Brain Research 887 (2000) 469–471
www.elsevier.com / locate / bres
Short communication
Coordinate enrichment of cranin (dystroglycan) subunits in synaptic
membranes of sheep brain
a b ,
*
Neil R. Smalheiser , Barbara J. Collins
a
Department of Psychiatry, University of Illinois at Chicago, MC 912, Chicago, IL 60612, USA
b
Department of Medicine, University of Illinois at Chicago, MC 640, Chicago, IL 60612, USA
Accepted 19 September 2000
Abstract
Cranin (dystroglycan), a mucin-like extracellular matrix receptor comprised of two subunits (a and b), is involved in regulating cell–matrix interactions in a variety of tissues, including brain. A basic issue remains unresolved concerning the distribution of cranin in brain: are the a and bsubunits coordinately expressed at the synapse? We report here that cranin is indeed enriched progressively in synaptosomes and synaptic membranes of sheep brain, as assessed by immunoblotting and laminin-blotting assays, and that the extent of enrichment is similar for bothaandbsubunits. These findings support the hypothesis that cranin (dystroglycan) contributes to synaptic function in the CNS. 2000 Elsevier Science B.V. All rights reserved.
Theme: Cellular and molecular biology
Topic: Membrane composition and cell-surface macromolecules
Keywords: Cranin; Dystroglycan; Laminin; Synaptic protein; Synaptosome; Subcellular fractionation
Cranin (dystroglycan) is a mucin-like extracellular ma- cerning the distribution of cranin (dystroglycan) in brain: trix receptor consisting of two subunits (a and b) and is Are theaandbsubunits co-localized at synapses? Several involved in regulating cell–matrix and cell–cell interac- studies have reported thata-dystroglycan can be expressed tions in many tissues (see [6] for a review). Immuno- as a free, soluble protein in brain [5] and Schwannoma cell cytochemistry of brain indicates that cranin is distributed conditioned medium [8]; thus, it remains possible that relatively diffusely throughout the parenchyma, as well as a-dystroglycan is selectively expressed at central synapses at basal laminae, glial end-feet and along dendritic mem- in the absence of an accompanying b-subunit. Although branes [9,14,15]. An immunoelectron microscopic study previous studies have indicated that bothaandbsubunits has demonstrated that cranin is expressed postsynaptically are detectable in synaptosomes and purified synaptic surrounding the active site of at least some central plasma membranes [2,10] these studies have not quantified synapses [15], and in the retina, cranin (dystroglycan) is the extent of enrichment relative to crude membranes nor present on the presynaptic side of synaptic contacts examined whether the a and b subunits are coordinately surrounding the active site at ribbon synapses [7,12]. A expressed. Therefore, we decided to take a closer look at loss of a-dystroglycan has been reported in the brains of this issue using sheep brain, whose expression of cranin patients with Duchenne muscular dystrophy, which may has been well characterized biochemically [11,14]. contribute to cognitive deficiency associated with this Subcellular fractionation
disorder [4]. These findings suggest a role for cranin Synaptosomes and crude synaptosomal membranes were (dystroglycan) in synaptic adhesion in the CNS. prepared from 80 g of sheep brains by a modification of Nevertheless, a basic issue remains unresolved con- the method of Cohen et al. [3]. Briefly, brains were homogenized in 0.32 M sucrose in 1 mM NaHCO buffer3 containing 1 mM MgCl2 and 0.5 mM CaCl . After2
*Corresponding author. Tel.: 11-312-413-1245; fax: 1
1-312-413-discarding material that pelleted at low speed (14753g), a
0437.
E-mail address: [email protected] (B.J. Collins). crude membrane pellet ‘P2’ was obtained by centrifugation
470 N.R. Smalheiser, B.J. Collins / Brain Research 887 (2000) 469 –471
at 17,3003g for 10 min. The P2 pellet was resuspended
and layered on a discontinuous gradient of 0.32, 0.85, 1.0, and 1.2 M sucrose in 1 mM NaHCO , and centrifuged at3 100,0003g for 2 h. The synaptosomal fraction (‘SYN’)
was recovered as a band at the 1.0–1.2 M interface, resuspended in 0.32 M Sucrose in bicarbonate buffer, and pelleted at 43,0003g. The pellet was subjected to
hypo-tonic lysis by stirring for 45 min in 6 mM Tris–HCl, pH 8.1, recentrifuged and the pellet resuspended in PBS alone or solubilized in PBS containing 1% Triton X-100 (‘SPM’, synaptosomal plasma membrane fraction). Based on Lowry protein assay, the total amounts of protein present in the various fractions were P2: 1.7 g; the synaptosome fraction: 0.45 g (28% of P2); and the SFM fraction: 0.24 g (14% of P2, or 54% of the synaptosome fraction). Equal amounts of
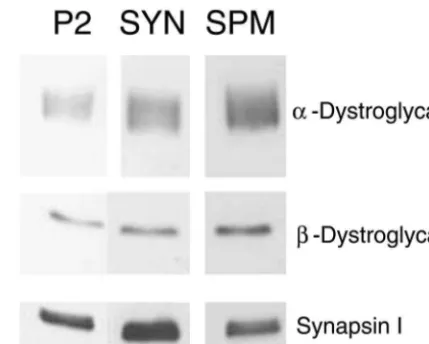
Fig. 1. Representative blot images from the crude membrane ‘P2’ protein in each fraction were precipitated with methanol,
fraction, synaptosomes (SYN), and synaptosomal membranes (SPM), dissolved in SDS–PAGE buffer under reducing conditions,
respectively, for immunoblots with various antibodies as shown. Equal separated on 6.5% or 7.5% PAGE gels with a 2.5%
amounts of protein were loaded in each lane. stacking gel, and transferred to PVDF membranes.
Laminin-binding assay was carried out as described [13].
Immunoblotting employed a mouse monoclonal antibody Mummery et al. [10], we did not detect any high molecular againsta-dystroglycan (Clone VIA-4, Upstate Biotechnol- weight bands cross-reactive witha-dystroglycan antibody, ogy, Inc.) at 1:500, and a mouse monoclonal antibody even in the SPM fraction (data not shown). As shown in against b-dystroglycan (raised against a 15-mer synthetic Fig. 1 and Table 1, a-dystroglycan and b-dystroglycan peptide corresponding to the extreme C-terminus; Clone were progressively enriched in the synaptosomal and SPM NCL-43, Novocastra Laboratories) was used at 1:200. fractions. The extent of enrichment across fractions, as Blots were blocked in 1% nonfat dry milk for 1 h, then monitored by immunoblot assay, was very similar for the incubated with primary antibody for 2 h and rinsed; anti- a- and b-subunits; furthermore, the enrichment of a-mouse IgG (peroxidase conjugated) was added at 1:4000 dystroglycan was similar whether monitored by immuno-for 2 h and rinsed; finally, blots were incubated in ECL blotting or by laminin-binding assay (Table 1). By multip-reagent (Amersham) and exposed to X-ray film (Hyperfilm lying the SPM:synaptosome enrichment by the ECL, Amersham). A rabbit polyclonal antibody against synaptosome:P2 enrichment, we estimated that the con-synapsin I (Calbiochem) was used at 1:14,000 followed by centration of b-dystroglycan in SPMs was 3.15-fold en-anti-rabbit IgG (peroxidase conjugated) at 1:10,000. riched relative to the P2 fraction. The concentration of
Immunoblot analysis a-dystroglycan in SPMs was also 3.15-fold enriched
Each subcellular fraction was examined at three differ- relative to the P2 fraction, as measured by immunoblotting ent loading volumes, and the optical density for each band (enrichment was slightly higher, 3.58-fold, as assessed by was measured using an image analyzer (Inquiry software, laminin-binding assay). In contrast, synapsin I, a marker of Loats, Inc.). All comparisons were run in parallel on the synaptic vesicles, was enriched in synaptosomes relative to same SDS–PAGE gels, and carried out pairwise (i.e., the P2 fraction, but was not enriched in the SPM fraction synaptosomes vs. P2 fraction and SPMs vs. synaptosomes). (Fig. 1); these results serve as a methodological control Contrast and brightness settings were adjusted to avoid
saturation of the more intense bands, and were kept Table 1
a
Relative enrichment ofa- andb-dystroglycan in synaptic membranes constant across the series of blots. For each band, the mean
intensity and area were measured, and the background was Comparison Percent enrichment (%)
subtracted by measuring an adjacent region within the Immunoblot Laminin-binding lane. Curves of band optical density vs. loading volume
b-subunit a-subunit a-subunit were plotted for each subcellular fraction; the extent to
Synaptosomes which these curves were shifted relative to each other,
vs. P2 fraction 14463 13367 14965 measured at three points along the curves, indicated how
SPMs
much they differed in their relative concentration of aand vs. synaptosomes 219630 23769 240631
bsubunits. a
Synaptic fractions were compared and the relative concentration of As reported previously, cranin (dystroglycan) isolated
N.R. Smalheiser, B.J. Collins / Brain Research 887 (2000) 469 –471 471
braina-dystroglycan in Duchenne muscular dystrophy but not in the and accord with expectation, since hypotonic lysis should
mdx animal model, Biochem. Biophys. Res. Commun. 249 (1998)
release synaptic vesicles from synaptosomes leaving SPMs
231–235.
relatively devoid of synapsin I. [5] S.H. Gee, R.W. Blacher, P.J. Douville, P.R. Provost, P.D. Yurchenco, We conclude that the a and b subunits of cranin S. Carbonetto, Laminin-binding protein 120 from brain is closely (dystroglycan) are significantly and coordinately enriched related to the dystrophin-associated glycoprotein, dystroglycan, and binds with high affinity to the major heparin binding domain of in synaptic membranes. These findings provide
experimen-laminin, J. Biol. Chem. 268 (1993) 14972–14980. tal support for the notion that the subunits of cranin
[6] M.D. Henry, K.P. Campbell, Dystroglycan: an extracellular matrix (dystroglycan) act as a single functional unit at central receptor linked to the cytoskeleton, Curr. Opin. Cell Biol. 8 (1996) synapses, and that its function there is related to modu- 625–631.
¨
lating cell–matrix or cell–cell interactions [1]. Cranin [7] P. Koulen, M. Blank, S. Kroger, Differential distribution of beta-dystroglycan in rabbit and rat retina, J. Neurosci. Res. 51 (1998) (dystroglycan) may contribute to the structure or
cyto-735–747. skeletal organization of synapses; as well, it may support
[8] K. Matsumura, A. Chiba, H. Yamada, H. Fukuta-Ohi, S. Fujita, T. synaptic signalling via its association with signal transduc- Endo, A. Kobata, L.V.B. Anderson, I. Kanazawa, K.P. Campbell, T. tion adaptor proteins and kinases [2]. Shimizu, A role of dystroglycan in schwannoma cell adhesion to
laminin, J. Biol. Chem. 272 (1997) 13904–13910.
[9] F. Montanaro, S. Carbonetto, K.P. Campbell, M. Lindenbaum, Dystroglycan expression in the wild type and mdx mouse neural
Acknowledgements
retina: synaptic colocalization with dystrophin, dystrophin-related protein but not laminin, J. Neurosci. Res. 42 (1995) 528–538. We thank Dr Rochelle Cohen (University of Illinois at [10] R. Mummery, A. Sessay, F.A. Lai, P.W. Beesley,b-dystroglycan: Chicago) for guidance in carrying out subcellular frac- subcellular localization in rat brain and detection of a novel immunologically related, postsynaptic density-enriched protein, J. tionation.
Neurochem. 66 (1996) 2455–2459.
[11] H.P. Peng, A.A. Ali, D.F. Daggett, H. Rauvala, J.R. Hassell, N.R. Smalheiser, The relationship between perlecan and dystroglycan and
References its implication in the formation of the neuromuscular junction, Cell Adhesion Commun. 5 (1998) 475–489.
[12] F. Schmitz, D. Drenckhahn, Localization of dystrophin and beta-[1] A.M. Belkin, N.R. Smalheiser, Localization of cranin (dystroglycan)
dystroglycan in bovine photoreceptor processes extending into the at sites of cell–matrix and cell–cell contact: recruitment to focal
postsynaptic dendritic complex, Histochem. Cell Biol. 108 (1997) adhesions is dependent upon extracellular ligands, Cell Adhesion
249–255. Commun. 4 (1996) 281–296.
[13] N.R. Smalheiser, N.B. Schwartz, Cranin: a laminin-binding protein [2] M. Cavaldesi, G. Macchia, S. Barca, P. Defilippi, G. Tarone, T.C.
of cell membranes, Proc. Natl. Acad. Sci. USA 84 (1987) 6457– Petrucci, Association of the dystroglycan complex isolated from
6461. bovine brain synaptosomes with proteins involved in signal
trans-[14] N.R. Smalheiser, E. Kim, Purification of cranin, a laminin binding duction, J. Neurochem. 72 (1999) 1648–1655.
membrane protein, J. Biol. Chem. 270 (1995) 15425–15433. [3] R.S. Cohen, F. Blomberg, K. Berzins, P. Siekevitz, The structure of
[15] M. Tian, C. Jacobson, S.H. Gee, K.P. Campbell, S. Carbonetto, M. postsynaptic densities isolated from dog cerebral cortex. I. Overall
Jucker, Dystroglycan in the cerebellum is a laminin alpha 2-chain morphology and protein composition, J. Cell Biol. 74 (1977) 181–
binding protein at the glial–vascular interface and is expressed in 203.