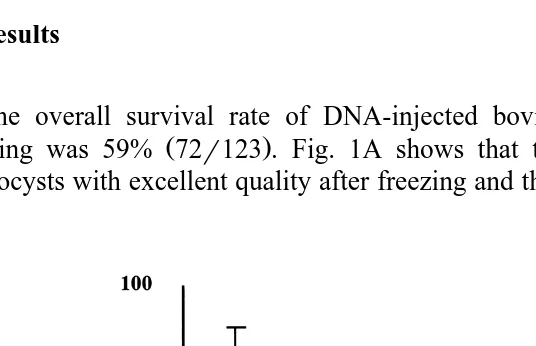www.elsevier.comrlocateranireprosci
Blastocyst viability and generation of transgenic
cattle following freezing of in vitro produced,
DNA-injected embryos
Y.M. Han
a, S.J. Kim
a, J.S. Park
a, I.Y. Park
a, Y.K. Kang
a,
C.S. Lee
a, D.B. Koo
a, T.H. Lee
a, D.Y. Yu
a, Y.H. Kim
b,
K.J. Lee
b, K.K. Lee
a,)a
Korea Research Institute of Bioscience and Biotechnology, P.O. Box 115, Yusong, Taejon 305-600, South Korea
b
Doosan DeÕelopment Business Group, Taean-Gun, Chungnam 357-960, South Korea
Received 4 January 2000; received in revised form 25 April 2000; accepted 15 May 2000
Abstract
This study examined whether the viability, determined in vitro, of DNA-injected bovine embryos produced in vitro was affected by freezing, and if the frozen embryos developed to term following transfer to recipients. In vitro fertilized zygotes were injected with the pBL1 gene and
Ž .
then co-cultured with mouse embryonic fibroblasts MEF in CR1aa medium. Embryos were
Ž .
prepared for cryopreservation by exposure to a 10% vrv glycerol solution, loaded into 0.25 ml straws and then frozen by conventional slow freezing. Thawing was by rapid warming in water
Ž378C. and embryos were rehydrated in PBS diluents of 6%, 3% and 0% Žvrv. glycerol
Ž .
supplemented with 0.25 M sucrose and 0.5% wrv BSA. In Experiment 1, blastocysts that developed from DNA-injected embryos were individually classified into three morphological groups and three stages of development prior to freezing. DNA-injected blastocysts of excellent
Ž .
quality at freezing showed a higher survival rate 78.8"10.6% after thawing than those of good
Ž60.9"16.4% or fair 12.5. Ž "5.9% quality P. Ž -0.05 . Post-thaw survival rate, judged in vitro,. Ž
increased with more advanced stage of blastocyst development at freezing early 48.8"15.9%,
.
mid 52.1"12.6% and expanded 71.2"1.1; P-0.05 . In Experiment 2, the frozenrthawed embryos were transferred to recipients to examine in vivo viability. Following transfer of one or
Ž .
two embryos per recipient, pregnancy rates at 60 days of gestation were 13.6% 13r96 for frozen
)Corresponding author. Tel.:q82-42-860-4420; fax:q82-42-860-4608.
Ž .
E-mail address: [email protected] K.K. Lee .
0378-4320r00r$ - see front matterq2000 Elsevier Science B.V. All rights reserved.
Ž .
Ž . Ž .
embryos and 26.5% 43r162 for fresh embryos P-0.05 . Of the 12 live calves born from the
Ž .
frozenrthawed embryos, two males 18.3% were transgenic. None of the live-born calves derived from fresh embryos exhibited the transgene. One of transgenic bulls did not produce transgenic
Ž .
sperm. Three out of 23 calves 13.0% produced from cows inseminated with semen of the other bull were transgenic, suggesting that this animal was a germ-line mosaic. These studies indicated that the viability of in vitro produced, DNA-injected bovine blastocysts was affected by freezing and by both the quality and stage of development of the embryo prior to freezing. The generation of transgenic cattle demonstrates that it is feasible to freeze DNA-injected, in vitro produced embryos.q2000 Elsevier Science B.V. All rights reserved.
Keywords: Cattle; Embryology; DNA injection; Cryopreservation; Transgenic; Transmission
1. Introduction
Ž . Ž . Ž .
In vitro maturation IVM , fertilization IVF and culture IVC , DNA injection and embryo transfer are complex techniques that have been used to produce transgenic cattle
Ž
in recent years Krimpenfort et al., 1991; Bowen et al., 1994; Hyttinen et al., 1994;
.
Eyestone et al., 1998 . Transgenic animals that produce human pharmaceutical proteins have many benefits compared to conventional production systems using microorganisms
Ž .
or animal cells Janne et al., 1992; Bremel, 1996 . The advantages include high productivity, low operating costs, appropriate post-translational modification of proteins, and the production of transgenic progeny by most transgenic animals. Problems associ-ated with production of transgenic cattle are the supply of in vivo fertilized zygotes, the low developmental potential of injected embryos and their high costs of production. Production of transgenic livestock by pronuclear injection is inefficient, with a low
Ž .
integration frequency around 1% of injected embryos Wall, 1996 . In vitro embryo production procedures have been used to provide a large number of synchronous zygotes
Ž .
for pronuclear microinjection Krimpenfort et al., 1991; Janne et al., 1992 . Embryos that develop successfully in culture can be biopsied or bisected to detect the transgene
Ž . Ž .
by DNA polymerase chain reaction PCR Janne et al., 1992 . Only those embryos containing the transgene are then transferred into recipients, thus increasing the probabil-ity of transgenic calves being born. Even with these advances, the process of producing transgenic cattle remains inefficient. Recently, revolutionary techniques giving rise to 100% transgenesis in mammals, the so-called nuclear transfer using transformed somatic
Ž .
cells, have been achieved Schnieke et al., 1997; Cibelli et al., 1998; Brink et al., 2000 . Alternatively, cryopreservation may increase utilization of DNA-injected bovine em-bryos. Studies on cryopreservation of IVF-derived bovine embryos have been reported
ŽSuzuki et al., 1993; Han et al., 1994 . Transfer of the frozen IVF embryos results in a. Ž
lower pregnancy rate than that of fresh IVF embryos Hasler et al., 1995; Agca et al.,
.
1998 . Factors affecting the in vivo viability after transfer of DNA-injected bovine
Ž .
blastocysts produced in vitro have been investigated Han et al., 1996 . However, little information has been reported on the in vitro and in vivo viabilities of DNA-injected bovine embryos after freezing and thawing.
exam-ined whether transgenic bulls derived from frozenrthawed embryos pass the transgenes to their progeny through the germ-line.
2. Materials and methods
2.1. IVM and IVF
Ž .
IVM and IVF of bovine oocytes were performed as described by Han et al. 1996 . Briefly, 10–20 immature oocytes obtained from slaughtered Holstein cows were
cul-Ž
tured in 0.5 ml of maturation medium containing 1mgrml estradiol Sigma, St Louis,
. Ž .
MO and 1 mgrml FSH-Pe Schering-Plough Animal Health, Kenilworth, NJ for 22–24 h at 38.58C, 5% CO2 in humidified air. The maturation medium consisted of
Ž .
TCM-199 with Eagle’s salts and L-glutamine supplemented with 10% vrv
heat-in-Ž .
activated fetal bovine serum FBS; Gibco BRL and 25 mM NaHCO . After IVM, 103 oocytes were fertilized with frozen–thawed sperm at a concentration of 2=106rml in
Ž .
50 ml of fertilization medium Bavister and Yanagimachi, 1977 . When sperm were added to the fertilization drops, 2 mgrml heparin, 20 mM penicillamine, 10 mM
Ž .
hypotaurine and 1mM epinephrine PHE were also added. After 18 h of insemination, cumulus-enclosed oocytes were stripped by vortexing for 2 min and placed in CR1aa medium before DNA injection. CR1aa medium was formulated according to the
Ž .
procedures of Rosenkrans et al. 1993 , and supplemented with 1 mM glutamine and
Ž .
1=Eagle’s essential amino acids solution Gibco BRL .
2.2. Microinjection of DNA
The DNA used for microinjection was 5.5 kbp fragments of an expression vector, pBL1, which consisted of the bovineb-casein gene promoter, human lactoferrin cDNA
Ž .
and SV40 polyadenylation signal Kim et al., 1994 . The DNA concentration was adjusted to 4mgrml in a buffer of 10 mM Tris–HCl, pH 8.0, and 0.1 mM EDTA. The microinjection technique was similar to the methods previously described for pig and
Ž .
sheep embryos Hammer et al., 1985 . In order to visualize the pronuclei, cumulus-free oocytes were centrifuged in 1 ml TL–Hepes medium at 15 000=g for 7 min in a
Ž .
microcentrifuge Vision Scientific, Korea . The embryos were then injected into one pronucleus with a DNA solution at 21–25 h after insemination. Swelling of the pronucleus indicated that the injection had been successful. After DNA injection, groups of 5–10 surviving zygotes were cultured for 2 days in 50 ml drops of CR1aa medium
Ž .
supplemented with 3 mgrml BSA under light mineral oil Sigma .
( )
2.3. Mouse embryonic fibroblasts MEF monolayer
Ž .
then finely minced with microscissors. The minced tissues were washed three times with PBS free of Ca2q and Mg2q ions, and trypsinized in 0.1% trypsinr0.05% EDTA solution while being stirred. Suspended cells were sieved through a stainless mesh
ŽIkemoto, Tokyo, Japan and centrifuged at 700. =g for 5 min. The cell pellet from each
Ž .
fetus was suspended in DMEM Gibco BRL containing 10% FBS and the cell suspension was placed in a 100 mm petri dish. The primary fibroblasts were cultured for 2 days to confluency at 378C, 5% CO2 in humidified air, proliferated through two subsequent passages as described above and then frozen at y708C. The freezing
Ž . Ž .
medium for the fibroblasts was 10% vrv glycerol plus 50% vrv FBS in PBS.
Ž .
Frozen MEF were thawed in 378C water and then grown in petri dishes 100 mm for 2
Ž .
days to confluency. MEF were treated with 10 mgrml mitomycin C Sigma to cause inactivation for 2.5 h and then harvested. To co-culture bovine embryos, mitomycin C-treated cells were rinsed three times with PBS, suspended in DMEM containing 10% FBS and then plated at a concentration of 6=105cellsrml in a four-well dish to be a monolayer. Approximately 1 h later, almost all DMEM was removed and replaced with 0.5 ml of CR1aa medium containing 10% FBS at least 2 h before use.
2.4. IVC
Ž .
Ten to 15 cleaved four- to eight-cell embryos were selected at approximately 48 h after DNA injection and then further co-cultured with MEF for 5 days in 0.5 ml of
Ž .
CR1aa medium supplemented with 10% vrv FBS at 38.58C, 5% CO in humidified2 air. At day 5 of culture, the culture medium was supplemented with 5.56 mM glucose
Ž .
and 1=GMS-X supplement Gibco BRL without medium change. GMS-X supplement solution consisted of 1.0 mgrml insulin, 0.67 mgrml sodium selenite, 0.55 mgrml transferrin and 0.2 mgrml ethanolamine. The day of in vitro fertilization was designated as day 0 of culture.
2.5. Freezing and thawing of embryos
The blastocysts that developed from DNA-injected bovine embryos were separately
Ž .
washed in PBS supplemented with 15% vrv FBS and then frozen by a modified
Ž .
conventional slow freezing method Massip et al., 1987 . Briefly, the embryos were
Ž .
equilibrated for 10 min in freezing medium, consisting of 10% vrv glycerol and 50% FBS in PBS at room temperature and then loaded into 0.25 ml straws with a maximum
Ž
of five embryos per straw. The straws were sealed with straw powder FHK, Fujihira
.
Industry, Japan and placed aty58C in the cooling chamber of an automatic cell freezer
M sucrose and 0.5% BSA. The embryos were stepwise washed in two fresh solutions of PBS containing 30% and 15% FBS, respectively, and then co-cultured for at least 12 h
Ž .
in CR1aa supplemented with 10% vrv FBS on MEF monolayers at 38.58C, 5% CO2 in air prior to embryo transfer. Survival of frozen–thawed bovine embryos were evaluated in terms of their morphological recovery and re-expansion of blastocysts after approximately 12 h.
2.6. Embryo transfer
Experiments were conducted according to The Animal Care and Use Committee guidelines of the Korean Research Institute of Bioscience and Biotechnology. DNA-in-jected blastocysts were transferred to recipients by a non-surgical method. All recipients were virgin Holstein heifers, 14 to 18 months old. One or two blastocysts, depending on the availability of embryos each day, were transferred to the unilateral uterine horn of a recipient heifer on day 7 or 8 after a spontaneous estrus. Following transfer, pregnancy was confirmed by rectal palpation at approximately 60 days of gestation. The pregnant recipients were normally managed to deliver their calves at the term.
2.7. Experimental designs
2.7.1. Experiment 1
It was investigated whether in vitro viability of DNA-injected bovine embryos produced in vitro was affected by freezing. All blastocysts that developed from
Ž
DNA-injected embryos were individually classified into three grades excellent, good
. Ž
and fair embryos and different developmental stages early, mid and expanded
blasto-. Ž .
cysts by morphological appearance Lindner and Wright, 1983; Han et al., 1994 . Embryos judged with the same quality and developmental stage were loaded into a straw and then frozen by a modified conventional slow freezing method. Following thawing, the survival of frozen embryos was evaluated in terms of their morphological recovery and re-expansion of blastocysts after approximately 12 h of culture.
2.7.2. Experiment 2
In vivo viability of DNA-injected, frozen blastocysts were examined following transfer to recipients. In this study, a total of 457 embryos was loaded into 217 straws at a maximum of five embryos per straw and frozen. The blastocysts surviving after thawing were transferred to recipients by a non-surgical method. In the frozen group, the number of single and twin transfers per recipient was 39 and 57, respectively. In the fresh group, single embryos were individually transferred to 82 recipients and transfers of two embryos per recipient were performed to 80 recipients. Following transfer, the recipients were observed for signs of estrus, and pregnancy was confirmed by palpation per rectum at approximately 60 days of gestation. Transfers of DNA-injected embryos
Ž .
were carried out over 21 2 years March 1994 to September 1996 .
2.8. Identification of transgene
Chromosomal DNAs from ear and blood samples of calves were isolated as described
Ž . Ž
.
mg from each calf was digested with DraI, EcoRI or HindIII and then separated on a 0.7% agarose gel. After transfer to a nylon membrane, the DNA was hybridized with a 32
P-labeled DNA probe for 24 h, rinsed with 0.1=SSCr0.1% SDS solution and then exposed to an X-ray film. The DNA used for the probe was the 2.0 kb SmaI–EcoRI
Ž .
fragments of human lactoferrin cDNA Kim et al., 1994 .
2.9. Statistical analysis
The pregnancy rate after transfer of DNA-injected bovine embryos was analyzed by the Student’s t-test. The survival rate of the DNA-injected bovine embryos after freezing and thawing was analyzed by the Duncan’s multiple range test using the general linear models in the statistical analysis system. Survival rates of DNA-injected bovine blasto-cysts after freezing and thawing according to embryo quality and developmental stage are described as mean"SD. All percentage data were subjected to arcsine transforma-tion. Probability of P-0.05 was considered to be statistically significant.
3. Results
The overall survival rate of DNA-injected bovine blastocysts after freezing and
Ž .
thawing was 59% 72r123 . Fig. 1A shows that the survival rate of DNA-injected
Ž .
blastocysts with excellent quality after freezing and thawing 78.8"10.6%, 37r46 was
Ž . Ž .
Fig. 1. Effects of embryo quality A and developmental stage B on the survival rate of DNA-injected bovine embryos after freezing and thawing. Differences were significantly detected in the survival rate of the embryos
Ž .
Ž . Ž . Ž
higher than those of good P-0.05 and fair P-0.05 quality 60.9"16.4%, 32r53
.
and 12.5"5.9%, 3r24, respectively . The difference between good and fair was
Ž .
significant P-0.05 . Survival rates of DNA-injected bovine blastocysts at different stages of development when frozen are shown in Fig. 1B. Expanded blastocysts
Ž71.2"1.1%, 35r49 showed higher survival rate than those of early 52.1. Ž "12.6%,
. Ž . Ž .
31r61 and mid- 48.8"15.9%, 6r13 blastocysts P-0.05 .
After IVM and IVF, 10 252 zygotes were injected with pBL1 and cultured in CR1aa
Ž
medium supplemented with 4 mgrml BSA for 48 h. When cleaved embryos 52.6%,
.
5391r10 252 were further co-cultured with MEF for 5 days, the developmental rate to
Ž .
blastocyst stage was 13.4% 722r5391 . Transfer of the frozen–thawed DNA-injected
Ž .
bovine embryos resulted in a pregnancy rate of 13.6% 13r96, Table 1 . This pregnancy
Ž . Ž .
rate was lower than that 26.5%, 43r162 of transferred fresh embryos P-0.05 . Most
Ž .
pregnant recipients transferred with frozen embryos delivered normal live calves 12r13 and one calf was still-born. In the fresh embryo transfer group, the number of abortions and still-births was 9r43. In this study, no histopathological tests were not performed on the still-born calves. A preponderance of male calves was produced from transfer of
Ž . Ž .
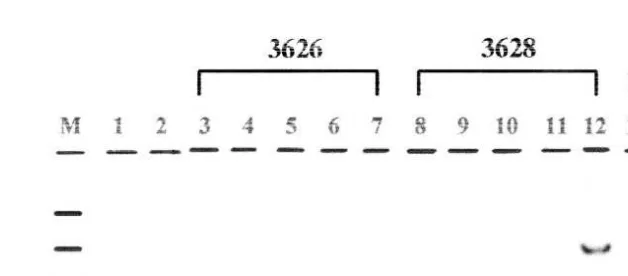
both fresh M:F 9:3 and frozenrthawed M:F 20:14 embryos. Two calves derived from DNA-injected embryos after freezing and thawing were identified as transgenic by Southern blot analysis, although no transgenic animals were detected in the calves resulting from fresh embryo transfers. Both the transgenic calves were males and appeared to be normal. Fig. 2 shows the Southern blot of the first transgenic calf. The
Ž .
transgene bovineb-caseinrhuman lactoferrin cDNA fusion gene has only one site for
Ž . Ž
a restriction enzyme HindIII. Genomic DNAs 10mg were obtained from blood lanes
. Ž .
3, 4, 7, 8, 9,12, 13, 14 and 17 and ear tissues lanes 5, 6, 10, 11, 15 and 16 of three
Ž .
calves a3626,a3629 anda3753 , and then digested with restriction enzymes of DraI Žlanes 1, 3, 5, 8, 10, 13, 15 and 18 , EcoRI lanes 2, 4, 6, 9, 11, 14, 16 and 19 and. Ž .
Ž .
HindIII lanes 5, 10 and 15 . After electrophoresis and transfer to a nylon membrane, the DNA samples were probed with 32P-labeled fragments of human lactoferrin cDNA.
Ž . Ž
Transgene was identified in both the blood lanes 8, 9 and 12 and ear samples lanes 10
. Ž .
and 11 of one calf a3628 . The density ratio of the upper and lower bands was
Ž .
approximately 1:3 determined by Phosphoimazer . This result shows that the founder transgenic bull has four copies of the transgene as a concatamer. The second transgenic
Ž .
calf had one copy of the transgene. To date, 3 out of 23 calves 13.0% born from cows
Table 1
Production of transgenic calves after the transfer of DNA-injected bovine embryos
Group No. of embryos Pregnant recipients Live-born Transgenic calves
a b c
Pregnant at 60 days of gestation.
b
Msmale, Fsfemale.
c
Transgenic calvesrlive-born calves=100.
)
Fig. 2. Identification of a transgenic calf by Southern blot analysis. The sizes of DNA marker, named as lrHindIII DNA fragments applied on lane M, were as follows; 2.0, 2.3, 4.3, 6.5, 9.4 and 23.1 kbp from
bottom to top. Lanes 1 and 2 are 5 pg of injected DNA fragment digested with DraI and EcoRI, respectively, as positive controls. Lanes 18 and 19 are 10mg of non-transgenic genomic DNAs digested with DraI and
EcoRI, respectively, as negative controls.
inseminated with the semen of the transgenic bull were transgenic. Thus, mosaicism of the founder bull was demonstrated. It was shown by PCR and Southern blot analyses
Ž .
that the second transgenic bull had no transgenic spermatozoa data not shown .
4. Discussion
In general, development to blastocyst stage of DNA-injected bovine zygotes
pro-Ž .
duced in vitro is considerably reduced compared to control non-injected embryos
ŽKrimpenfort et al., 1991; Peura et al., 1994; Han et al., 1996 . The reduced develop-.
ment of DNA-injected bovine zygotes may be due to the pronuclear injection itself
Ž
rather than injection-related handling or the damage caused by zygote piercing Peura et
.
al., 1994 . This study was to examine whether bovine embryos microinjected with
Ž .
foreign DNA were affected by freezing. The overall survival rate 59% of DNA-in-jected bovine blastocysts after freezing and thawing was similar to the previous data which showed a survival rate of 61% for IVF-derived blastocysts after freezing and
Ž . Ž .
thawing Han et al., 1994 . Vajta et al. 1996 reported that 79% of vitrified blastocysts produced in vitro without DNA injection re-expanded after thawing. As shown in Fig. 1A, in vitro viability of DNA-injected blastocysts after thawing was significantly
Ž .
affected by embryo quality and developmental stage at freezing P-0.05 . There was a higher survival rate of DNA-injected embryos after freezing and thawing in the group of
Ž .
Pregnancy rates of IVF-derived bovine embryos after transfer are lower than those of
Ž .
in vivo embryos Reichenbach et al., 1992; Farin and Farin, 1995; Hasler et al., 1995 . In particular, the pregnancy rate of DNA-injected bovine embryos produced in vitro is
Ž
lower than that of in vivo or in vitro produced embryos without injection Krimpenfort
.
et al., 1991; Han et al., 1996 . However, little information is available on in vivo viability of DNA-injected bovine embryos after freezing and thawing. The pregnancy rate achieved by DNA-injected embryos after freezing and thawing was lower than that
Ž .
of fresh embryos P-0.05 , indicating that their viability was damaged by freezing. Two transgenic calves were born from DNA-injected bovine embryos after freezing and thawing, although none were produced following the transfer of fresh embryos. Transgenic livestock are expensive to produce, primarily because the process is ineffi-cient. Pronuclear injection method has been unable to achieve transgenesis in more than
Ž .
1% of injected embryos in farm animals such as cattle, sheep and pigs Wall, 1996 .
Ž
Some techniques including selection of transgenic embryos Bowen et al., 1994;
. Ž .
Hyttinen et al., 1994 , the retroviral vector system Kim et al., 1993 and the sperm
Ž .
vector system Lauria and Gandolfi, 1993 have been tried to enhance the production efficiency of transgenic animals. It has been suggested that reverse-transcribed gene
Ž .
transfer is a more efficient method Chan et al., 1998 . Nuclear transfer using
trans-Ž
formed somatic cells allows to generate 100% transgenic animals Schnieke et al., 1997;
.
Cibelli et al., 1998; Brink et al., 2000 . To our knowledge, this study is the first report of the generation of transgenic cattle from DNA-injected embryos after freezing and thawing.
The first founder bull was determined to be a mosaic because there was a low
Ž .
transmission rate approximately 13% of the transgene among calves born from recipients artificially inseminated with semen of the transgenic bull. This lower trans-mission rate means that the transgene might be integrated into the genome at the early two-cell stage rather than the pronuclear stage. Mosaicism, possibly arising from delayed integration, could account for a reduced frequency of genetic transmission to
Ž
successive generations below Mendelian levels Gordon and Ruddle, 1981; Palmiter et
. Ž .
al., 1984 . This mosaic frequency will be high in transgenic cattle. Eyestone 1999 reported that seven of eight transgenic founder cattle passed their transgenes to embryos
Ž .
at low transmission rates -30% , showing varying degrees of mosaicism. In bovine zygotes, pronuclei do not become visible by Normarski optics until 16–18 h
post-in-Ž .
semination, when DNA replication is already in progress Wall, 1996 . Thus, most of the zygotes in the present work were injected during DNA synthesis, increasing the probability that the resulting transgenic offspring would be mosaic and transmit their transgenes only to 25% of their offspring. Nuclear transfer technique using transgenic
Ž .
somatic cells Schnieke et al., 1997; Cibelli et al., 1998 could be an efficient method of overcoming these disadvantages of mosaic founders, greatly reducing the time and cost involved.
indicated that freezing is useful for saving DNA-injected embryos in the production of transgenic livestock.
Acknowledgements
Ž .
This study was supported by grants HS2550 and HS2705 from the Ministry of
Ž
Science and Technology, Korea. We thank Mr. Y.K. Lee National Livestock Research
.
Institute, Suwon, Korea for statistical analysis.
References
Agca, Y., Monson, R.L., Northey, D.L., Abas Manzi, O., Schaefer, D.M., Rutledge, J.J., 1998. Transfer of fresh and cryopreserved IVP bovine embryos: normal calving, birth weight and gestation lengths. Theriogenology 50, 147–162.
Bavister, B.D., Yanagimachi, R., 1977. The effects of sperm extracts and energy sources on the motility and acrosome reaction of hamster spermatozoa in vitro. Biol. Reprod. 16, 228–237.
Bowen, R.A., Reed, M.L., Schnieke, A., Seidel, G.E. Jr., Stacey, A., Thomas, W.K., Kajikawa, O., 1994. Transgenic cattle resulting from biopsied embryos: expression of c-ski in a transgenic calf. Biol. Reprod. 50, 664–668.
Bremel, R.D., 1996. Potential role of transgenesis in dairy production and related areas. Theriogenology 45, 51–56.
Brink, M.F., Bishop, M.D., Pieper, F.R., 2000. Developing efficient strategies for the generation of transgenic cattle which produce biopharmaceuticals in milk. Theriogenology 53, 139–148.
Chan, A.W., Homan, E.J., Ballou, L.U., Burns, J.C., Bremel, R.D., 1998. Transgenic cattle produced by reverse-transcribed gene transfer in oocytes. Proc. Natl. Acad. Sci. U.S.A. 95, 14028–14033.
Cibelli, J.B., Stice, S.L., Golueke, P.J., Kane, J.J., Jerry, J., Blackwell, C., Ponce de Leon, F.A., Robl, J.M., 1998. Cloned transgenic calves produced from nonquiescent fetal fibroblasts. Science 280, 1256–1258. Eyestone, W.H., Gowallis, M., Monahan, J., Sink, T., Ball, S.F., Cooper, J.D., 1998. Production of transgenic
cattle expressing human alpha-lactalbumin in milk. Theriogenology 49, 386, abstract.
Eyestone, W.H., 1999. Production and breeding of transgenic cattle using in vitro embryo production technology. Theriogenology 51, 509–517.
Farin, P.W., Farin, C.E., 1995. Transfer of bovine embryos produced in vivo or in vitro: survival and fetal development. Biol. Reprod. 52, 676–682.
Gordon, J.W., Ruddle, F.H., 1981. Integration and stable germ line transmission of genes injected into mouse pronuclei. Science 214, 1244–1246.
Hammer, R.E., Pursel, V.G., Rexroad, C.E. Jr., Wall, R.J., Bolt, D.J., Ebert, K.M., Palmiter, R.D., Brinster, R.L., 1985. Production of transgenic rabbits, sheep and pigs by microinjection. Nature 315, 680–683. Han, Y.M., Yamashina, H., Koyama, N., Lee, K.K., Fukui, Y., 1994. Effects of quality and developmental
stage on the survival of IVF-derived bovine blastocysts cultured in vitro after freezing and thawing. Theriogenology 42, 645–654.
Han, Y.M., Park, J.S., Lee, C.S., Lee, J.H., Kim, S.J., Choi, J.T., Lee, H.T., Chung, B.H., Chung, K.S., Shin, S.T., Kim, Y.H., Lee, K.S., Lee, K.K., 1996. Factors affecting in vivo viability of DNA-injected bovine blastocysts produced in vitro. Theriogenology 46, 769–778.
Hasler, J.F., Henderson, W.B., Hurtgen, P.J., Jin, Z.Q., McCauley, A.D., Mower, S.A., Neely, B., Shuey, L.S., Stokes, J.E., Trimer, S.A., 1995. Production, freezing and transfer of bovine IVF embryos and subsequent calving results. Theriogenology 43, 141–152.
Hyttinen, J.-M., Peura, T., Tolvanen, M., Aalto, J., Alhonen, L., Sinervirta, R., Halmekyto, M., Myohanen, S., Janne, J., 1994. Generation of transgenic dairy cattle from transgene-analyzed and sexed embryos produced in vitro. BiorTechnology 12, 606–608.
Janne, J., Hyttinen, J.-M., Peura, T., Tolvanen, M., Alhohen, L., Halmekyto, M., 1992. Transgenic animals as bioproducers of therapeutic proteins. Ann. Med. 24, 273–280.
Joyner, A.L., 1993. Gene Targeting: A Practical Approach. Oxford Univ. Press, New York, pp. 36–39. Kim, S.J., Cho, Y.Y., Lee, K.W., Yu, D.Y., Lee, C.S., Han, Y.M., Lee, K.K., 1994. Expression of human
lactoferrin in milk of transgenic mice using bovineb-caseinrhuman lactoferrin cDNA fusion genes. Mol. Cells 4, 355–360.
Kim, T., Leibfried-Rutledge, M.L., First, N.L., 1993. Gene transfer in bovine blastocysts using replication-de-fective retroviral vectors packaged with Gibbon ape leukemia virus envelopes. Mol. Reprod. Dev. 35, 105–113.
Krimpenfort, P., Rademakers, A., Eyestone, W., van der Schans, A., van der Broek, S., Kooiman, P., Kootwijk, E., Platenburg, G., Pieper, F., Strijker, R., de Boer, H., 1991. Generation of transgenic dairy cattle using in vitro embryo production. BiorTechnology 9, 844–847.
Lauria, A., Gandolfi, F., 1993. Recent advances in sperm cell mediated gene transfer. Mol. Reprod. Dev. 36, 255–257.
Lindner, G.M., Wright, R.W. Jr., 1983. Bovine embryo morphology and evaluation. Theriogenolgy 20, 407–416.
Massip, A., van der Zwalmen, P., Ectors, F., 1987. Recent progress in cryopreservation of cattle embryos. Theriogenology 27, 69–79.
Palmiter, R.D., Wilkie, T.M., Chen, H.Y., Brinster, R.L., 1984. Transmission distortion and mosaicism in an unusual transgenic mouse pedigree. Cell 36, 869–877.
Peura, T.T., Hyttinen, J.-M., Tolvanen, M., Janne, J., 1994. Effects of microinjection-related treatments on the subsequent development of in vitro-produced bovine oocytes. Theriogenology 42, 433–443.
Reichenbach, H.D., Leibrich, J., Berg, U., Brem, G., 1992. Pregnancy rates and births after unilateral or bilateral transfer of bovine embryos produced in vitro. J. Reprod. Fertil. 95, 363–370.
Rosenkrans, C.F. Jr., Zeng, G.Q., McNamara, G.T., Schoff, P.K., First, N.L., 1993. Development of bovine embryos in vitro as affected by energy substrates. Biol. Reprod. 49, 459–462.
Schnieke, A.E., Kind, A.J., Ritchie, W.A., Mycock, K., Scott, A.R., Ritchie, M., Wilmut, I., Colman, A., Campbell, K.H.S., 1997. Sheep transgenic for human factor IX produced by transfer of nuclei from transfected fetal fibroblasts. Science 278, 2130–2133.
Suzuki, T., Takagi, M., Yamamoto, M., Boediono, A., Saha, S., Sakakibara, H., Oe, M., 1993. Pregnancy rate and survival in culture of in vitro fertilized bovine embryos frozen in various cryoprotectants and thawing using a one-step system. Theriogenology 40, 651–659.
Vajta, G., Holm, P., Greve, T., Callesen, H., 1996. Overall efficiency of in vitro embryo production and vitrification in cattle. Theriogenology 45, 683–689.