www.elsevier.com / locate / bres
Interactive report
Characterisation of vasopressin V , angiotensin AT and AT receptor
1A 1 2distribution and density in normotensive and hypertensive rat brain
1
stem and kidney: effects of restraint stress
*
Stuart J. McDougall, Carlie A. Roulston, Robert E. Widdop, Andrew J. Lawrence
Department of Pharmacology, Monash University, Wellington Rd., Clayton, Victoria, Australia 3168
Accepted 14 September 2000
Abstract
In the present study, we have examined neurochemical correlates that may be involved in the differential cardiovascular responses observed in normotensive and hypertensive rats during stress. Using a restraint stress paradigm, both normotensive Wistar Kyoto (WKY) and Spontaneously Hypertensive rats (SHR) underwent acute (1 h restraint in a perspex tube), chronic (1 h restraint for ten consecutive days) or no restraint (control) stress. Following cessation of restraint, rats were processed by incubating sections of brain stem and kidney
125 125 1 8
with [ I]-HO-LVA (0.03 nM) or [ I]Sar Ile -AngiotensinII (0.5 nM), in the presence of PD123319 (10mM) or losartan (10mM), to determine the distribution and density of vasopressin V , angiotensin AT and AT receptors, respectively. Analysis of autoradiograms1A 1 2 indicated changes in the density of radioligand binding in acutely and chronically-stressed rats, as compared to controls. For example, V1A
binding in the medial nucleus tractus solitarius (SolM) decreased in the WKY but increased in the SHR. AT binding in SolM did not1 significantly change in the WKY but decreased in the SHR with repeated restraint. In kidney slices, AT binding decreased with stress in1
the WKY (217%) but increased in SHR (110–15%). AT binding in the kidney showed a pattern similar to that of AT binding in SHR,2 1 but not WKY. Graded increases in V1A binding were measured in kidney medulla and cortex of both strains (150–60% with chronic restraint). These results suggest that physiological adaptation to restraint is associated with specific changes in V , AT and AT receptor1A 1 2 density within brain nuclei and kidney. 2000 Elsevier Science B.V. All rights reserved.
Theme: Neurotransmitters, modulators, transporters, and receptors
Topic: Receptor modulation, up- and down-regulation
Keywords: Restraint; WKY; SHR; Angiotensin; Vasopressin; Receptor autoradiography
1. Introduction telemetry, that Spontaneously Hypertensive rats (SHR)
have an impaired capacity to cope with the stress-induced Acute stressors, such as restraint are well characterised tachycardia compared to normotensive Wistar–Kyoto rats to cause immediate and profound tachycardia; however, (WKY) [22]. Considering that coping (adaptation or such responses may diminish with subsequent stress habituation) occurs due to changes within the central encounters [6]. Associated with this adaptation are also nervous system (CNS) nuclei that regulate the stress alterations in the activity of the hypothalamo–pituitary– response [15], it would appear that SHR may have a adrenal axis (HPA) and sympatho-adrenal system (SAS) reduced plasticity within the CNS compared to WKY. [14,17]. We have recently demonstrated, using radio- Central control of the cardiovascular system is achieved
via a complex network of interconnected nuclei. While the basic control is primarily mediated at the level of the
1
Published on the World Wide Web on 2 October 2000. medulla oblongata, significant supramedullary modulation *Corresponding author. Tel.: 161-3-9905-4855; fax: 1
61-3-9905-also occurs [8]. For example, the locus coeruleus (LC)
5851.
makes up a large portion of the central noradrenergic
E-mail address: [email protected] (A.J.
Law-rence). system, which is thought to play an important role in the
initiation of the stress response [15] and can regulate in diameter, Plastic Labs, Lansing, MI) for 60 min between sympathetic vasomotor outflow [24,41]. A number of 900 and 1200 h. This procedure of restraint stress was neurotransmitters have been implicated in central car- performed once for the acute study and for 10 consecutive diovascular control including arginine vasopressin (AVP) days in the chronic study, as previously described [37,22]. and angiotensin II (Ang II) [18]. Elevations in plasma AVP
reset arterial baroreflex control of sympathetic nerve 2.2. Receptor autoradiography activity and heart rate (HR) to lower pressures, an effect
mediated by V1A receptors in rabbits [20]. Similarly in rats, Frozen brainstems and kidneys were equilibrated from AVP is known to facilitate the baroreceptor heart-rate 2808C to2178C and then sectioned in a cryostat (Cryocut reflex and sensitize low and high pressure baroreceptors 1800, Leica). Coronal sections (14 mm) were collected [13]. Conversely, Ang II inhibits baroreflex function at the from specific brainstem regions. In the kidney, sagittal level of the NTS [5]. AVP and Ang II are also involved in sections incorporating both cortex and medulla were cut on the control of the stress response. Thus AVP is known to a cryostat and sections were thaw-mounted onto gelatin-become a major ACTH stimulator with repeated restraint / chrome alum coated slides. All tissue slices were stored at immobilisation stress [32], while it has been suggested that 2808C until autoradiography experiments. For the three Ang II plays a role in the control of SAS activity due to autoradiography assays, tissue slices from all treatment immobilisation-induced stress response [16]. groups (day 0, 1 and 10 days of stress) and from both Since we have recently documented the differential strains (WKY and SHR) were processed simultaneously effects of restraint on cardiovascular regulation between for any particular receptor assay, to ensure a valid com-WKY and SHR [22], the present study was designed to parison between groups.
investigate whether this differential coping between WKY The vasopressin V1A receptor autoradiography technique
125
and SHR with chronic restraint was associated with using [ I]HO-LVA (0.03 nM) (sequence: 4-(HO)-differential alterations in neuropeptide receptor binding. To Phenylacetyl-D-Tyr(Me)-Phe-Gln-Asn-Arg-Pro-Arg-NH ;2
achieve this aim, quantitative in vitro autoradiography was specific activity: 2000 Ci / mmol; Auspep, Parkville, Aus-employed to assess the effect of restraint stress upon the tralia) was adapted from a published protocol [2] by density of V , AT1A 1 and AT2 receptors in selected increasing bovine serum albumin concentration in the brainstem regions and the kidneys of acutely and chroni- incubation medium from 0.1 to 1%; the addition of a 15 cally stressed WKY and SHR. min pre-incubation of tissue in incubation medium at room temperature; 1mM AVP (Sigma, St. Louis, USA) was used to define non-specific binding. Following the assay
incuba-2. Methods tion, brain sections were washed for 735 min in ice-cold
Tris–HCl (50 mM, pH7.4) while kidney sections had 635 Experimental procedures were approved by the Monash min washes.
125
University Animal Ethics Committee and performed ac- For angiotensin receptor autoradiography, [ I]Sar-Ile cording to the National Health and Medical Research Ang II (0.5 nM) (specific activity: 2000 Ci / mmol; Auspep, Council of Australia guidelines for animal experimenta- Parkville, Australia) binding was performed according to a tion. All rats were obtained from the Austin Hospital, published protocol [9], except that the assay incubation Heidelberg, Victoria and maintained on a 12 h light / dark was for 60 min and the post incubation wash consisted of cycle with standard laboratory rat chow and water avail- 433 min washes. AT1 and AT2 receptor binding was able ad libitum. determined in the presence of PD123319 (10 mM) (gift from Dr J. Keiser, Parke-Davis, USA) and losartan (10 2.1. Restraint stress mM) (gift from Dr. R. Smith, DuPont, USA), respectively. Non-specific binding was defined by 20 mM Ang II Age matched (15–16 weeks) male WKY (n515) and (Auspep, Parkville, Australia).
SHR (n515) rats were divided into three groups (n55 per After washing and rinsing, all tissue sections were dried group): (i) control (no-stress), (ii) acute (60 min) and (iii) under a gentle stream of cool air and desiccated overnight. chronic (60 min daily for 10 days) stress groups. Those Subsequently, all slides were apposed to X-ray film rats designated for the ‘chronic’ paradigm had telemetry (Kodak XAR-5) alongside standard microscales (American probes implanted 11 days prior to the onset of restraint; Radiolabelled Chemicals, Inc.). Film apposition time dif-telemetry data are published elsewhere [22]. Rats subjected fered for various regions as determined by preliminary to restraint were housed singularly while control rats assays, and autoradiograms were subsequently developed maintained social contact. Previous studies have demon- in a Kodak automatic developer (100 Plus). Binding of
125 125
Canada), under constant illumination, as previously de- strains (Fig. 2 and Table 1). Binding densities varied scribed [19,37]. Briefly, images were captured and regions significantly, with SHR showing greater V1A density in the
2
of interest delineated such that unit activity (dpm) per mm hypoglossal nucleus (17%) and kidney cortex (129%), could be determined with reference to the standard mi- but lower V1A density in the LC (221%) and Sp5 (215%) croscales. Brain nuclei were defined using counterstained as compared to WKY. Differences in binding to Ang II (0.1% thionin) slices and reference to a stereotaxic atlas receptors were also found with SHR showing a greater [26]. All slices were of a constant thickness, cut on the density of AT1 receptors in the medial nucleus tractus same cryostat, thereby negating any potential confounding solitarius (SolM) (112%), commissural nucleus tractus factor of tissue thickness on signal density. solitarius (SolC) (126%), AP (137%) and RVLM (19%), but decreased AT density in the kidney medulla1
2.3. Statistical analysis (219%) and cortex (212%) as compared to WKY (Fig. 2 and Table 1). Binding to AT receptors was significantly2
All data are represented as mean6standard error of the higher in the LC (111%), SolM (121%), and IO (122%) mean. For analysis of quantitative autoradiography data, an of SHR as compared to WKY (Fig. 2).
unpaired t-test was employed for a strain comparison
(controls only). A one-way ANOVA, followed by post-hoc 3.2. Effect of restraint Dunnett’s test, was used to compare different stress
paradigms within strains, while a 2-way ANOVA was used Changes in V , AT and AT receptor binding within1A 1 2
to assess the effect of strain on the temporal nature of the the brainstem and kidney of WKY and SHR associated response to the stressor. In this instance data were normal- with ongoing restraint are shown in Fig. 2 and Table 1, ised to preclude any potentially confounding effects of respectively. Significant decreases in V1A binding after one different basal values between strains. P,0.05 was consid- restraint session (acute group) occurred in the LC and ered significant. hypoglossal nucleus of the WKY, while in the SHR significant increases were observed in the SolM, hypo-glossal nucleus and Sp5. Within the WKY group subjected
3. Results to chronic restraint stress, V1A density in the SolM and
hypoglossal nucleus was decreased whereas in the SHR the
125
High affinity binding of [ I]HO-LVA (0.03 nM) to V1A SolM, hypoglossal nucleus, IO and Sp5 showed signifi-receptors was observed in regions including the LC of the cantly increased V1A binding during chronic stress com-pons, NTS, area postrema (AP), spinal trigeminal nucleus pared with controls. With respect to the AT1 binding, (Sp5), inferior olive (IO), and hypoglossal nucleus of the significant increases subsequent to chronic restraint medulla oblongata, and in kidney medulla and cortex (Fig. occurred in the SolC and CVLM in both strains, while a 1). Although not quantified, there was also specific binding decrease in AT binding was only seen in the SolM of the1
in the choroid plexus. With the exception of the cere- SHR (Fig. 2). There were no significant changes in central
125
bellum, where non-specific binding for [ I]HO-LVA has AT receptor binding as stress was repeated.2
been previously observed [2], non-specific binding (in the The most notable changes in binding, as restraint presence of 1mM vasopressin) over the rest of the tissue continued from acute to chronic, were within the kidney. sections was too faint to quantify, confirming the spe- V1A binding showed graded increases in both kidney
125 1 8
cificity of the radioligand. Binding of [ I]Sar Ile -AII medulla and cortex, with binding in some instances (0.5 nM) in the presence of PD123319 (10 mM) (AT1 increasing greater than 50% of control values (Table 1). receptors) was observed in the LC, NTS, AP, rostral By contrast, AT binding significantly differed between the1
ventrolateral medulla (RVLM), caudal ventrolateral medul- strains with repeated restraint, where AT1 binding de-la (CVLM), kidney and although not quantified, also in creased in the WKY while increasing in the SHR. Similar-Sp5 (Fig. 1). In the presence of losartan (10 mM) (AT2 ly, stress increased AT binding in SHR, but there were2
125 1 8
receptors), binding of [ I]Sar Ile -Ang II (0.5 nM) variable changes in kidney of WKY (Table 1). occurred in the LC, NTS, IO and kidney cortex. In the
125 1 8
presence of Ang II (20mM), binding of [ I]Sar Ile -Ang
II was too faint to quantify in the brainstem and kidney 4. Discussion
regions examined.
The present study represents the first quantitative com-3.1. Strain comparison parison of central vasopressin V1Areceptors between WKY and SHR. Furthermore, these data represent the first ¨
A comparison of tissue sections from naıve WKY and systematic comparison of the responses of WKY and SHR SHR not subjected to any stress revealed that region- to repeated restraint stress in terms of plasticity of specific
125
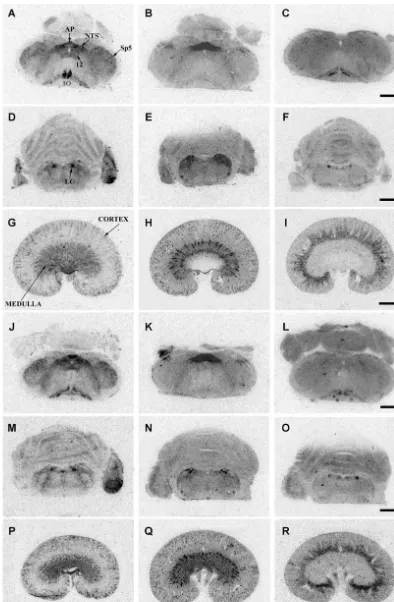
Fig. 1. Representative autoradiograms of sections of brain stem and kidney from control WKY (A–I) and SHR (J–R). Left column represents
125 125 1 8
[ I]HO-LVA (V1A binding), the middle column represents [ I]Sar Ile -AII in the presence of PD123319 (AT1 binding), while the right column
125 1 8
represents [ I]Sar Ile -AII in the presence of losartan (AT binding). A–C and J–L: medulla oblongata at the level of area postrema (bregma –13.682
125 125 1 8
Fig. 2. [ I]HO-LVA (0.03 nM)(V1 binding)[A and B]; [A I]Sar Ile -AII (0.5 nM) in the presence of PD123319 (10mM) (AT binding)[C and D]; and1 125 1 8
[ I]Sar Ile -AII in the presence of losartan (10mM) (AT binding) [E and F] binding density in WKY [A, C and E] and SHR [B, D and F] control (open2
columns); acute (60 min only) restraint (hatched columns); and chronic (10 consecutive days) restraint (filled columns) groups in brainstem regions quantified by MCID M4 Image Analyser (n55 per strain per group). *P,0.05 versus control (one-way ANOVA followed by post-hoc Dunnett’s test).
¨
†P,0.05 versus naıve (no stress) WKY (unpaired t-test).
differential ability to cope with restraint stress between the explain our previous physiological findings, indicating that WKY and SHR rats was manifested by a slowly-adapting there was differential regulation of vasopressin V , and1A
reduction in tachycardia in the SHR [22]. The present Ang II AT and AT receptor density in brainstem nuclei1 2
Table 1
a
V , AT and AT binding changes in the kidney medulla and cortex of WKY and SHR due to repeated restraint stress1A 1 2
Region Stress V1Abinding AT binding1 AT binding2
day
Kidney medulla 1 21.2061.01* 25.5265.97* 152.1063.01 1.1662.00 62.7061.608 13.7762.92* 10 26.1061.18* 54.3466.99* 166.1064.72* 10.4663.14* 65.2060.79* 18.3661.46*
V , AT and AT binding determined using [1A 1 2 I]HO-LVA (0.03 nM), [ I]Sar Ile -AII (0.5 nM) in the presence of PD123319 (10mM) or losartan (10
†
mM), respectively (all n55). Stress day 0, 1 and 10 refer to no stress, acute stress and 10 consecutive days of restraint stress, respectively. P,0.05 versus
[
WKY (unpaired t-test). *P,0.05 versus control (one-way ANOVA followed by post-hoc Dunnett’s test). P,0.05, different temporal response versus equivalent region of WKY (two-way ANOVA).
restraint stress. While unlikely, it is possible that the studies is unknown, but may relate to the source of rat altered densities of binding observed reflect changes in the stocks.
affinity of the radioligands caused by alterations in the Since the initial observations of specific binding of conformational states of the receptors. labeled angiotensin II to brain regions of normotensive and hypertensive rats [30,12], subtypes of angiotensin receptors have been identified. Binding to AT and AT receptors in1 2
4.1. Strain comparisons this study was consistent with previous findings [29,35]. For example, AT receptors were predominantly located in1 125
Binding of the radioligand [ I]HO-LVA, shown to be NTS and AP, while AT receptors were mainly localised in2
highly selective for vasopressin V1A receptors [2], was LC and IO, while both subtypes were present in the localised in brainstem nuclei and kidney. The distribution ventrolateral medulla and kidney. Moreover, the SHR of vasopressin V1A receptors in these regions has been controls had increased AT1 binding as compared to the reported previously [38,27,33]. While renal V1A binding WKY control group in the NTS (both SolM and SolC), AP has previously been compared between normotensive and and in RVLM, a finding consistent with previous studies hypertensive rats [23], there has been no previous com- [35,1]. In addition, there was increased binding with parison of central V1A binding. Compared with control respect to AT receptors in the LC, SolM and IO of control2
WKY rats, the present study has shown that V1A binding SHR as compared to that of the control WKY. AT1
densities varied significantly with control SHR showing Receptor binding in the control SHR kidney medulla and greater density in the hypoglossal nucleus (17%) and cortex was significantly lower than that of the control lower density in the LC (221%) and Sp5 (215%). WKY, in contrast to previous reports [35], where differ-Whether these differences in V1A density between WKY ences were found in the outer medulla only.
and SHR are physiologically significant remain to be
established; however, it is of interest to note that the 4.2. Effect of restraint hypoglossal nucleus, locus coeruleus and Sp5 are all
findings clearly indicate that the SHRs have a substantially pressor responses did not show the same pattern of reduced adaptation of stress-induced tachycardia compared adaptation [22], that may be reflective of a relatively to WKY rats [22]. In the present study, a number of unchanged sympathetic vasomotor outflow from the differences between strains were noted in brainstem and RVLM. In a similar manner, we have previously demon-kidney in response to chronic stress which may relate to strated that restraint stress caused dramatic effects in the the differential stress-induced cardiovascular responses expression of the mRNA encoding preprogalanin in the previously observed [22]. For example, restraint stress supuroptic nucleus [37] without any change in the RVLM. evoked opposite changes in V1A and AT receptors in the1 Thus it is possible that restraint-induced influences in the SolM of SHR (increases and decreases, respectively) cardiovascular system are at least partially mediated by compared with WKY rats, while AT1 receptor binding higher brain centres. Despite the lack of change in AT1
increased in SolC of both WKY and SHR with restraint. binding in the RVLM, there is considerable evidence Low doses of AVP injected into the NTS of SHR result in linking central angiotensin receptors to both sympathetic bradycardia without a change in blood pressure [36], and neuroendocrine responses to stressors [40,10]. For whereas Ang II injected at the same site inhibits baroreflex example, central administration of the AT1 receptor an-function [5]. Therefore, increased V1A and decreased AT1 tagonist, losartan, attenuated both the increase in plasma receptors could cause directionally similar alterations in catecholamines and the increased expression of the mRNA heart rate at the level of the NTS which could contribute to encoding corticotropin releasing factor subsequent to im-the slowly-adapting reduction in stress-induced tachycardia mobilization stress in rats [16]. These data were suggested observed in the SHR [22]. to indicate that the central release of endogenous angioten-The LC is pivotal to the activation of the stress response sin II is necessary to mediate sympathoadrenal activation [15]. Significant changes in binding with restraint stress during stress, and may also act as a modulator of hypo-only occurred with the vasopressin V1A receptor in the thalamic neurosecretory cells.
WKY LC after one restraint session. AVP injections into The intracerebroventricular administration of a vasopres-the LC cause dose-related increases in heart rate and blood sin V1A antagonist did not alter the adaptive heart rate pressure [3] via modulating sympathetic outflow [4]. Thus, response to restraint in Lister Hooded rats, suggesting that a decrease in V1A receptor binding in the LC within the AVP does not play a major role in cardiovascular adapta-restraint session may contribute to the cardiovascular tion in this rat strain [7]. However, as central V1A receptor coping exhibited by the WKY, i.e. heart rate and arterial densities are not homogenous between normotensive and pressure returning towards resting levels during the re- hypertensive strains, the role of AVP in the SHR coping straint period [22]. However, the role of AII receptors in mechanism cannot be ruled out, particularly since icv the LC would seem to be limited in terms of coping with administration may not sufficiently access all central nuclei restraint, since binding did not change in the either strain containing V1A receptors. Furthermore, considerable evi-as restraint wevi-as repeated. These findings are in contrevi-ast to dence exists to link vasopressin systems with the central a recent study which demonstrated an increase in the integration of various stress responses, such as a hy-expression of the mRNA encoding AT receptors in the2 pertonic saline challenge [21] and swim stress [11]. In LC of Wistar rats subjected to either immobilisation or addition, central administration of a vasopressin V re-1
air-jet stress [10]. This apparent paradox may be explained ceptor antagonist blocked the suppression of immune by species variation (in the present study SHR had a function in rats exposed to intermittent electrical footshock higher density of AT2 receptors in the LC compared to [34]. While the present study has concentrated upon WKY), plus it is clearly possible that an increase in the vasopressin V1A receptors in autonomic nuclei, it must also expression of mRNA may not necessarily be paralleled by be remembered that stressors are likely to modulate an increase in transcription. vasopressin V1B receptors, particularly since activation of The CVLM and RVLM play an important role in the V1B receptors in the pituitary gland stimulates ACTH sympathetic vasomotor efferent pathway [8], and appreci- secretion [28].
able levels of AT receptors are located in these nuclei. In1 Therefore, while the reported cardiovascular changes terms of the current restraint paradigm, no significant occurring subsequent to restraint stress [22] may not changes in AT1 binding occurred in the RVLM with necessarily be paralleled by all of the, observed changes in restraint. In the CVLM on the other hand, increases in brain stem neuropeptide receptors, it is clearly possible binding to AT receptors were observed in both strains as1 that: (i) other neurotransmitter(s) and receptors are also restraint was repeated over time; a pattern that does not involved in mediating cardiovascular coping; and (ii) clearly fit with differential coping between strains. Thus, higher brain centres may also be implicated in mediating the role of AII receptors within CVLM and RVLM with some of the observed cardiovascular adaptation, such as respect to restraint is equivocal. As such, the present the paraventricular nucleus of the hypothalamus and observations may reflect secondary effects due to altered cortical regions [8].
tion of vasopressin into the locus coeruleus of conscious rats, Am. J.
following chronic restraint stress were found. V1A receptors
Physiol. 247 (1984) H675–H681.
in the kidney are predominantly localised on vascular
[4] K.H. Berecek, Role of central vasopressin in cardiovascular
regula-structures [33,25], inferring that other vascular (non-renal) tion, J. Cardiovasc. Pharmacol. 8 (1986) S76–S80.
V1A binding may have also increased. In contrast, the [5] R. Casto, M.I. Phillips, Angiotensin II attenuates the baroreflex at
stress-induced changes in binding for AT1 receptors in nucleus tractus solitarius of rats, Am. J. Physiol. 250 (1986) R193– R198.
kidney were markedly different in WKY rats and SHR,
[6] X. Chen, J. Herbert, Regional changes in c-fos expression in the
since there were decreases in both cortical and medullary
basal forebrain and brainstem during adaptation to repeated stress:
AT binding in the former, but increased AT binding in1 1 correlation with the cardiovascular, hypothermic and endocrine
the latter. AT receptors are located extensively throughout1 responses, Neuroscience 64 (1995) 675–685.
the kidney and conceivably a similar increase in vascular [7] X. Chen, J. Herbert, Alterations in sensitivity to intracerebral vasopressin and the effects of a V receptor antagonist on cellular,
AT1 receptors may contribute to the well-maintained 1a
autonomic and endocrine responses to repeated stress, Neuroscience
pressor activity throughout each restraint session in the
64 (1995) 687–697.
SHR group only. AT receptors are localised predominant-2 [8] R.A. Dampney, Functional organization of central pathways
regulat-ly in glomeruli [39], which is consistent with the greater ing the cardiovascular system, Physiol. Rev. 74 (1994) 323–364. cortical binding in the present study. Moreover, in WKY [9] A.M. De Oliveria, M. Viswanathan, F.M.J. Heemskerk, J.M.
Saavedra, Expression of a novel angiotensin II receptor subtype in
rats, there was differential AT and AT cortical binding1 2
gerbil brain, Brain Res. 705 (1995) 177–187.
due to stress since there was actually an increase in AT2
[10] E.C. Dumont, S. Rafrafi, S. Laforest, G. Drolet, Involvement of
and a concomitant decrease in AT density in this strain.1 central angiotensin receptors in stress adaptation, Neuroscience 93
However, similar increases in renal cortical AT and AT1 2 (1999) 877–884.
binding were observed in SHR. Thus, stress caused [11] K. Ebner, C.T. Wotjak, F. Holsboer, R. Landgraf, M. Engelmann,
opposite changes in renal AT receptors between strains,1 Vasopressin released within the septal brain area during swim stress modulates the behavioural stress response in rats, Eur. J. Neurosci.
whereas for the most part, stress increased renal AT2
11 (1999) 997–1002.
receptors in both strains. Interestingly, differential
regula-[12] D.R. Gehlert, R.C. Speth, J.K. Wamsley, Quantitative
autoradiog-tion of renal AT1 and AT2 receptors has recently been raphy of angiotensin II receptors in the SHR brain, Peptides 7 reported under various treatment conditions. For example, (1986) 1021–1027.
renal AT , but not AT , receptor protein was down-1 2 [13] B.N. Gupta, A.L. Abboud, J.S. Floras, P.E. Aylward, F.M. Abboud, Vasopressin facilitates inhibition of renal nerve activity mediated
regulated by Ang II-induced hypertension [40]. However,
through vagal afferents, Am. J. Physiol. 253 (1987) H1–H7.
given the variety of hormonal factors altered by stress, the
[14] R.L. Hauger, M. Lorang, M. Irwin, G. Aguilera, CRF receptor
possible differential modulation of renal AT1 and AT2 regulation and sensitization of ACTH responses to acute ether stress
receptors by individual hormones involved in the stress during chronic intermittent immobilization stress, Brain Res. 532
response awaits further investigation. (1990) 34–40.
[15] G. Huether, The central adaptation syndrome: psychosocial stress as
In conclusion, the present study found that highly
a trigger for adaptive modifications of brain structure and brain
specific alterations occur in the binding properties of V ,1A function, Prog. Neurobiol. 48 (1996) 569–612.
AT and AT receptors between WKY and SHR following1 2 [16] D. Jezova, T. Ochedakski, A. Kiss, G. Aguilera, Brain angiotensin II
exposure to restraint stress. Whether or not these changes modulates sympathoadrenal and hypothalamic pituitary
adreno-are mediating or consequential of the adaptation of the cortical activation during stress, J. Neuroendocrinol. 10 (1998) 67–72.
stress response remains to be elucidated.
[17] J. Lachuer, I. Delton, M. Buda, M. Tappaz, The habituation of brainstem catecholaminergic groups to chronic daily restraint stress is stress specific like that of the hypothalamo–pituitary–adrenal
Acknowledgements axis, Brain Res. 638 (1994) 196–202.
[18] A.J. Lawrence, B. Jarrott, Neurochemical modulation of car-diovascular control in the nucleus tractus solitarius, Prog. Neurobiol.
These studies were supported, in part, by grants from the
48 (1996) 21–53.
National Health and Medical Research Council of Aus- [19] A.J. Lawrence, E. Krstew, B. Jarrott, Functional dopamine D
2
tralia, and the Clive & Vera Ramaciotti Foundations. receptors on rat vagal afferent neurones, Br. J. Pharmacol. 114 (1995) 1329–1334.
[20] J. Luk, I. Ajaelo, V. Wong, J. Wong, D. Chang, L. Chou, I.A. Reid, Role of V1 receptors in the action of vasopressin on the
baro-References receptor control of heart rate, Am. J. Physiol. 265 (1993) R524–
R529.
[1] C.O. Andrews, J.W. Crim, D.K. Hartle, Angiotensin II binding in [21] X.M. Ma, G. Aguilera, Transcriptional responses of the vasopressin area postrema and nucleus tractus solitarius of SHR and WKY rats, and corticotropin-releasing hormone genes to acute and repeated Brain Res. Bull. 32 (1993) 419–424. intraperitoneal hypertonic saline injection in rats, Mol. Brain Res. 68 [2] C. Barberis, M.N. Balestre, S. Jard, E. Tribollet, Y. Arsenijevic, J.J. (1999) 129–140.
Dreifuss, K. Bankowski, M. Manning, W.Y. Chan, S.S. Schlosser, F. [22] S.J. McDougall, J.R.A. Paull, R.E. Widdop, A.J. Lawrence, Re-Holsboer, J. Elands, Characterization of a novel, linear radioiodi- straint stress: differential cardiovascular responses in WKY and nated vasopressin antagonist: an excellent radioligand for vasopres- SHR, Hypertension 35 (1999) 126–129.
receptors in spontaneously hypertensive rats and DOCA-salt hy- [33] C. Serradeil-Le Gal, D. Raufaste, E. Marty, C. Garcia, J.P. Maffrand, pertensive rats using computerized quantification for macro-auto- G. Le Fur, Autoradiographic localization of vasopressin V1a
re-3
radiogram, Res. Commun. Mol. Pathol. Pharmacol. 95 (1997) 43– ceptors in the rat kidney using [ H]-SR 49059, Kidney Int. 50
56. (1996) 499–505.
[24] T. Miyawaki, H. Kawamura, K. Hara, K. Suzuki, W. Usui, T. [34] T. Shibasaki, M. Hotta, H. Sugihara, I. Wakabayashi, Brain vaso-Yasugi, Differential regional haemodynamic changes produced by pressin is involved in stress-induced suppression of immune func-L-glutamate stimulation of the locus coeruleus, Brain Res. 600 tion in the rat, Brain Res. 808 (1998) 84–92.
(1993) 56–62. [35] K. Song, Y. Kurobe, H. Kaneharam, H. Okunishi, T. Wada, Y. Inada, [25] N.L. Ostrowski, S.J. Lolait, A.-M. O’Carroll, M.J. Brownstein, W.S. K. Nishikawa, M. Miyazaki, Quantitative localization of angiotensin Young, Distribution of V1Aand V vasopressin receptor messenger2 II receptor subtypes in spontaneously hypertensive rats, Blood Press. ribonucleic acids in rat liver, kidney, pituitary and brain, Endocrinol- Supp. 5 (1994) 21–26.
ogy 131 (1992) 533–535. [36] M. Sonntag, W. Schalike, A. Brattstrom, Cardiovascular effects of [26] G. Paxinos, C. Watson, The Rat Brain in Stereotaxic Co-ordinates, vasopressin micro-injections into the nucleus tractus solitarii in 2nd Edition, Academic Press, Australia, 1986. normotensive and hypertensive rats, J. Hypertens. 8 (1990) 417– [27] P.A. Phillips, R.E. Widdop, S.Y. Chai, J. Kelly, V. Mooser, D. 421.
Trinder, C.I. Johnston, Reduced V vasopressin binding in the rat1 [37] B. Sweerts, B. Jarrott, A.J. Lawrence, Expression of preprogalanin nucleus solitarii after nodose ganglionectomy, Clin. Exp. Pharmacol. mRNA following acute and chronic restraint stress in brains of Physiol. 17 (1990) 321–325. normotensive and hypertensive rats, Mol. Brain Res. 69 (1999) [28] C. Rabadandiehl, G. Aguilera, Glucocorticoids increase vasopressin 113–123.
V1B receptor coupling to phospholipase C, Endocrinology 139 [38] E. Tribollet, C. Barberis, S. Jard, M. Dubois-Dauphin, J.J. Dreifuss, (1998) 3220–3226. Localization and pharmacological characterization of high affinity [29] B.P. Rowe, D.L. Saylor, R.C. Speth, Analysis of angiotensin II binding sites for vasopressin and oxytocin in rat brain by light
receptor subtypes in individual rat brain nuclei, Neuroendocrinology microcopy autoradiography, Brain Res. 442 (1988) 105–118. 55 (1992) 563–573. [39] Z.Q. Wang, L.J. Millatt, N.T. Heiderstadt, H.M. Siragy, R.A. Johns, [30] J.M. Saavedra, F.M. Correa, L.M. Plunkett, A. Israel, M. Kurihara, R.M. Carey, Differential regulation of renal angiotensin subtype K. Shigematsu, Binding of angiotensin and atrial natriuretic peptide AT1Aand AT receptor protein in rats with angiotensin-dependent2
in brain of hypertensive rats, Nature 320 (1986) 758–760. hypertension, Hypertension 33 (1999) 96–101.
[31] S. Schenk, K. Gorman, Z. Amit, Age-dependent effects of isolation [40] T. Watanabe, T. Fujioka, M. Hashimoto, S. Nakamura, Stress and housing on the self-administration of ethanol in laboratory rats, brain angiotensin II receptors, Crit. Rev. Neurobiol. 12 (1998)
Alcohol 7 (1990) 321–326. 305–317.
![Fig. 2. [125†125I]HO-LVA (0.03 nM)(V1 binding)[A and B]; [I]Sar Ile -AII (0.5 nM) in the presence of PD123319 (1018 mM) (AT binding)[C and D]; and[125I]Sar Ile -AII in the presence of losartan (1018A1 mM) (AT binding) [E and F] binding density in WKY [A, C](https://thumb-ap.123doks.com/thumbv2/123dok/3139069.1382705/5.612.51.546.54.606/binding-presence-binding-presence-losartan-binding-binding-density.webp)