimum pH range (6.0–6.5) for the attachment ofBradyrhizobium japonicumUSDA110 to soybean roots. By contrast, root surface modifications occurring when 50 mM NaCl was added to the rooting system might be strong grounds for some differences in bacterial root attachment under saline conditions.
Root attachment was dose-dependent in the case ofE. frediiSMH12 strain, and optimum adhesion was found at a concentration of 108–109CFU mL 1, in agreement with other results (Jjemba & Alexander, 1999). However, in the case of R. tropici CIAT899, a large inoculum gave poor attachment to bean roots. Differences in the optimum attachment of these strains with varying inoculum size might be attributable to differential bacterial traits rather than to a limitation of binding sites in these root-segment assays, as most of the attachment trials have been performed with saturated bacterial inocula or in the presence of other bacteria (preinoculated C. balustinum roots), and small percentages of rhizobial populations were consistently found to adhere firmly to roots.
The presence ofC. balustinumAur9 strain in the rooting media 15–20 h before rhizobial-attachment assays did not affect the adhesion of bean-nodulating species, but did lessen the attachment ofE. frediistrains; perhaps the higher level of Aur9 in the soybean rooting media was the reason for this difference.
The study has demonstrated that the coinoculation of Aur9 and rhizobial strains provides the same growth capa-city in root exudates as that of single inocula, but further studies of proper root colonization dynamics, using whole root systems, are necessary to reveal the actual binding points on the roots, and to demonstrate persistence of the studied strains in the rhizosphere.
Acknowledgements
This research was founded by the Ministerio de Educaci ´on y Ciencia (Spain). Project: AGL2002-04188-CO6.
References
Bacilio-Jim´enez M, Aguilar-Flores S, Ventura-Zapata E, P´erez-Campos E, Bouquelet S & Zenteno E (2003) Chemical characterization of root exudates from rice (Oryza sativa) and their effects on the chemotactic response of endophytic bacteria.Plant Soil249: 271–277.
Beringer JE (1974) R factor transfer inRhizobium leguminosarum.
Bohlool BB & Schmidt EL (1974) Lectins: a possible basis for specificity in theRhizobium–legume symbiosis.Science185: 269–271.
Caetano-Anolles G & Favelukes G (1986) Quantitation of adsorption of rhizobia in low number to small legume roots.
Appl Environ Microbiol52: 371–376.
Camacho M, Santamar´ıa C, Temprano F,et al. (2002) Soils of the Chinese Hubei province show a very high diversity of
Sinorhizobium frediistrains.Syst Appl Microbiol25: 592–602. Chabot R, Antoun H, Kloepper JW & Beauchamp CJ (1996) Root
colonization of maize and lettuce by bioluminescent
Rhizobium leguminosarumbiovarphaseoli.Appl Environ Microbiol62: 2767–2772.
Cleyet-Marel JC (1987) Dynamique des populations de
Rhizobiumet deBradyrhizobiumdans le sol et la rhizosphere, PhD thesis, University Claude Bernard-Lyon, France. Dardanelli M, Angelini J & Fabra A (2003) A calcium-dependent
bacterial surface protein is involved in the attachment of rhizobia to peanut roots.Can J Microbiol49: 399–405. D´eziel E, Comeau Y & Villemur R (2001) Initiation biofilm
formation byPseudomonas aeruginosa57RP correlates with emergence of hyperpiliated and highly adherent phenotype variants deficient in swimming and swarming, and twitching motilities.J Bacteriol183: 1195–1204.
D¨obereiner J & Baldani VLD (1979) Selective infection of maize roots by streptomycin-resistantAzospirillum lipoferumand other bacteria.Can J Microbiol25: 1264–1269.
Dolhem-Biremon C, Mary P & Tailliez R (1993) Comp´etition entre souches deRhizobiaceae: mise en ´evidence de diff´erents modes d’adh´esion sur les racines de soja.Can J Microbiol39: 982–986.
Dowdle SF & Bohlool BB (1985) Predominance of fast growing
Rhizobium japonicumin a soybean field in the People’s Republic of China.Appl Environ Microbiol50: 1171–1176. Guti´errez-Ma˜nero FJ, Probanza A, Ramos B, Col ´on-Flores JJ &
Lucas-Garc´ıa JA (2003) Effects of culture filtrates of rhizobacteria isolated from wild lupine on germination, growth and biological nitrogen fixation of lupine seedlings.
J Plant Nutr26: 1101–1115.
Jjemba PK & Alexander M (1999) Possible determinants of rhizosphere competence of bacteria.Soil Biol Biochem31: 623–632.
Kos B, Sˇusˇkovi´c J, Vukovi´c S, Sˇimpraga M, Frece J & Matosˇic S (2003) Adhesion and aggregation ability of probiotic strain
Lactobacillus acidophilusM92.J Appl Microbiol94: 981–987. Law IJ, Yamamoto Y, Mort AJ & Bauer WD (1982) Nodulation of
soybeanRhizobium japonicummutants with altered capsule synthesis.Planta154: 100–109.
Mandimba G, Heulin T, Bally R, Guckert A & Balandreau J (1986) Chemotaxis of free-living nitrogen-fixing bacteria towards maize mucilage.Plant Soil90: 129–139.
Mart´ınez-Romero E, Segovia L, Mercante FM, Franco AA, Graham P & Pardo MA (1991)Rhizobium tropicia novel
species nodulatingPhaseolus vulgarisL. beans andLeucaena
spp. trees.Int J Syst Bacteriol41: 417–426.
Meyer MC & Pueppke SG (1980) Differentiation ofRhizobium japonicumderivatives by antibiotic sensitivity partners, lectin binding and utilization of biochemicals.Can J Microbiol26: 606–612.
Parmar N & Dadarwal KR (1999) Stimulation of nitrogen fixation and induction of flavonoids like compounds by rhizobacteria.J Appl Microbiol86: 36–44.
Pieterse CMJ, van Wees SCM, Hoffland E, van Pelt JA & van Loon LC (1996) Systemic resistance inArabidopsisinduced by biocontrol bacteria is independent of salicylic acid
accumulation and pathogenesis-related gene expression.Plant Cell8: 1225–1237.
Pueppke SG (1984) Adsorption of slow- and fast-growing rhizobia to soybean and cowpea roots.Plant Physiol75: 924–928.
Rigaud J & Puppo A (1975) Indole-3-acetic acid catabolism by soybean bacteroids.J Gen Microbiol88: 223–228.
Rodr´ıguez-Navarro DN, Buend´ıa AM, Camacho M, Lucas MM & Santamar´ıa C (2000) Characterization ofRhizobiumspp. bean isolates from South-West Spain.Soil Biol Biochem32: 1601–1613.
Smith GB & Wollum AG (1991) Bacterial culture history affects the attachment ofBradyrhizobium japonicumto hostGlycine maxroots.Can J Microbiol37: 730–736.
Smith GB & Wollum AG (1993) Physicochemical andD
-galactose-mediated interactions in the attachment of
Bradyrhizobium japonicumto roots ofGlycine max.Can J Microbiol39: 245–251.
Spaink HP, Aarts A, Stacey G, Bloemberg GV, Lugtemberg BJJ & Kennedy EP (1992) Detection and separation of
RhizobiumandBradyrhizobiumNod metabolites using thin-layer chromatography.Mol Plant-Microbe Interact5: 72–80.
Tarrand JJ, Krieg NR & D¨obereiner J (1978) A taxonomic study of theSpirillum lipoferumgroup, with descriptions of a new genus,Azospirillumgen. nov. and two species,Azospirillum lipoferum(Beijerinck) comb. nov. andAzospirillum brasilense
sp. nov.Can J Microbiol24: 967–980.
Vesper SJ & Bauer WD (1985) Characterization of
Rhizobiumattachment to soybean roots.Symbiosis1: 139–162.
Vesper SJ & Bauer WD (1986) Role of pili (fimbriae) in attachment ofBradyrhizobium japonicumto soybean roots.
Appl Environ Microbiol52: 134–141.
Villacieros M, Power B, S´anchez-Contreras M,et al. (2003) Colonization behaviour ofPseudomonas fluorescensand
Sinorhizobium melilotiin the alfalfa (Medicago sativa) rhizosphere.Plant Soil251: 47–54.
Zaady E & Okon J (1990) Cultural conditions affecting