www.elsevier.com / locate / bres
Short communication
Peptidergic input to immunohistochemically-identified Renshaw cells
*
Patrick A. Carr , Matthew J. Roller, Richard A. Zaruba
Department of Anatomy and Cell Biology, University of North Dakota, Grand Forks, ND 58202, USA Accepted 3 October 2000
Abstract
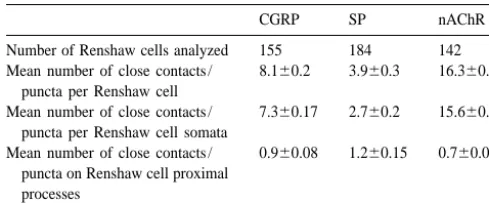
Peptidergic influences on Renshaw cells were assessed in rat using gephyrin-immunoreactivity, as a Renshaw cell specific marker, in combination with substance P, calcitonin gene-related peptide- and nicotinic acetylcholine receptor-immunolabelling. An average of 3.9 substance P-, and 8.1 calcitonin gene-related peptide-, and 16.3 nicotinic acetylcholine receptor-immunoreactive close contacts or puncta were observed per Renshaw cell. Most appositions were somatic. These results provide neuroanatomical support for the peptidergic modulation of Renshaw cells. 2000 Elsevier Science B.V. All rights reserved.
Theme: Motor systems and sensorimotor integration
Topic: Spinal cord and brainstem
Keywords: Substance P; Calcitonin gene-related peptide; Interneuron; Motor circuitry; Recurrent inhibition
The control of Renshaw cell activity involves a complex Adult, male Sprague–Dawley rats (n59) were perfused interaction of numerous excitatory and inhibitory influ- with 0.9% saline containing 0.1% sodium nitrite followed ences utilizing cholinergic, glycinergic, and serotonergic by either 4% paraformaldehyde alone or 4% paraformal-signalling molecules [1,2,8,9]. Evidence suggests that dehyde and 0.16% picric acid in 0.1 M phosphate buffer peptidergic modulation of this classical neurotransmitter (pH 7.4). The spinal cords were quickly removed and input may also play a significant role in the neurochemical placed in the same fixative for 2 h followed by immersion, signal transduced by Renshaw cells. There is abundant for at least 2 days, in cold (48C) 25% sucrose and 10% pharmacological evidence describing the modulation of glycerol in 50 mM phosphate buffer. Twenty micron cholinergic-mediated Renshaw cell activity via substance P transverse sections of midlumbar spinal cord were then cut (SP) / nicotinic receptor interactions [6,16–18]. In addition, on a freezing sliding microtome and collected into 0.1 M the localization of calcitonin gene-related peptide (CGRP) phosphate buffer with 0.9% saline (PBS). The tissue was in a-motoneurons and its release at the neuromuscular then incubated for 48–72 h at 48C in a primary antibody junction [3] suggests that CGRP is a motoneuronal neuro- cocktail consisting of anti-gephyrin (mouse; 1:400; Cedar-signalling molecule in the periphery. Centrally, there is lane Labs) primary antibody combined with either anti-SP electron microscopic evidence to suggest that CGRP may (rabbit; 1:1000; Chemicon), anti-CGRP (rabbit; 1:10 000; be present in recurrent axon collateral terminals presynap- Peninsula) or anti-nAChR (rat; 1:10; Developmental tic to putative Renshaw cells [10]. Studies Hybridoma Bank; see Refs. [20,21] for complete Here, we examined the abundance and localization of characterization) primary antibody diluted in 0.1 M phos-peptidergic input to, and the organization of nicotinic phate buffer with 0.9% saline and 0.3% Triton X-100 acetylcholine receptors (nAChR) upon, immunohistoch- (PBS–T). Following the primary incubation, sections were emically-identified Renshaw cells [1,2,8,9]. A portion of washed twice in PBS–T and incubated 1.5 h in FITC-these results have been reported in abstract form [7]. conjugated donkey anti-mouse IgG (for gephyrin visualiza-tion) and CY3-conjugated donkey anti-rat (for nAChR visualization) or CY3-conjugated donkey anti-rabbit (for
*Corresponding author. Tel.: 11-701-777-2576; fax: 1
1-701-777-SP and CGRP visualization). All secondary incubations
2477.
E-mail address: [email protected] (P.A. Carr). were conducted at 1:100 dilution in PBS–T using Jackson
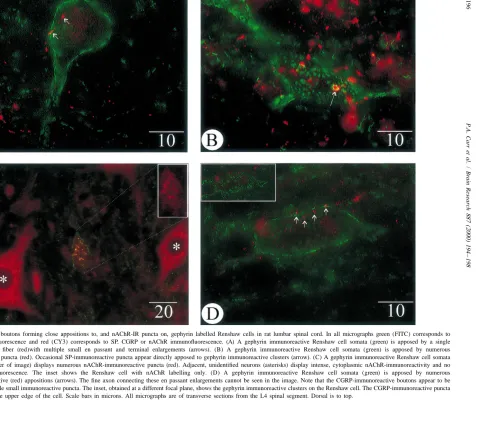
staining pattern of gephyrin [1,2,8,9]. Qualitative and (,0.5mm) puncta.
quantitative analyses of the presence and distribution of As motoneuronal recurrent axon collateral input is a SP-immunoreactive (-IR) or CGRP-IR close contacts, and defining characteristic of Renshaw cells [13], numerous nAChR-IR puncta, on immunohistochemically identified post- and pre-synaptic aspects of this cholinergic signalling Renshaw cells were conducted on neuronal profiles iden- mechanism have been investigated using electrophysiologi-tified in single spinal cord sections using standard fluores- cal, pharmacological and neuroanatomical methodologies. cence microscopy (Olympus BX–60) and video images Evidence suggests that, in addition to motoneuron-captured at 8 bits per pixel (framegrabber;) with a Dage- Renshaw signalling via classic acetylcholine / cholinergic MTI CCD-300-RC video camera or laser scanning con- receptor interactions, neuropeptides could play important focal microscopy (Olympus Fluoview). In order to resolve modulatory roles in this motor pathway. The presence of discrete puncta, all quantification of immunofluorescent CGRP in motoneurons has been well described and it has puncta or close appositions on Renshaw cell profiles were been demonstrated to have both transmitter-like and conducted using a 1003 oil immersion UPlanFl iris- trophic effects at the motor end plate axon terminal [3]. equipped objective. Figures were generated using Image Despite abundant evidence for the role of motoneuron-Pro Plus (Media Cybernetics) and CorelDRAW (Corel). derived CGRP in the periphery, data supporting the Statistical analysis of the data was conducted using presence of CGRP in, or its release from, recurrent axon SigmaStat (Jandel). collateral terminals in the ventral horn of the spinal cord is In single, transverse sections of rat lumbar spinal cord, sparse. A brief report has described moderate CGRP Renshaw cell profiles were identified by characteristic immunoreactivity in axon collaterals impinging upon large, abundant and intense gephyrin-IR clusters on their putatively identified Renshaw cells [10]. These ‘Renshaw perikarya and proximal dendrites. All putative Renshaw elements’ were also found to be depleted of immuno-cell profiles were similar in appearance, abundance and reactive CGRP following supramaximal antidromic stimu-location to that previously described [1,2,8,9]. The somata lation of sciatic nerve. Here, we provide quantitative and proximal dendrites of 481 Renshaw cells were ex- evidence regarding the abundance and distribution of amined to determine the distribution and abundance of CGRP-IR varicosities closely apposed to Renshaw cells. either close contacts arising from immunoreactive pep- Although these close contacts are consistent with both the tidergic terminal enlargements or anti-nicotinic receptor- presence of this peptide ina-motoneurons and the known immunofluorescent puncta. motoneuron–Renshaw cell circuitry, it has been shown In gephyrin and substance P immunoreacted tissue, 730 that the majority of cholinergic terminals, as labelled by SP-IR axonal varicosities were localized in close apposi- vesicular acetylcholine transporter (VAChT), are apposed tion to 184 Renshaw cell somata or proximal dendrites to Renshaw cell dendrites [2]. As 89% of the CGRP-IR (Fig. 1A, 1B; Table 1); 69% of the boutons made close appositions detailed here were localized on Renshaw cell contacts with Renshaw cell somata while the remainder somata, this suggests that CGRP- and VAChT-IR terminals were present adjacent to proximal processes. SP-IR close may represent different axonal systems. Although a direct contacts ranged in size from 0.5 to 3 mm in diameter. primary afferent input to Renshaw cells has not been Occasional SP-IR boutons were observed immediately described [5], non-motoneuronal sources for the CGRP-IR apposed to large gephyrin clusters (Fig. 1B). In tissue terminals, such as descending pathways [14], must be simultaneously reacted with gephyrin and nicotinic acetyl- acknowledged.
P
.A
.
Carr
et
al
.
/
Brain
Research
887
(2000
)
194
–
198
Mean number of close contacts / 0.960.08 1.260.15 0.760.08 puncta on Renshaw cell proximal
processes References
[1] F.J. Alvarez, D.E. Dewey, D.A. Harrington, R.E.W. Fyffe, Cell type-specific organization of glycine receptor clusters in the mam-malian spinal cord, J. Comp. Neurol. 379 (1997) 150–170.
substance P on Renshaw cells [18]. Both nicotinic and [2] F.J. Alvarez, D.E. Dewey, P. McMillin, R.E.W. Fyffe, Distribution of muscarinic acetylcholine receptors have been shown to cholinergic contacts on Renshaw cells in the rat spinal cord: a light
microscopic study, J. Physiol. 515.3 (1999) 787–797.
have powerful excitatory effects on Renshaw cells
[3] U. Arvidsson, F. Piehl, H. Johnson, B. Ulfake, S. Cullheim, T.
[11,12,15,19]. Our demonstration here of
nAChR-immuno-¨
Hokfelt, The peptidergic motoneurone, NeuroReport 4 (1993) 849–
reactivity associated with Renshaw cells lends anatomical
856.
support for the presence of nicotinic receptors on these [4] U. Arvidsson, S. Cullheim, B. Ulfake, G.W. Bennett, K.C.F. Fone, cells and suggests that they have an somatic localization. A.C. Cuello, A.A.J. Verhofstad, T.J. Visser, T. Hokfelt, 5-hydroxy-¨
The apparent incongruity between the dendritic distribution tryptamine, substance P, and thyrotropin-releasing hormone in the adult cat spinal cord segment L7: immunohistochemical and
chemi-of VAChT labelled cholinergic terminals and the somatic
cal studies, Synapse 6 (1990) 237–270.
distribution of nAChR-IR puncta may be a function of a
[5] F. Baldissera, H. Hultborn, M. Illert, Integration in spinal neuronal
differential distribution between muscarinic and nicotinic systems, in: V.B. Brooks (Ed.), Handbook of Physiology, Sec. I, Part acetylcholine receptors or a selective difference in anti- I, American Physiological Society, Vol. II, Bethesda, 1981, pp.
body recognition due to receptor subunit composition of 509–595.
[6] G. Belcher, R.W. Ryall, Substance P and Renshaw cells: a new
the nicotinic receptors.
concept of inhibitory synaptic interactions, J. Physiol. 272 (1977)
Renshaw cells appear to receive a higher density of
105–119.
substance P-IR close contacts (3.9 close contacts / RC) as [7] P.A. Carr, S.J. Shefchyk, M.J. Roller, The distribution of substance compared to 5-HT-IR boutons (2.8 close contacts / RC) [9]. P-immunoreactive boutons on immunohistochemically-identified In the ventral spinal cord it has been demonstrated that not Renshaw cells in cat and rat lumbar spinal cord, Soc. Neurosci.
Abstr. 25 (1999) 656.
all substance P immunoreactive fibers contain
5-HT-im-[8] P.A. Carr, F.J. Alvarez, E.A. Leman, R.E.W. Fyffe, Calbindin D28 k
munoreactivity and vice versa [4,22]. This agrees with our
expression in immunohistochemically-identified Renshaw cells,
observations that not all substance P immunoreactive fibers NeuroReport 9 (1998) 2657–2661.
in the immediate vicinity of Renshaw cells were double [9] P.A. Carr, J.C. Pearson, R.E.W. Fyffe, Distribution of 5-hydroxy-labelled for 5-HT transporter (unpublished observations). tryptamine-immunoreactive boutons on immunohistochemically-identified Renshaw cells in cat and rat lumbar spinal cord, Brain
This, in combination with the quantitative difference
Res. 823 (1999) 198–201.
between the number of substance P and 5-HT close
´
[10] B. Csillick, E. Knyihar-Csillik, G.W. Kreutzberg, L. Tajti, A.
contacts on Renshaw cells, suggests that not all substance
´
Kereszturi, T. Kovacs, Calcitonin gene-related peptide is released
P-IR input to Renshaw cells is derived from descending from cholinergic synapses, Ann. NY Acad. Sci. 657 (1992) 466– serotonergic fibers. Efforts at labelling substance P re- 468.
[11] D.R. Curtis, R.W. Ryall, The excitation of Renshaw cells by
ceptors (NK-1) on Renshaw cells did not reveal labelled
cholinomimetics, Exp. Brain Res. 2 (1966) 49–65.
puncta or cytoplasmic / membrane labelling as observed in
[12] D.R. Curtis, R.W. Ryall, The acetylcholine receptors of Renshaw
the dorsal horn (not shown). The complementary
dis-cells, Exp. Brain Res. 2 (1966) 66–80.
tribution between substance P and VAChT-IR close con- [13] J.C. Eccles, P. Fatt, K. Koketsu, Cholinergic and inhibitory synapses tacts on Renshaw cells may indicate that the pharmaco- in a pathway from motor-axon collaterals to motoneurones, J.
Physiol. 126 (1954) 524–562.
logically-mediated substance P/ cholinergic interactions
˚
¨ ´
[14] T. Hokfelt, U. Arvidsson, S. Ceccatelli, R. Cortes, S. Cullheim, A.
occur via a presynaptic or paracrine influence. However,
Dagerland, H. Johnson, C. Orazzo, F. Piehl, V. Pieribone, M.
the correspondence between the somatic distribution of
Schalling, L. Terenius, B. Ulfhake, V.M. Verge, M. Villar, Z.
both substance P- and nAChR-IR suggests that peptidergic Wiesenfeld-Hallin, X.-J. Xu, Z. Xu, Calcitonin gene-related peptide modulation of cholinergic neurotransmission could occur in the brain, spinal cord, and some peripheral systems, Ann. N.Y.
[15] K.T. King, R.W. Ryall, A re-evaluation of acetylcholine receptors on [20] L.W. Swanson, D.M. Simmons, P.J. Whiting, J. Lindstrom, Immuno-feline Renshaw cells, Br. J. Pharm. 73 (1981) 455–460. histochemical localization of neuronal nicotinic receptors in the [16] K. Krnjevic, D. Lekic, Substance P selectively blocks excitation of rodent central nervous system, J. Neurosci. 7 (1987) 3334–3342.
Renshaw cell by acetylcholine, Can. J. Physiol. Pharmacol. 55 [21] P.J. Whiting, J. Lindstrom, Purification and characterization of a (1977) 958–961. nicotinic acetylcholine receptor from rat brain, Proc. Natl. Acad. Sci. [17] R.W. Ryall, Modulation of cholinergic transmission by substance P, USA 84 (1987) 595–599.
Ciba Found. Symp. 91 (1982) 267–280. [22] W. Wu, M.W. Wessendorf, I. Organization of the serotonergic [18] R.W. Ryall, G. Belcher, Substance P selectively blocks nicotinic innervation of spinal neurons in rats, Neuropeptide coexistence in receptors on Renshaw cells: a possible synaptic inhibitory mecha- varicosities innervating some spinothalamic tract neurons but not in nism, Brain Res. 137 (1977) 376–380. those innervating postsynaptic dorsal column neurons, Neuroscience [19] R.W. Ryall, H.L. Haas, On the physiological significance of mus- 50 (1992) 885–898.