www.elsevier.com / locate / bres
Research report
Acute protective effect of nimodipine and dimethyl sulfoxide against
hypoxic and ischemic damage in brain slices
a ,
*
b a a¨
Christoph Greiner
, Andrea Schmidinger , Swen Hulsmann , Dag Moskopp ,
a b b a
¨
¨
¨
Johannes Wolfer , Rudiger Kohling , Erwin-Josef Speckmann , Hansdetlef Wassmann
a
¨ ¨
Klinik und Poliklinik f ur Neurochirurgie, Albert-Schweitzer-Strasse 33, 48142 Munster, Germany
b
¨ ¨
Institut f ur Physiologie, Robert-Koch-Strasse 27a, 48149 Munster, Germany
Accepted 19 September 2000
Abstract
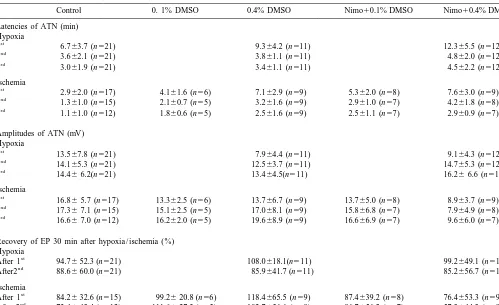
Nimodipine and dimethyl sulfoxide (DMSO) were tested (alone and in combination) regarding their ability to increase hypoxic tolerance of brain slices under ‘hypoxic’ (deprivation of oxygen) or ‘ischemic’ (hypoxia1withdrawal of glucose) conditions. Direct current (DC) and evoked potentials were recorded in the CA1 region of hippocampal slices of adult guinea pigs. After induction of hypoxia or ischemia, the latency of anoxic terminal negativity (ATN) of the DC potential was determined during superfusion with artificial cerebrospinal fluid alone (aCSF), and during superfusion with aCSF containing DMSO [0.1% (14.1 mmol / l) and 0.4% (56.3 mmol / l)] with the addition of nimodipine (40 mmol / l). Latencies of ATN with first hypoxia were 6.763.7 min in the control group, 9.364.2 min in the 0.4% DMSO group and 12.365.5 min (P50.007) in the nimodipine / 0.4% DMSO group. Latencies of ATN with first ischemia were 2.962 min in the control group, 4.161.6 min in the 0.1% DMSO group, 7.163.9 min in the 0.4% DMSO group (P50.006), 5.361.5 min in the nimodipine / 0.1% DMSO group and 7.663 min (P,0.001) in the nimodipine / 0.4% DMSO group. DMSO (0.4%), either alone or in combination with nimodipine, increase the latency of the ATN after acute onset of hypoxia and ischemia. 2000 Elsevier Science B.V. All rights reserved.
Theme: Disorders of the nervous system
Topic: Ischemia
Keywords: Ischemia; Brain slice; Neuroprotection; Nimodipine; Dimethyl sulfoxide
1. Introduction membrane calcium movements, particularly through L-type calcium channels [15,45]. Several investigations Subarachnoid hemorrhage often causes cerebral artery showed protective effects of nimodipine both in vitro and spasms from day 3 after the event onwards. These spasms in vivo, as well as in clinical trials, although, in the latter,
21
are due to an influx of Ca into the smooth muscle cells controversial findings were also published [48,49]. Often causing vessel contraction and, finally, a critically reduced these studies were focused on cerebral artery spasm or cerebral perfusion [12,14,27,33,35,50]. Dihydropyridine effects of nimodipine on the regional cerebral blood flow calcium antagonists, such as nimodipine, inhibit trans- [8,16,17,20,25,26,34,37]. In most of these investigations, membrane calcium influx into vascular smooth muscle an interfering and possibly neuroprotective effect of the cells and thus influence cerebral artery spasm [13,54]. solvents used as vehicle was not taken into account. Furthermore, calcium antagonists can directly interfere The aim of these experiments was to analyze the acute with neuronal excitability, which is influenced by trans- effect of nimodipine on the bioelectric activity of isolated hippocampal brain slices during hypoxia and ischemia. Nimodipine and dimethyl sulfoxide (DMSO), the latter being widely applied as a solvent for a variety of drugs, *Corresponding author. Tel.:149-251-834-7472; fax: 1
49-251-834-were tested in combination and alone [23]. 7479.
E-mail address: [email protected] (C. Greiner). Hypoxic conditions were induced by deprivation of
oxygen, whereas ischemic conditions were imitated by ischemic episode were 30 min. Latency of ATN was combined deprivation of oxygen and glucose. determined from the beginning of the N application to the2 Bioelectrical changes in brain tissue during hypoxia / point at which 10% of the ATN peak amplitude had been ischemia are well described. Under critical hypoxic / is- reached [43]. The amplitude of the DC potential was chemic conditions, a sudden negative shift of the direct measured from baseline to peak. The amplitudes of the EP current (DC) potential, so-called anoxic terminal negativity were measured from the interpolated baseline to peak. (ATN), is brought about by the anoxic depolarization of Thirty min after hypoxia / ischemia, the EP amplitude was neurons. This sudden depolarization, which is accom- compared with the initial amplitude at the beginning of the panied both in vivo and in vitro by an excessive trans- experiment. Statistical analysis was performed using the membranous influx of calcium and sodium ions, a release Student’s t-test and the Mann-Whitney-Rank-Sum test, of glutamate, an increase in extracellular potassium con- with P,0.05 considered to be significant. All data are centrations (up to 60 mmol / l), and a reduction of the given as means and standard deviation (S.D.).
extracellular volume fraction, is the endpoint of energy Parallel experiments were done in two recording cham-depletion of neuronal tissue during persistent hypoxia / bers of similar construction to compare two slices from the ischemia. These changes are at least partially reversible if same animal under both hypoxic and ischemic conditions. hypoxia / ischemia is terminated upon reaching the peak of In some experiments, the pO of the slice surface during2 ATN [1,10,23,24,28,31,50]. the whole experiment was monitored online with an optical monitoring system (phosphorescence quenching method) [29].
2. Material and methods Besides control experiments without the addition of drugs, different solutions containing nimodipine and Adult guinea pigs (n554; 340–440 g) were anesthetized DMSO were added to the aCSF. Solutions given under with the short acting barbiturate methohexital (250 mg / kg hypoxic conditions: 40 mmol / l nimodipine with 0.4% i.p.). The brain was rapidly removed and chilled in (56.3 mmol / l) DMSO, and 0.4% DMSO alone. Solutions artificial cerebrospinal fluid (aCSF) at 48C. The aCSF given under ischemic conditions: 40 mmol / l nimodipine contained (in mmol / l): NaCl 124, KCl 4, NaH PO 1.24,2 4 with 0.1% (14.1 mmol / l) DMSO, 40 mmol / l nimodipine MgSO 1.3, NaHCO 26, glucose 10 and CaCl 2). The4 3 2 with 0.4% DMSO, 0.1% DMSO and 0.4% DMSO alone. hippocampus was dissected, transverse slices were cut Experiments with nimodipine were performed in a dar-(500 mm) using a McIlwain chopper (Mickle Lab Eng. kened room. The concentration of nimodipine (40mmol / l) Co., Gomshall, UK). The slices (n592) were immediately was chosen in view of the well known protein-binding placed in a submerged-type preincubation chamber con- properties of the substance [30] and results of experiments taining aCSF. The solution was equilibrated with 95% O2 demonstrating massive diffusion barriers and substance and 5% CO (pH 7.35–7.45). The temperature was held at2 accumulation in the uppermost cut surface of the slices, 288C. which even preclude fast diffusion of small ions [2,46].
After a preincubation period of 1–2 h, slices were transferred into the recording chamber [21] at the interface
of aCSF and humidified gas (95% O and 5% CO ; 348C,2 2 3. Results
measured adjacent to the slice). A constant flow rate of 2
ml / min was maintained (chamber volume51.4 ml). One 3.1. Latencies and amplitudes of ATN under hypoxic slice was used for each experiment. Schaffer collaterals conditions
were stimulated with bipolar electrodes (2 ms / 1 s) that
were placed in the stratum radiatum between areas CA2 With hypoxic conditions (deprivation of oxygen), ATN and CA3. Direct current and evoked potentials (EPs) were occurred in all slices (n544). Latencies of ATN with the recorded using NaCl-filled glass micropipettes (0.5–1 MV) first episode of hypoxia in the nimodipine / 0.4% DMSO from CA1 (str. pyramidale). In all experiments, the am- group were significantly longer than in the control group plitudes of EP were stable for 30 min prior to initiation of (P50.007). Latencies of ATN generally lasted longer in hypoxia or ischemia. Slices with an EP amplitude,4 mV the nimodipine / 0.4% DMSO group also with the second were excluded. and third episodes of hypoxia, although the differences
Hypoxic conditions (n544) were established by replac- were not statistically significant.
Table 1
Latencies and amplitudes of anoxic terminal negativity and amplitudes of evoked potentials after inducing three hypoxic or three ischemic episodes in
a
Recovery of EP 30 min after hypoxia / ischemia (%) Hypoxia
Hypoxia was defined as oxygen deprivation only, ischemia was defined as oxygen deprivation and glucose reduction. DMSO, dimethyl sulfoxide; Nimo, nimodipine. All data are given as the mean6S.D.
3.2. Recovery of evoked potentials under hypoxic ischemia than in the control group. Although there is no conditions statistically significant difference of ATN amplitudes with the 2nd and 3rd hypoxia, amplitudes of the nimodipine / With the beginning of hypoxia, amplitudes of the EP DMSO 0.4% group were smaller than in the other groups decreased and disappeared before the occurrence of ATN. (Table 1).
Recovery of evoked potentials was determined 30 min
after termination of the first and second episodes of 3.4. Recovery of evoked potentials under ischemic hypoxia. The initial amplitude before the first episode of conditions
hypoxia was taken as 100%. No statistically significant
differences were seen among the groups (Table 1). No statistically significant differences could be observed concerning the recovery of the evoked potentials.
3.3. Latencies and amplitudes of ATN under ischemic conditions
4. Discussion
With ischemic conditions (deprivation of oxygen and
decrease in glucose), ATN occurred in all slices (n548). Hippocampal slice preparations of either rats or guinea st
Latencies of ATN with 1 ischemia lasted significantly pigs are frequently used to evaluate cerebroprotective longer in the DMSO 0.4% group (P50.006) and in the effects of drugs on neurons and glia. This technique has nimodipine / DMSO 0.4% group (P,0.001) than in the the advantage that experimental parameters such as pH, control group. Prolongations of ATN latency after applica- flow and temperature of the bath solution can be effective-tion of DMSO 0.4% and nimodipine / DMSO 0.4% were ly manipulated and monitored, and that the neuro-glial also evident with the following ischemias. compartment can be observed in isolation, without being In the nimodipine / DMSO 0.4% group amplitude of dependent on regional cortical blood flow
st
ex-Fig. 1. Time course of anoxic terminal negativity (ATN) of the DC-potential during repetitive hypoxia (deprivation of oxygen) and ‘ischemia’ (deprivation of oxygen and reduction of glucose) in hippocampal slices of guinea pigs. Fig1a: * indicates P50.007; Fig. 1b: * indicates P50.006, ** indicates
P,0.001. Mean1S.D., Student’s t-test, Mann-Whitney-Rank-Sum test.
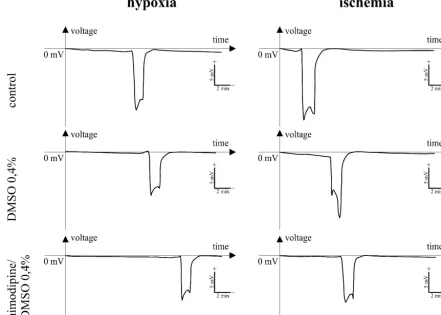
perimental conditions such as pO2 during hypoxic epi- intracellular calcium, causing an extracellular increase in sodes, which declines to 260.1 kPa [29], are uniform excitatory amino acids, a depletion of high energy phos-throughout the experiments. At the same time, changes of phates, failure of membrane ion pumps with subsequent
1 21 1
Fig. 2. Changes of the DC-potentials during repetitive hypoxia (deprivation of oxygen) and ‘ischemia’ (deprivation of oxygen and reduction of glucose). Guinea pig, hippocampal slice, CA1 region. DMSO, dimethyl sulfoxide.
hypoxia was not statistically significant. The increasing synaptic activity and, therefore, reduced ATP
consump-1 1
21
by the sarcoplasmic reticulum Ca -ATPase: ATP hydrolysis, ATP function by a direct action on nerve cells [15,53]. By
synthesis and heat production, FEBS Lett. 406 (1997) 201–204. limiting, and thus decelerating, excessive neuronal calcium
[12] N.W. Dorsch, The effect and management of delayed vasospasm entry, the application of nimodipine increases the latency after aneurysmal subarachnoid hemorrhage, Neurol. Med. Chir. of ATN. In our experiments, this effect became obvious Tokyo 38 (Suppl.) (1998) 156–160.
with a concentration of 40 mmol / l nimodipine. A small [13] V.L. Feigin, G.J. Rinkel, A. Algra, M. Vermeulen, J. van Gijn, Calcium antagonists in patients with aneurysmal subarachnoid unpublished series on four hippocampal slices of guinea
hemorrhage: a systemic review, Neurology 50 (1998) 876–883. pig was performed with 80 mmol / l nimodipine dissolved
[14] A. Fleckenstein, Specific pharmacology of calcium in myocardium, in 0.4% DMSO under hypoxic conditions. This series of cardiac pacemakers, and vascular smooth muscle, Annu. Rev. experiments showed no differences compared to the pres- Pharmacol. Toxicol. 17 (1977) 149–166.
ent data with 40mmol / l nimodipine, which thus appears to [15] G. Fleckenstein-Grun, A. Fleckenstein, Prevention of cerebrovascu-¨ lar spasms with nimodipine, Stroke 21 (Suppl. IV) (1990) 64–71. be the saturating dose.
[16] H.J. Gelmers, K. Gorter, C.J. de Weerdt, H.J. Wiezer, A controlled In conclusion, a vessel-independent neuroprotective
trial of nimodipine in acute ischemic stroke, N. Engl. J. Med. 318 effect of nimodipine and DMSO, and of DMSO alone,
(1988) 203–207.
could be observed. The effect of DMSO on the latency and [17] H.J. Gelmers, M. Hennerici, Effects of nimodipine on acute amplitude of ATN can be augmented by the administration ischemic stroke. Pooled results from five randomized trials, Stroke
12 (Suppl. IV) (1990) 81–84. of 40 mmol / l nimodipine.
[18] J.H. Greenberg, D. Uematsu, N. Araki, W.F. Hickey, M. Reivich, Cytosolic free calcium during focal cerebral ischemia and the effects of nimodipine on calcium and histologic damage, Stroke 21 (Suppl. IV) (1990) 72–77.
Acknowledgements
¨
[19] C. Greiner, S. Hulsmann, H. Wassmann, D. Moskopp, A.
Schmid-¨ ¨ ¨
inger, J. Wolfer, R. Kohling, A. Lucke, E.J. Speckmann, Neuro-With support of the Deutsche Forschungsgemeinschaft protection of mild hypothermia: differential effects, Brain Res. 786
(1998) 267–269. (AZ: Wa 604 / 2-1).
[20] R. Grobe-Einsler, Clinical aspects of nimodipine, Clin. Neuro-pharmacol. 16 (Suppl 1) (1993) S39–S45.
[21] H.L. Haas, B. Schaerer, M. Vosmansky, A simple perfusion chamber for the study of nervous tissue slices in vitro, J. Neurosci. Methods 1
References
(1979) 323–325.
[22] A.J. Hansen, Effects of anoxia on ion distribution in the brain, [1] P.G. Aitken, M. Balestrino, G.G. Somjen, NMDA antagonists: lack Physiol. Rev. 65 (1985) 101–148.
of protective effect against hypoxic damage in CA1 region of [23] S. Hulsmann, C. Greiner, R. Kohling, J. Wolfer, D. Moskopp, B.¨ ¨ ¨ hippocampal slices, Neurosci. Lett. 89 (1988) 187–192. Riemann, A. Lucke, H. Wassmann, E.J. Speckmann, Dimethyl¨ [2] D. Albrecht, U. Heinemann, Low calcium-induced epileptiform sulfoxide increases latency of anoxic terminal negativity in hip-activity in hippocampal slices from infant rats, Dev. Brain Res. 48 pocampal slices of guinea pig in vitro, Neurosci. Lett. 261 (1999)
(1989) 316–320. 1–4.
[3] L.M. Auer, M. Mokry, Effect of topical nimodipine versus its [24] S. Hulsmann, R. Kohling, C. Greiner, D. Moskopp, A. Lucke, H.¨ ¨ ¨ ethanol-containing vehicle on cat pial arteries, Stroke 17 (1986) Wassmann, E.J. Speckmann, Neuroprotection by 21-aminosteroids: 225–228. insights from latencies of anoxic terminal negativity in hippocampus [4] L.M. Auer, R.W. Oberbauer, H.V. Schalk, Human pial vascular slices of guinea pig, Neurol. Res. 21 (1999) 305–308.
reactions to intravenous nimodipine infusion during EC–IC bypass [25] B. Infeld, S.M. Davis, G.A. Donnan, M. Yasaka, M. Lichtenstein, surgery, Stroke 14 (1983) 210–213. P.J. Mitchell, G.J. Fitt, Nimodipine and perfusion changes after [5] L.M. Auer, A. Suzuki, N. Yasui, Z. Ito, Intraoperative topical stroke, Stroke 30 (1999) 1417–1423.
nimodipine after aneurysm clipping, Neurochirurgia 27 (1984) 36– [26] A. Kakarieka, E.H. Schakel, J. Fritze, Clinical experiences with
38. nimodipine in cerebral ischemia, J. Neural. Transm. Suppl. 43
[6] M. Balestrino, Pathophysiology of anoxic depolarization: new (1994) 13–21.
findings and a working hypothesis, J. Neurosci. Methods 59 (1995) [27] N.F. Kassel, T. Sasaki, A.R. Colohan, G. Nazar, Cerebral vasospasm
99–103. following aneurysmal subarachnoid hemorrhage, Stroke 16 (1985)
¨
[7] G.W. Bielenberg, M. Burniol, R. Rosen, W. Klaus, Effects of 562–572.
¨ ¨ ¨
nimodipine on infarct size and cerebral acidosis after middle [28] R. Kohling, A. Schmidinger, S. Hulsmann, S. Vanhatalo, A. Lucke, cerebral artery occlusion in the rat, Stroke 21 (Suppl. IV) (1990) H. Straub, E.J. Speckmann, I. Tuxhorn, P. Wolf, R. Lahl, H. Pannek,
90–92. F. Oppel, C. Greiner, D. Moskopp, H. Wassmann, Anoxic terminal
[8] J. Bogousslavsky, F. Regli, V. Zumstein, W. Kobbering, Double- negative DC-shift in human neocortical slices in vitro, Brain Res. blind study of nimodipine in non-severe stroke, Eur. Neurol. 30 741 (1996) 174–179.
¨ ¨
(1990) 23–26. [29] R. Kohling, C. Greiner, J. Wolfer, H. Wassmann, E.J. Speckmann, [9] R. Busto, W.D. Dietrich, M.Y.T. Globus, I. Valdes, P. Scheinberg, Optical monitoring of p02 changes and simultaneous recording of M.D. Ginsberg, Small differences in intraischemic brain temperature bioelectric activity in human and animal brain slices, J. Neurosci. critically determine the extent of ischemic neuronal injury, J. Cereb. Methods 85 (1998) 181–186.
Blood Flow Metab. 13 (1987) 729–738. [30] M.S. Langley, E.M. Sorkin, Nimodipine: A review of its pharmaco-[10] H. Caspers, E.J. Speckmann, Cortical DC-shifts associated with dynamic and pharmacokinetic properties, and therapeutic potential
changes of gas tension and blood and tissue, in: A. Remond (Ed.), in cerebrovascular disease, Drugs 37 (1989) 669–699.
to the study of focal cerebral ischemia in the rat; an evaluation, J. Pannek, F. Oppel, Flat and steep terminal negativity in the DC-Neurosci. Methods 28 (1989) 1–6. potential after deprivation of oxygen and glucose in human neocorti-[33] N.A. Martin, C. Doberstein, C. Zane, M.J. Caron, K. Thomas, D.P. cal slices, Brain Res. 794 (1998) 28–34.
Becker, Posttraumatic cerebral arterial spasm: transcranial doppler [44] S. Shimizu, R.P. Simon, S.H. Graham, Dimethylsulfoxide (DMSO) ultrasound, cerebral blood flow, and angiographic findings, J. treatment reduces infarction volume after permanent focal cerebral Neurosurg. 77 (1992) 575–583. ischemia in rats, Neurosci. Lett. 239 (1997) 125–127.
¨
[34] E. Martinez-Vila, F. Guillen, J.A. Villanueva, J. Matias-Guiu, J. [45] B.K. Siesjo, F. Bengtsson, Calcium fluxes, calcium antagonists, and Bigorra, P. Gil, A. Carbinell, J.M. Martinez-Lage, Placebo-con- calcium-related pathology in brain ischemia, hypoglycemia, and trolled trial of nimodipine in the treatment of acute ischemic spreading depression: a unifying hypothesis, J. Cereb. Blood Flow cerebral infarction, Stroke 21 (1990) 1023–1028. Metab. 9 (1989) 127–140.
¨
[35] K. Mori, H. Arai, K. Nakajima, A. Tajima, M. Maeda, Hemor- [46] H. Straub, H. Kuhlmann, D. Kohling, E.J. Speckmann, Accumula-¨
heological and hemodynamic analysis of hypervolamic hemodilution tion of nifedipine in hippocampal slices, Pfluger’s Arch. 437 (Suppl. therapy for cerebral vasospasms after aneurysmal subarachnoid 5) (1999) R136.
hemorrhage, Stroke 26 (1995) 1620–1626. [47] C.P. Taylor, M.L. Weber, Effect of temperature on synaptic function [36] B.A. Moursi, B.A. Luyckx, L.G. D’Alecy, The role of ethanol in after reduced oxygen and glucose in hippocampal slices,
Neuro-diluents of drugs that protect mice from hypoxia, Stroke 14 (1983) science 52 (1993) 555–562.
791–796. [48] The American Nimodipine Study Group, Clinical trial of nimodipine [37] G.D. Murray, G.M. Teasdale, H. Schmitz, Nimodipine in traumatic in acute ischemic stroke, Stroke 23 (1992) 3–8.
subarachnoid haemorrhage: a reanalysis of the HIT I and HIT II [49] Trust Study Group, Randomised, double-blind, placebo-controlled trials, Acta Neurochir. 138 (1996) 1163–1167. trial of nimodipine in acute stroke, Lancet 336 (1990) 1205–1209. [38] M. Nakahiro, O. Arakawa, T. Narahashi, S. Ukai, Y. Kato, K. [50] M. Vermeulen, Subarachnoid haemorrhage: diagnosis and treatment,
Nishinuma, T. Nishimura, Dimethyl sulfoxide (DMSO) blocks J. Neurol. 243 (1996) 496–501. ¨
GABA-induced current in rat dorsal root ganglion neurons, Neuro- [51] H. Wassmann, C. Greiner, S. Hulsmann, D. Moskopp, E.J. Spec-sci. Lett. 138 (1992) 5–8. kmann, J. Meyer, H. Van Aken, Hypothermia as cerebroprotective [39] T. Ogura, L.M. Shuba, T.F. Mc Donald, Action potentials, ionic measure. Experimental hypoxic exposure of brain slices and clinical currents and cell water in guinea pig ventricular preparations application in critically reduced cerebral perfusion pressure, Neurol. exposed to dimethyl sulfoxide, J. Pharmacol. Exp. Ther. 273 (1995) Res. 20 (Suppl. 1) (1998) S61–S65.
¨
1273–1286. [52] H. Wassmann, D. Moskopp, B. Woesler, A. Lucke, E.J. Speckmann,
¨ ¨
[40] J.W. Phillis, A.Y. Esterez, M.H. O’Regan, Protective effects of the R. Kohling, H. Straub, S. Hulsmann, C. Greiner, Repetitive hypoxic free radical scavengers, dimethyl sulfoxide and ethanol, in cerebral exposure of brain slices and electrophysiological responses as an ischemia in gerbils, Neurosci. Lett. 244 (1998) 109–111. experimental model for investigation of cerebroprotective measure-[41] M. Sawada, M. Sato, The effect of dimethyl sulfoxide on the ments, Neurol. Res. 18 (1996) 367–369.
neuronal excitability and cholinergic transmission in Aplysia gang- [53] M. Welsch, J. Nuglisch, J. Krieglstein, Neuroprotective effect of lion cells, Ann. NY Acad. Sci. 243 (1975) 337–357. nimodipine is not mediated by increased cerebral blood flow after [42] S.J. Schiff, G.G. Somjen, The effects of temperature on synaptic transient forebrain ischemia in rats, Stroke 21) ((Suppl. IV)) (1990)
transmission in hippocampal tissue slices, Brain Res. 345 (1985) 105–107.
279–284. [54] M.H. Zornow, D.S. Prough, Neuroprotective properties of
calcium-¨ ¨