www.elsevier.com / locate / bres
Research report
Administration of muscimol into the posterior hypothalamus reduces
hyperthermia induced by hippocampal neostigmine injection
*
M. Monda , A. Viggiano, V. De Luca
Department of Human Physiology and Integrated Biological Functions‘‘F. Bottazzi’’, Second University of Naples, via Constantinopoli 16, 80138-Naples, Italy
Accepted 26 September 2000
Abstract
The firing rate of the sympathetic nerves innervating interscapular brown adipose tissue (IBAT), IBAT and colonic temperatures (TIBAT
and T ) and oxygen (O ) consumption were monitored in urethane-anesthetized male Sprague–Dawley rats. These variables wereC 2
27
measured for 40 min before (baseline values) and 40 min after an injection of neostigmine (5310 mol in 1ml of saline) into the hippocampus and a bilateral administration of a GABA -agonist, muscimol (28 ng in 0.5a ml of saline, per side) into the posterior hypothalamus. The same variables were recorded in other rats, but the muscimol was replaced by saline. Control animals were used with muscimol or saline alone. The results show an increase of sympathetic firing rate, TIBAT, TC and O consumption after neostigmine2 injection. Muscimol significantly reduces this enhancement. The findings suggest that hippocampus controls the sympathetic and thermogenic activation induced by neostigmine through an influence on GABAergic tone of the posterior hypothalamus. 2000 Elsevier Science B.V. All rights reserved.
Theme: Other systems of the CNS
Topic: Limbic system and hypothalamus
Keywords: Body temperature; Oxygen consumption; Sympathetic activity; Rat
1. Introduction sympathetic activation [16,24]. The posterior
hypo-thalamus also controls the thermogenic activity of IBAT.
The hippocampus controls various functions, including Injections of pyrogens cause increases in activity of the
those which are vegetative. Indeed, the hippocampus is posterior hypothalamic neurons [12] and in body
tempera-involved in the control of body temperature. An injection ture [11], while an injection of muscimol, a GABAa
of neostigmine, an acetylcholine esterase inhibitor, into the agonist, in the posterior hypothalamus reduces the
sympa-hippocampus induces an increase in heat production due to thetic activation induced by PGE [13].1
an activation of interscapular brown adipose tissue (IBAT) The present experiment tested the hypothesis that the
which contributes to induce a rise in body temperature hippocampus controls the sympathetic and thermogenic
[14]. IBAT is the principal effector of non-shivering activation induced by neostigmine through an influence on
thermogenesis [2,8] and this tissue is under the control of GABAergic tone of the posterior hypothalamus.
the sympathetic nervous system [7]. The hypothalamic areas regulate the firing rate of sympathetic nerves to the
IBAT and its temperature. Lateral hypothalamic lesions 2. Materials and methods
increase the sympathetic discharge to IBAT [15], while
disruption of the ventromedial hypothalamus reduces the 2.1. Animals
We used male inbred Sprague–Dawley rats (n548), 3
*Corresponding author. Tel.:139-081-566-5833; fax: 1
39-081-566-months old and weighing 270–300 g. These were housed
5846.
E-mail address: [email protected] (M. Monda). in pairs at controlled temperature (22618C) and humidity
(70%) with a 12:12 h light–dark cycle from 07:00 to 19:00 tobarbital sodium (50 mg / kg b.w. i.p.) and a 20-gauge
h. The experiments were in conformity with the European stainless guide cannula was positioned stereotaxically
Convention for the Protection of Vertebrate Animals used above the dorsal hippocampus at the following
coordi-for Experimental and Scientific Purpose (Council of nates: 1.5 mm lateral to the midline, 2.0 mm posterior to
Europe No. 123, Strasbourg 1985). the bregma, 2.2 mm from the surface [23]. In addition, two
20-gauge stainless guide cannulas were positioned
stereo-2.2. Apparatus taxically 0.1 mm above the posterior hypothalamus at the
following coordinates: 0.6 mm lateral to midline, 1 mm
The firing rate of sympathetic nerves to IBAT was posterior to the bregma, 8.0 mm from the cranial theca.
recorded by a pair of silver wire electrodes. The electrical The guide cannulas were secured to the skull by screws
pulses were amplified by a condenser-coupled amplifier and dental cement. Stylets were inserted into the guide
and were filtered by band-path filters (NeuroLog System, tubes and removed only during drug administration. Rats
Digitimer). The raw pulses were displayed on a oscillos- were given 7–10 days to recover from surgery as judged
cope (Tektronix) and sent to a window discriminator. by recovery of preoperative body weight.
Square waves from the discriminator were sent to an
analog–digital converter (DAS system, Keithley) and 2.5. Procedure
stored on a computer (Personal Computer AT, IBM) every
5 s. Furthermore, a rate meter with a reset time of 5 s was After recovery, six animals (1st group) were
anes-used to observe the time course of the nerve activity thetized with urethane (1.2 g / kg b.w. i.p.) and mounted in
recorded by pen recorder (Dynograph, Beckman). Because a stereotaxic instrument (Stoelting). The level of
anes-the signal-to-noise ratio depended on anes-the number of nerve thesia was kept constant as evaluated by skeletal muscle
filaments and the condition of contact between nerve and relaxation, eye and palpebral responses to stimuli. Nerve
electrodes, the basal burst rates were different for each rat. activity was recorded by small nerve bundles dissected
The threshold level of the event detector was fixed during from the nerve branches innervating the right side of
the experiment at 50% of the peaks of the largest pulses IBAT. Nerve filaments were isolated from the central cut
and above background noise. end of these nerve bundles under a dissecting microscope
Thermocouples (Ellab) were used to monitor colonic to record the efferent activity with a pair of silver wire
and IBAT temperatures (TC and TIBAT) and the values electrodes. The nerve filaments were covered with a
were stored on a chart recorder. mixture of vaseline and liquid petroleum at 378C to avoid
Resting oxygen (O ) consumption was determined with2 dehydration. T was measured by inserting the thermocou-C
an indirect calorimeter. The closed circuit apparatus was an ple into the colon at 4 cm from the anus, while TIBAT was
adaptation of Benedict and MacLeod’s calorimeter. Air monitored by inserting the thermocouple into the left side
was continuously circulated through a drying column of IBAT. Firing rate, TIBAT and TC were recorded for 40
(CaSO Drierite), a respiratory chamber 2.5 l and CO trap4 2 min before and for 40 min after injection of neostigmine
27
(soda lime), by a peristaltic pump at a rate of 2 l / min. A (5310 M in 1 ml of saline) into the hippocampus and
1-l cylindrical metal bell, fitted in a concentric cylinder bilateral administration of muscimol (28 ng in 0.5 ml of
filled with water forming an air-tight seal, served as the O2 saline for side) into the posterior hypothalamus. The same
reservoir. The 5-ml graduated cylinder was connected to variables were recorded in another six rats (2nd group),
the respiratory chamber. Respiratory chamber temperature but the muscimol was substituted with saline. The same
was maintained constant at 298C by circulating water, and procedure used with the 1st group was carried out with
was monitored by an internal thermometer. The volume of another six (3rd group) animals except that saline was
O consumed by each animal was corrected for tempera-2 injected into the hippocampus. In the rats of the 4th group,
0.75
ture and pressure and was expressed as ml / min / kg b.w. saline was injected into the hippocampus and the posterior
hypothalamus. The baseline values of T from all animalsC
2.3. Drugs used were maintained constant by a heating pad. The
electrical energy supplied to the pad was not altered during
Neostigmine and muscimol, a GABA receptor agonist,a the experimental period. In other words, a servo system for
were purchased from Sigma. The neostigmine was dis- controlling the animal’s temperature was not used.
27
solved in a pyrogen-free saline solution (5310 M of In the second part of the experiment, four other groups
neostigmine in 1 ml of 0.9% NaCl sterile pyrogen free of six animals each were used to determine O consump-2
solution). The muscimol (28 ng) was dissolved in 0.5ml of tion in the metabolic chamber, using the same anesthesia
saline. and the same procedure of cerebral injections.
2.4. Surgery 2.6. Histology
cannulas was identified. A dye (bromophenol blue) was between the neostigmine1muscimol group and other
injected in the hippocampus and the posterior hypo- groups at 15 and 20 min.
thalamus in the same volume as used for drug administra- The baseline absolute values were 42.2166.12 spikes / 5
tions. The rats were then injected with an overdose of s in the 1st group, 39.2667.11 in the 2nd group,
pentobarbital (200 mg / kg b.w. i.p.) and were perfused 40.2766.13 in the 3rd group and 41.2868.11 in the 4th
with 0.9% NaCl followed by 10% formalin solution. The group. There were no differences in the baseline absolute
brain was removed and stored in formalin solution. After a values of all groups. Examples of the changes in firing rate
few days, 50-mm coronal sections of the fixed brain were are shown in Fig. 2.
cut and stained with neutral red. In the hippocampus, the Fig. 3 illustrates the TIBAT changes. Neostigmine
in-dye was confined within the dorsal region, which is rich of jection caused a rise that peaks at 20 min in the rats
cholinergic receptors [6,26]. The histological control showed that the hypothalamic injection sites were within the posterior hypothalamic area.
3. Results
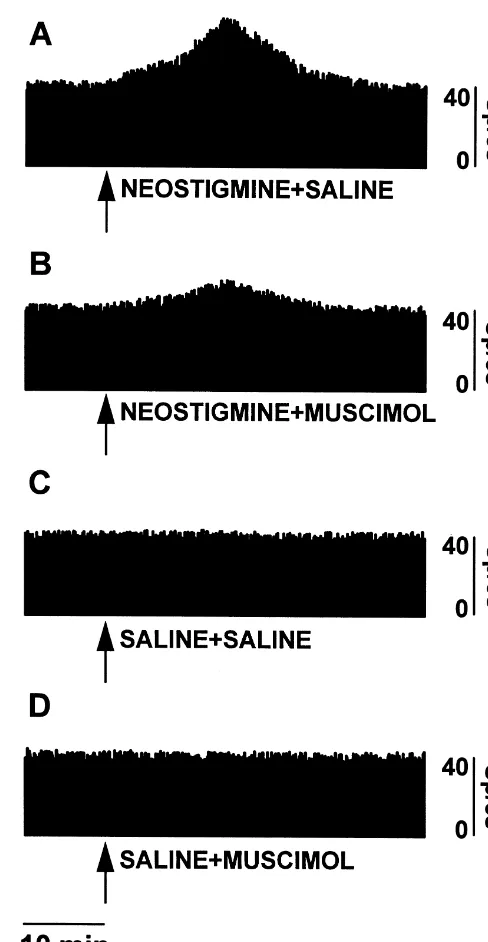
Fig. 1 shows the percentage changes in firing rate of the sympathetic nerves to the IBAT. Neostigmine injection caused a rise that peaks at 15 min in the rats without muscimol. This increase was reduced by muscimol in-jection. Muscimol or saline alone did not cause any
modification. Three-way ANOVA (neostigmine3
muscimol3time, 23239) with repeated measures on the
last factor showed significant main effects of neostigmine
[F(1, 20)562.541, P,0.01], of muscimol [F(1,20)5
10.696, P,0.05], of time [F(8, 160)527.237, P,0.01], as well as significant first order interactions, neostigmine3
muscimol [F(1, 20)510.638, P,0.05], neostigmine3time
[F(8, 160)526.519, P,0.01], muscimol3time [F(8,
160)55.250, P,0.01] and second order interaction,
neostigmine3muscimol3time [F(8, 160)54.929, P,
0.01]. A Newman–Keuls posthoc test showed that the neostigmine group without muscimol was different from other groups at 10–20 min. Differences were demonstrated
Fig. 5. Means6S.E. of oxygen consumption after an hippocampal Fig. 3. Means6S.E. of the interscapular brown adipose tissue
tempera-injection of neostigmine or saline and a posterior hypothalamic tempera-injection ture (IBAT) after an hippocampal injection of neostigmine or saline and a
of muscimol or saline. n56 for each group. posterior hypothalamic injection of muscimol or saline. The injections
were carried out at time 0; n56 for each group.
5.762, P,0.05], of time [F(8, 160)55.702, P,0.01], as
without muscimol. This increase was reduced by mus- well as significant first order interactions, neostigmine3
cimol. No change was induced by the muscimol or saline muscimol [F(1, 20)55.610, P,0.05], neostigmine3time
alone. Three-way ANOVA (neostigmine3muscimol3time, [F(8, 160)53.874, P,0.01]. The muscimol or saline
23239) with repeated measures on the last factor showed alone did not cause any change. Newman–Keuls posthoc
significant main effects of neostigmine [F(1, 20)518.956, test showed that the neostigmine group without muscimol
P,0.01], of muscimol [F(1,20)55.69, P,0.05], of time was different from other groups at 15–40 min. Differences
[F(8, 160)54.014, P,0.01], as well as significant first were demonstrated between the neostigmine1muscimol
order interactions, neostigmine3time [F(8, 160)55.718, group and other groups at 15 and 20 min.
P,0.01]. Newman–Keuls posthoc test showed that the Fig. 5 shows effects on O consumption. The neostig-2
neostigmine group without muscimol was different from mine injection induced an increase in O consumption, that2
other groups at 10–40 min. Differences were demonstrated was reduced by muscimol injection. No change was found
between the neostigmine1muscimol group and other in the rats with injection of muscimol or saline alone.
groups at 15 and 20 min. Three-way ANOVA (neostigmine3muscimol3time, 23
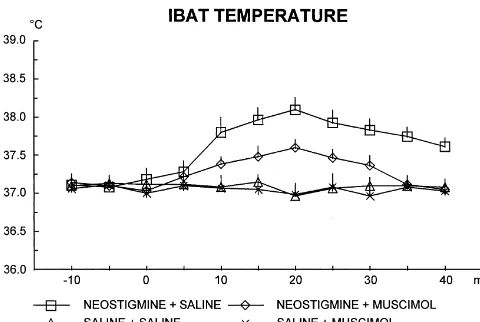
Fig. 4 illustrates the TC changes. Neostigmine injection 232) with repeated measures on the last factor showed
caused a rise that peaks at 20 min. This increase was significant main effects of neostigmine [F(1, 20)591.630,
reduced by muscimol. Three-way ANOVA (neostigmine3 P,0.01], of muscimol [F(1,20)510.118, P,0.01], of
muscimol3time, 23239) with repeated measures on the time [F(1, 20)5104.58, P,0.01], as well as significant
last factor showed significant main effects of neostigmine first order interactions, neostigmine3muscimol [F(1,
[F(1, 20)522.642, P,0.01], of muscimol [F(1,20)5 20)514.119, P,0.01], neostigmine3time [F(1, 20)5
91.930, P,0.01], muscimol3time [F(1, 20)510.762, P,
0.01], and second order interaction, neostigmine3
muscimol3time [F(1, 20)56.979, P,0.01]. Newman–
Keuls posthoc test showed that the neostigmine group without muscimol was different from other groups.
Differ-ences were demonstrated between the neostigmine1
muscimol group and control groups.
4. Discussion
These findings are the first to demonstrate that the sympathetic and thermogenic response to cholinergic stimulation of the hippocampus is reduced by an adminis-tration of muscimol into the posterior hypothalamus. This
Fig. 4. Means6S.E. of the colonic temperature after an hippocampal
indicates that the posterior hypothalamus is part of
hip-injection of neostigmine or saline and a posterior hypothalamic hip-injection
pocampal–sympathetic pathway and the posterior
hypo-of muscimol or saline. The injections were carried out at time 0; n56 for
to IBAT. The key role of GABAergic control on posterior Although this experiment has not tested food intake, we
hypothalamic activity is corroborated by other demonstra- can affirm that one of the two factors influencing body
tions. Bicuculline methiodide and picrotoxin, both post- weight is modified by the hippocampal administration of
synaptic GABA antagonists, induce an increase in heart neostigmine. We can therefore suppose that the
sympa-rate when injected in the posterior hypothalamus of thetic stimulation due to hippocampal neostigmine
ad-urethane-anesthetized rats [5]. The posterior hypothalamic ministration should reduce food ingestion, as reported in
level of GABA is modified by an intracerebroventricular other experiments [1,15,19]. On the other hand, the
injection of PGE which induces an increases in the firing1 posterior hypothalamus is involved in the control of eating
rate of nerves to IBAT [20]. Then, the sympathetic behavior. Peptides which control food intake, such as the
activation induced by neostigmine injected into the hip- orexins, have been detected in the secretory vesicles at
pocampus is modulated by GABA. synapses of fibers that project to the posterior
hypo-This experiment provides further evidence for the role of thalamus [25].
hippocampus in the control of vegetative functions, such as In this experiment, the increased O2 consumption
in-the control of body temperature. There is oin-ther evidence duced by neostigmine underlines the importance of
extra-showing the role of hippocampus in metabolic parameters, hypothalamic areas in the control of energy expenditure. It
such as glycemia; an injection of neostigmine in the has been demonstrated that cerebral neocortex influences
hippocampus increases blood glucose [9,27] and muscimol energy expenditure. Indeed, stimulation of frontal
neocor-injected in the ventromedial hypothalamus reduces hy- tex increases metabolic rate [4,17], while functional
de-perglycemia [22]. This indicates that vegetative changes cortication reduces energy expenditure [3,21]. This
experi-can be included in the response to stress that is controlled ment indicates that not only the neocortex, but also the
by hippocampus, through an influence on hypothalamic paleocortex is involved in the control of energy
expendi-areas, including the posterior hypothalamus. ture, through an influence on hypothalamic areas. Further
The influence of the posterior hypothalamus on the experiments need to test directly the effect of hippocampal
IBAT activity is directly demonstrated by reductions of neostigmine injection on eating behavior and energy
sympathetic discharge to IBAT and related temperature, intake, but the evidence of this paper strongly supports the
both induced by muscimol. This suggests that the increase hypothesis that the hippocampus is a key structure in the
in the sympathetic discharge induced by hippocampal control of energy balance.
neostigmine could be induced by a reduction of the In conclusion, these findings suggest that the
hippocam-posterior hypothalamic GABA level, so that the injection pus controls the sympathetic and thermogenic activation
of muscimol compensates partially for this reduction. In induced by neostigmine through an influence on
GABAer-other words, a reduced decrease in GABA levels is gic tone of the posterior hypothalamus.
associated with a reduced increase in the sympathetic and In perspective, modification of GABA concentration in
thermogenic activity. The injection of this GABA agonist the posterior hypothalamus induced by hippocampal
stimu-modifies the sympathetic activity and body temperature lation could be measured by microdialysis so that a
only in the rats who received the neostigmine injection, but temporal relation between neostigmine injection and
not in the control animals who received a saline injection. GABA modification should be evaluated. Furthermore,
This seems to indicate that the GABA receptors in the electrophysiological studies showing connectivities and of
posterior hypothalamus are almost saturated in the basal the responses of the connecting neurons to the
pharmaco-condition, so that an injection of muscimol does not logical agents could be performed.
modify the sympathetic discharge and the thermogenic variables. This explanation is corroborated by other
ob-servations. Muscimol injection in the posterior hypo- Acknowledgements
thalamus reduces the thermogenic activation induced by
sucrose-rich diet [18] or PGE1 [13] that induces an The support of the Italian National Research Council is
increase in sympathetic activity, while no modification is acknowledged.
induced by muscimol under basal conditions. We used 28 ng of muscimol per side, because this dose has been well
established in other studies on GABAergic influences on References
the sympathetic activity [10,13,18]. A GABA antagonist,
such as the bicuculline, has not been injected into the [1] G.A. Bray, Reciprocal relation between the sympathetic nervous
posterior hypothalamus, because bicuculline per se caused system and food intake, Brain Res. Bull. 27 (1991) 517–520.
[2] B. Cannon, J. Houstek, J. Nedergaard, Brown adipose tissue. More
an increase in the sympathetic discharge [5]. Then, the
than an effector of thermogenesis, Ann. NY Acad. Sci. 856 (1998)
effects of bicuculline would confuse the effects of
neostig-171–187.
mine which also stimulates the sympathetic activity [3] B. De Luca, M. Monda, M.P. Pellicano, A. Zenga, Cortical control
Energy expenditure (expressed as O consumption) and2 of thermogenesis induced by lateral hypothalamic lesion and
[4] B. De Luca, M. Monda, S. Amaro, M.P. Pellicano, L.A. Cioffi, [16] M. Monda, A. Sullo, B. De Luca, Lesions of ventromedial hypo-Thermogenic changes following frontal neocortex stimulation, Brain thalamus reduce post-ingestional thermogenesis, Physiol. Behav. 61
Res. Bull. 22 (1989) 1003–1007. (1997) 687–691.
[5] J.A. Di Micco, V.M. Abshire, K.D. Hankins, R.H.B. Sample, J.H. [17] M. Monda, A. Sullo, V. De Luca, A. Viggiano, Procaine injection Wible, Microinjection of GABA antagonists into the posterior into the paraventricular nucleus reduces sympathetic and ther-hypothalamus elevates heart rate in anesthetized rats, Neurophar- mogenic activation induced by frontal cortex stimulation in the rat, macology 25 (1986) 1063–1066. Brain Res. Bull. 47 (1998) 657–662.
[6] S.E. File, P.J. Kenny, S. Cheeta, The role of the dorsal hippocampus [18] M. Monda, A. Sullo, V. De Luca, A. Viggiano, Sucrose rich diet serotonergic and cholinergic systems in the modulation of anxiety, modifies thermogenic response to injection of muscimol into the Pharmacol. Biochem. Behav. 66 (2000) 65–72. posterior hypothalamus, Acta Physiol. Scand. 163 (1998) 379–384. [7] D.O. Foster, F. Depocas, G. Zaror-Behrens, Unilaterality of the [19] M. Monda, A. Sullo, A. Viggiano, V. De Luca, Eating behavior is sympathetic innervation of each pad of rat interscapular brown related to sympathetic activation induced by icv injection of adipose tissue, Can. J. Physiol. Pharmacol. 60 (1982) 107–113. prostaglandin E in the rat, Nutr. Neurosci. 2 (1999) 69–74.1 [8] J. Himmis-Hagen, Non-shivering thermogenesis, Brain Res. Bull. 12 [20] M. Monda, A. Viggiano, A. Sullo, G. Manzi, V. De Luca,
Intracereb-(1984) 151–160. roventicular injection of prostaglandin E increases1 g-aminobutyric [9] A. Iguchi, K. Uemura, H. Miura, T. Ishiguro, K. Nonogaki, T. acid level in the posterior hypothalamus, J. Therm. Biol. 24 (1999)
Tamagawa, K. Goshima, N. Sakamoto, Mechanism of intrahip- 359–363.
pocampal neostigmine-induced hyperglycemia in fed rats, Neuroen- [21] M. Monda, A. Viggiano, A. Sullo, V. De Luca, Cortical spreading docrinology 55 (1992) 44–50. depression reduces paraventricular activation induced by hippocam-[10] M. Lisa, E. Marmo, J.H. Wible, J.A. DiMicco, Injection of mus- pal neostigmine injection, Brain Res. 824 (1999) 119–124.
cimol into posterior hypothalamus blocks stress-induced tachycar- [22] K. Ozawa, H. Miura, T. Tamagawa, Y. Hiyoshi, K. Nonogaki, N. dia, Am. J. Physiol. 257 (1989) R246–R251. Maeda, G. Watanabe, N. Sakamoto, A. Iguchi, Intrahypothalamic but [11] T.J. Malkinson, W.L. Veale, K.E. Cooper, Experimental characteriza- not hippocampal administration of muscimol suppresses hy-tion and applicahy-tions of an anesthetized animal model for ther- perglycemia induced by hippocampal neostigmine in anesthetized moregulatory investigations, Biomed. Sci. Instrum. 29 (1993) 369– rats, Life Sci. 25 (1993) 1093–1099.
376. [23] L.J. Pellegrino, A.S. Pellegrino, A.J. Cushman (Eds.), A Stereotaxic [12] M. Monda, S. Amaro, A. Sullo, B. De Luca, Posterior hypothalamic Atlas of the Rat Brain, Plenum Press, New York, 1979.
activity and cortical control during the PGE -hypertermia, NeuroRe-1 [24] T. Sakaguchi, G.A. Bray, G. Eddlestone, Sympathetic activity port 6 (1994) 135–139. following paraventricular or ventromedial hypothalamic lesions in [13] M. Monda, S. Amaro, A. Sullo, B. De Luca, Injection of muscimol rats, Brain Res. Bull. 20 (1988) 461–465.
in the posterior hypothalamus reduces the hyperthermia induced by [25] J.G. Sutcliffe, L. de Lecea, Novel neurotransmitters for sleep and prostaglandin E in the rat, Brain Res. Bull. 37 (1995) 575–580.1 energy homeostasis, Results Probl. Cell Differ. 26 (1999) 239–255. [14] M. Monda, A. Papa, G. Brizzi, B. De Luca, Neostigmine injection in [26] L.K. Takahashi, C.S. Goh, Presynaptic muscarinic cholinergic the hippocampus activates thermogenesis by an increase in sympa- receptors in the dorsal hippocampus regulate behavioral inhibition of thetic activity and T -to-T conversion, Am. J. Physiol. 270 (1996)4 3 preweanling rats, Brain Res. 731 (1996) 230–235.
R1215–R1219. [27] Y. Uemura, A. Iguchi, A. Yatomi, H. Miura, A. Honmura, M. [15] M. Monda, A. Sullo, E. De Luca, M.P. Pellicano, Lysine Yanase, N. Sakamoto, Involvement of the hippocampus in central acetylsalycilate modifies aphagia and thermogenic changes induced nervous system-mediated glucoregulation in rats, Endocrinology 124 by lateral hypothalamic lesion, Am. J. Physiol. 271 (1996) R1638– (1989) 2449–2455.