Short communication
Accumulation of heavy metals in food plants and grasshoppers
from the Taigetos Mountains, Greece
B. Devkota
a, G.H. Schmidt
b,∗aDepartment of Ecology, Faculty-5, University of Osnabrueck, Barbarastr. 11, D-49076 Osnabrueck, Germany bDepartment of Zoology–Entomology, University of Hanover, Herrenhaeuserstr. 2, D-30419 Hanover, Germany
Received 11 November 1998; received in revised form 18 June 1999; accepted 9 September 1999
Abstract
Geogenic, as well as anthropogenic heavy metals from distant sources, gradually increase the level of toxic metals in natural environments and these will be increasingly taken up by the plants and transferred further up the food chain. The level of different heavy metals (Hg, Cd, Pb) was studied in the producers (food plants) and consumers [four species of acridid grasshoppers: Calliptamus italicus (L.), Oedipoda caerulesens (L.), O. germanica (Latr.) and Chorthippus(Glyptobothrus) crassiceps(Ramme, 1926)] of a grassland located 1200 m above the sea level in the Taigetos Mountains, Peloponnesus, Greece. The concentrations of heavy metals in the food plants and grasshoppers were in the order Pb > Cd > Hg and the mean concentration of Pb was about 55 and 20 times the concentrations of Hg and Cd, respectively. The solely herbivorous C.(G.) crassiceps had a significantly higher Hg-concentration than in the food plants, but it did not exceed that of Cd and Pb. Cd-concentration in the grasshoppers was significantly higher than in food plants, and female grasshoppers had higher Cd accumulation than males. Lead accumulation in grasshoppers was always lower than in their food plants. The accumulation factors of these elements in the grasshoppers were found in the order Cd > Hg > Pb, thus showing greater affinity to Cd accumulation. Significantly higher concentration of Hg in both sexes of C.(G.) crassiceps than in other three grasshoppers proved this species to be a comparatively better bioindicator of Hg pollution. Elevated concentrations of Cd in both, females and males of all four grasshopper species suggested that any grasshopper, irrespective of the sex, could equally play the role of bioindicator. Studies on the bioaccumulation and biotransfer of different heavy metals showed that the organisms of such distantly located ecosystems were also exposed to measurable amounts of toxic heavy metals. ©2000 Elsevier Science B.V. All rights reserved.
Keywords: Heavy metals; Hg; Cd; Pb; Accumulation; Bioaccumulation factors; Grasshoppers
1. Introduction
The occurrence of toxic heavy metals in the soil is of geogenic or anthropogenic origins. The natural content
∗Corresponding author. Tel.:+49-511-7625548; fax:+49-511-7625381.
of heavy metals in the soil is dependent on geochemi-cal and geophysigeochemi-cal processes. Heavy metals from the point and other sources of emission can be transported to distant environments (Steinnes, 1980). Transport of heavy metals from the atmosphere to the soil and veg-etation takes place by dust fall, bulk precipitation and gas or aerosol adsorption processes (Andersen et al.,
1978). The bulk of the elements is naturally bound as insoluble compounds in rock and sediments, and a multitude of ions can be released from sediments by redox changes (Lieth and Markert, 1990). The in-put of anthropogenic toxic metals in distantly located mountain ecosystems is generally lower than in val-leys and settlement areas, but due to different natural (geophysical and geochemical) processes, the amount of pedogenic and lithogenic metals is also gradually increasing and is capable of interacting with biota. Some elements, such as mercury (Hg), zinc (Zn), cad-mium (Cd), lead (Pb), and arsenic (As), may be in-creasingly taken up by the crop and transferred further to the food chain (Beijer and Jernelöv, 1986).
About 20–30% of the total arthropod biomass dur-ing summer in a grassland ecosystem is due to acri-did grasshoppers (Schmidt, 1986). Being herbivorous (primary consumer) and being preyed upon by other insectivorous vertebrates and arthropods, they play a significant role in accumulating and further transfer-ring toxic metals to higher trophic levels. The studies on the transfer of heavy metals in an aquatic ecosys-tem by Jamil and Hussain (1992), and in a copper (Cu) and Cd contaminated grassland by Hunter et al. (1987) showed that the accumulation and biotransfer of anthropogenic heavy metals can be very high.
Organisms, like grasshoppers, occurring in such en-vironments are also exposed to gradually increasing toxic metals and contribute to the accumulation and biotransfer of heavy metals. Hunter et al. (1987) stud-ied the influence of toxic heavy metals on the acri-dids Chorthippus(Glyptobothrus) brunneus (Thunbg.) from a Cd and Cu contaminated grassland. Higher ac-cumulation of Hg in males than in females of
Eypre-pocnemis plorans (Charp.), but more Hg in females
than in males of Aiolopus thalassinus (Fabr.), and no clear difference in Cd and Pb concentrations be-tween the two sexes of A. thalassinus exposed to con-taminated food under laboratory conditions showed that different grasshoppers accumulate different heavy metals in various concentrations and the accumula-tion of toxic metals might be sex dependent (De-vkota, 1992). This study was undertaken to evaluate the dynamics of toxic heavy metals in a relatively less polluted grassland ecosystem and to determine whether some species are more suitable bioindicators and whether there is any sex-specific metal accumu-lation in the grasshoppers of such grasslands.
2. Materials and methods
Using insect nets, males and females, ten each, of four species of acridid grasshoppers Calliptamus
italicus (L.), Oedipoda caerulcesens (L.), O. german-ica (Latr.) and Chorthippus (Glyptobothrus) crassi-ceps (Ramme, 1926) were collected from a grassland
(1200 m above the sea level) in the Taigetos Moun-tains, Peloponnesus, Greece, during the summer of 1991. A mixture of food plants (grass) from the same grassland was also collected. During transportation to the Department of Zoology–Entomology, Univer-sity of Hanover (Germany), the grasshoppers were fed the same grass. The grass samples were washed with deionised water to remove the heavy metals attached on the surface and were freeze-dried. Five samples each of both, males and females, and two grasshop-pers in each sample, of all four grasshopper species were ground in a vibrating mill (Retsch, Germany) to a homogenous powder. For the food plants, only one sample was ground.
2.1. Determination of elements
Cadmium (Cd) and lead (Pb) were determined with a Zeemann AAS SM30 (Gruen Optiker, Wetzlar, Ger-many) equipped with graphite furnace and suitable for element determinations in solid samples (Steubing et al., 1980; Grobecker and Kurfürst, 1990; Devkota and Schmidt, 1992). About 40–60mg of a homogenous sample weighed in a graphite boat was introduced into graphite tube furnace using special tweezers. After stepwise heating and ashing, the samples were atom-ised at 2000◦C (for Cd) and 2300◦C (for Pb) in an ar-gon atmosphere. Atomic absorption was measured at wavelengths of 228.3 nm during Cd and 283.3 nm dur-ing Pb determination usdur-ing respective hollow cathode lamps. BCR reference materials CRM 060 (Aquatic plant) and CRM 061(Aquatic plant) for plant sam-ples and CRM 185 (Bovine liver) and CRM 186 (Pig kidney) for insect samples were used for calibration. Along with the samples, the solid reference materials were also determined for Cd and Pb concentrations. The obtained data were within the range of certified values.
1985) using a Perkin–Elmer AAS 1100 equipped with a mercury hydride system (MHS 10). About 200 mg of finely ground sample was wet digested in 3 ml aqua regia (conc. H2SO4 and conc. HNO3 in 2 : 1 V/V of spectroscopic grade (Riedel-de Haen) at 140–150◦C for 4 h; one digestion per sample of grasshoppers and three digestions of the grass sample were carried in parallel. Sample aliquots were diluted to 20 ml with deionised water. From diluted sample solution, 10 ml was pipetted for each determination and reduced by 3% NaHBO4 and 1% NaOH in the presence of KMnO4. The atomic absorption of the elemental mercury was measured at 253.6 nm using a hollow cathode mercury lamp. Nitrogen (extra pure) was used as carrier gas and Fixanal mercury standard solution (manufacturer: Riedel-de Haen, Germany) was used for calibration. The Hg concentration in the blank sample was also determined and this value was subtracted from sample values to avoid any unwanted contamination during sample preparation.
2.2. Analysis and presentation of data
Mean and standard deviation of five determinations (one measurement per sample of five separate samples
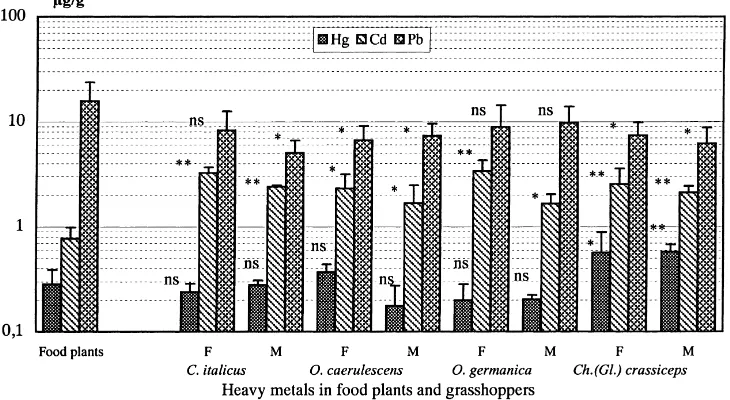
Fig. 1. Concentrations (mean±SD inmg/g dry weight) of heavy metals (Hg, Cd, Pb) in food plants and in females (F) and males (M) of four species of grasshoppers from the Taigetos Mountains. Each bar represents the mean of five measurements and the differences in the mean concentrations between food plants and grasshoppers are indicated by ***p<0.001, **p<0.01, *p<0.05 and ns — not significant differences.
in case of grasshoppers, but five measurements of one grass sample) of each heavy metal was calculated and the data are given inmg g−1dry weight of the sample. Differences between the mean concentrations in food plants and in grasshoppers were calculated using Stu-dent’s t-test (Wardlaw, 1985). The data were subjected to analysis of variance to calculate the F-ratio of in-tergroups variance (variations in metal concentrations between species means) to intragroups variance (varia-tions of individual concentra(varia-tions within each species) of heavy metal concentrations in grasshoppers.
3. Results
As shown in Fig. 1, heavy metals (Hg, Cd, Pb) were found in remarkably high concentrations in the grass samples from the Taigetos Mountains, where the concentrations of these heavy metals were in the order Pb > Cd > Hg. The mean concentration of Pb was about 55 and 20 times the concentrations of Hg and Cd, respectively.
Table 1
Accumulation factors (concentration in grasshoppers/concentration in food plants) of three heavy metals (Hg, Cd, Pb) in females (F) and males (M) of four grasshopper species, where the factor >1 means higher concentration in grasshoppers than in food plants
Metal C. italicus O. caerulescens O. germanica C.(G.) crassiceps Remarks
F M F M F M F M concentration in acridids
Hg 0.84 <0.99 1.30 >0.62 0.70 ∼=0.71 2.00 ∼=2.04 either∼=or > or > in food plants Cd 4.19 >3.09 2.97 >2.16 4.35 >2.15 3.27 >2.72 always > in food plants Pb 0.53 >0.32 0.42 <0.47 0.57 <0.62 0.47 >0.39 always<in food plants
concentration of Hg in these grasshoppers showed no sex-specific difference in bioaccumulation. The solely herbivorous C.(G.) crassiceps had Hg-concentration significantly higher than in food plants and both sexes of this grasshopper had the same level of accumula-tion (accumulaaccumula-tion factor 2.00 in female and 2.04 in male) (Table 1).
All four species of grasshoppers had signifi-cantly higher Cd concentration than the food plants. Cadmium accumulation in female grasshoppers was always higher (accumulation factors 2.97–4.35) than in males (accumulation factors 2.15–3.09) and it was remarkably high in O. germanica and C. italicus.
In the case of Pb, the accumulation in grasshoppers (herbivores) never exceeded that of food plants (pro-ducers). In all grasshoppers, the concentration of Pb was higher than Hg and Cd. Though O. germanica was found to accumulate the highest amount of Pb, the accumulation factor was still<1. Like Hg, accu-mulation behaviour towards Pb in these grasshoppers was also quite different, and neither the females nor the males were found to have higher concentrations of Pb than food plants. In C.(G.) crassiceps, the accu-mulation behaviour of Hg and Cd was different (sig-nificantly higher) than of Pb (sig(sig-nificantly low), but in
Table 2
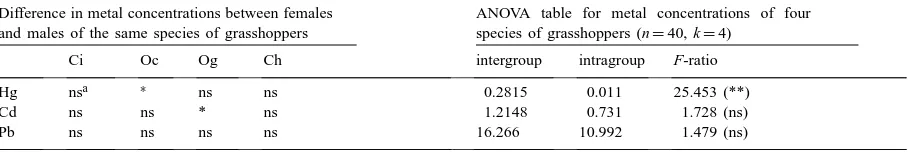
Differences in the mean concentrations of Hg, Cd and Pb in grasshoppers (Ci: C. italicus; Oc: O. caerulescens; Og: O. germanica; Ch: C.(G.) crassiceps) between females and males of the same species and F-ratio of intergroup (four different species) and intragroup (males and females of all four species of grasshoppers) variances in metal concentrations
Difference in metal concentrations between females and males of the same species of grasshoppers
ANOVA table for metal concentrations of four species of grasshoppers (n=40, k=4)
Ci Oc Og Ch intergroup intragroup F-ratio
Hg nsa ∗ ns ns 0.2815 0.011 25.453 (**)
Cd ns ns * ns 1.2148 0.731 1.728 (ns)
Pb ns ns ns ns 16.266 10.992 1.479 (ns)
∗Levels of significance are indicated as: **p<0.01, *p<0.05.
aNot significant differences.
other grasshoppers such differences between different heavy metals could not be observed within the same species. The accumulation factors of these elements in the grasshoppers were in the order Cd > Hg > Pb, with Cd bioaccumulation being significantly higher in all grasshoppers.
Mercury in females of O. caerulescens was found in significantly higher concentrations than in males, but in other grasshoppers there was no intraspecific difference between the females and the males (Table 2). Though both sexes of all grasshoppers accumu-lated significantly high amounts of Cd, the difference in Cd concentration between females and males was significantly high only in O. germanica, where fe-males had higher concentration than fe-males. Signifi-cantly lower concentrations of Pb in O. caerulescens and C.(G.) crassiceps than in food plants could not deliver any intraspecific difference in Pb accumu-lation between the females and the males of these species.
of Cd and Pb, such inter- and intragroup variations did not vary significantly and the variations between different species and within the species were same.
4. Discussion
The concentration of heavy metals in the food plants and grasshoppers from the Taigetos mountains was proportional in the order Pb > Cd > Hg, with the same pattern of occurrence, both in producers and in herbi-vores. The concentrations of Cd, Cu, Pb and Zn in the larvae of four ephemeroptera species from a polluted stream were also proportional to the concentrations in water and sediment in the order Cd<Pb<Cu<Zn (Jop, 1991). Plants have a key function in the bio-transformation of chemical elements from soil, wa-ter and air (Ernst, 1990), but green plants are un-able to take up much mercury from contaminated soil (Lodenius, 1990). Rauter (1976) found only 4.6mg Hg kg−1 in grasses of an industrially contaminated area and its concentration in grasses of distantly lo-cated grassland of the Taigetos Mountains was still lower (0.284mg g−1). In grasshoppers, the accumula-tion factor was in the order Cd > Hg > Pb, where Cd was in significantly higher concentrations than Hg and Pb. This might indicate that different metals have dif-ferent affinities leading to bioaccumulation in differ-ent organisms. Among these three elemdiffer-ents, the con-centration of Pb in grass was high (about 55 and 20 times higher than Hg and Cd, respectively), but it was comparatively low (10–49 times of Hg and only 2–6 times of Cd) in the grasshoppers.
The higher bioaccumulation of Cd could be respon-sible for its higher toxicity, whereas the poor accu-mulation of Pb in the organisms could be one cause to its less toxicity. The difference in the accumulation behaviour towards Cd and Pb may be due to the oppo-site solubility properties and to the chemical similari-ties between Cd and the essential Zn (Roth-Holzapfel, 1990). Cadmium can replace the Zn in the enzyme car-bonic anhydrase (Hopkin, 1989), changing the prop-erties and activity of this enzyme.
Jamil and Hussain (1992) studied the transfer of heavy metals through water — aquatic plants — aquatic insect system using the plant Eichhornia
crassipes Solms. and its specific feeder Neochetina eichhorniae. The study showed the biotransfer of
metals by a simple food chain model representing the transfer of metals from polluted waters to insects via aquatic plants. Clark (1992) measured the concentra-tions of organochlorine compounds and heavy metals in the 17-year cicada to determine the possible food chain hazards to birds. This homopteran contained metal concentrations similar to or less than other local invertebrates. Roth-Holzapfel (1990) could not find an enrichment of elements, except Cd and Ni, with increasing trophic level. Also during the present study, Cd was found to be enriched in the grasshop-pers, but the concentration of Hg in all four species of grasshoppers was lower than Cd. Mukherjee and Nuorteva (1994) likewise reported lower concentra-tions of Hg than Cd in bark beetles and ants from a forest surrounding the steel works in northern Fin-land. In the beetles and ants, the concentrations of these metals were near background levels. Copper and Zn, being essential for insect metabolism, are ac-cumulated and Cd, due its chemical property related to Zn, is also accumulated (Roth-Holzapfel, 1990).
Devkota and Schmidt (1992) after feeding Hg-, Cd-and Pb-contaminated food to adult Aiolopus
thalass-inus (Fabr.) found very high concentrations of Cd in
midgut, malpighian tubules, muscles, fat bodies and gonads of these grasshoppers, but concentration of Hg was high only in midgut and malpighian tubules (sites of absorption and excretion, respectively), and Pb was very low in these organs. With the growth of muscle tissues and fat bodies during post-embryonic develop-ment (nymph — adult) the concentration of Cd was found to be steadily increasing (Devkota, 1992).
found a higher accumulation of Cd in the European pine sawfly Neodiprion sertifer (Hymenoptera; Dipri-onidae) than in pine needles, whereas the levels of Cu, Ni and Fe were higher in pine needles than in the insects. Because N. sertifer might be one of the most abundant forest insects, this might be an impor-tant pathway for these metals to higher trophic levels. Likewise, grasshoppers constitute significantly large amounts of the arthropod biomass of the grassland (Schmidt, 1986) and. thus, the biotransfer of geogenic heavy metals, especially Cd, via the grasshoppers to higher trophic levels may be very important.
Insignificant differences in the metal concentrations between the females and the males of the same species of grasshoppers showed that both sexes could equally accumulate the heavy metals in their bodies and, for the purpose of biomonitoring, the preference to one sex of grasshopper might not be necessary. Signifi-cantly higher concentrations of Hg in both sexes of
C.(G.) crassiceps than in those of other three
grasshop-pers showed that this species might serve better as test animal for the evaluation of Hg pollution. Signif-icantly higher concentrations of Cd in both females and males of all four grasshopper species would sug-gest that any grasshopper irrespective of the sex could equally play the role of a bioindicator. Although the degrees of bioaccumulations and biotransfers were dif-ferent for difdif-ferent elements, the grasshoppers of this mountain grassland can be regarded as the bioindica-tors of heavy metal pollution.
References
Andersen, A., Hovmand, M.F., Johnsen, I., 1978. Atmospheric heavy metal deposition in the Copenhagen area. Environ. Pollut. 17 (2), 113–132.
Beijer, K., Jernelöv, A., 1986. General aspects of and specific data on ecological effects of metals. In: Friberg, L., Nordberg, G.F., Vouk, V. (Eds.), Handbook on the Toxicology of Metals, pp. 253–268.
Clark, D.R., 1992. Organochlorines and heavy metals in 17-year cicadas pose no apparent dietary threat to birds. Environ. Monitor. Assess. 20 (1), 47–54.
Devkota, B., 1992. Wirkung einer Dauerbelastung von Schwerme-tallen (Hg, Cd, Pb) auf Feldheuschrecken-Generationen (Insecta, Orthoptera, Acrididae). Dr. rer. nat. Thesis, FB Biologie, University of Hannover, Germany.
Devkota, B., Schmidt, G.H., 1992. Bioaccumulation of heavy metals (Hg, Cd, Pb) in different organs of the grasshopper, Aiolopus thalassinus (Fabr.)(Acrididae). In: Bohac,
J. (Ed.), Proc. VI International Conference — Bioindicatores Deteriorisationis Regionis, Ceske Budejovice, Czech Republic, pp. 368–376.
Ernst, W.H.O., 1990. Element allocation and (re)transportation in plants and its impact on representative sampling. In: Lieth, H., Markert, B. (Eds.), Element Concentration Cadasters in Ecosystems, VCH Verlagsgesellschaft, Weinheim, Germany, pp. 17–40.
Grobecker, K.-H., Kurfürst, U., 1990. Solid sampling by Zeeman Graphite-Furnace-AAS, a suitable tool for environmental analysis. In: Lieth, H., Markert, B. (Eds.), Element Concentration Cadasters in Ecosystems, VCH Verlagsgesellschaft, Weinheim, Germany, pp. 121–137. Heliovaara, K., 1990. Concentrations of heavy metals in the food,
faeces, adults, adults and empty cocoons of Neodiprion sertifer (Hymenoptera: Diprionidae). Bull. Environ. Contam. Toxicol. 45 (1), 13–18.
Hopkin, S.P., 1989. Ecophysiology of Metals in Terrestrial Invertebrates. Elsevier Applied Science, England.
Hunter, B.A., Hunter, L.M., Johnson, M.S., Thompson, D.J., 1987. Dynamics of metal accumulation in the grasshopper Chorthippus brunneus in contaminated grasslands. Arch. Environ. Contam. Toxicol. 16, 711–716.
Jamil, K., Hussain, S., 1992. Biotransfer of metals to the insect Neochetina eichhornae via aquatic plants. Arch. Environ. Contam. Toxicol. 22 (4), 459–463.
Jop, K.M., 1991. Concentration of metals in various larval stages of four Ephemeroptera species. Bull. Environ. Contam. Toxicol. 46 (6), 901–905.
Lieth, H., Markert, B., 1990. Element concentration cadasters in ecosystems. State of the art and plans for the further development of an international research program till 1990. In: Lieth, H., Markert, B. (Eds.), Element Concentration Cadasters in Ecosystems, VCH Verlagsgesellschaft, Weinheim, Germany, pp. 3–14.
Lodenius, M., 1990. Environmental mobilisation of mercury and cadmium. Publication of the Department of Environmental Conservation at the University of Helsinki, No. 13: ISBN 0782-2790.
Mukherjee, A.B., Nuorteva, P., 1994. Toxic metals in forest biota around the steel works of Rautaruukki Oy, Finland. Sci. Total Environ. 151 (3), 191–204.
Rauter, W., 1976. Aufnahme von Quecksilber aus der Umgebungsluft durch Pflanzen und seine Speicherung im pflanzlichen Gewebe. Z. Lebensm.Unters. Forsch. 162, 1–6. Roberts, R.D., Johnson, M.S., Firth, J.N.M., 1979. Predator prey
relationships in the food chain transfer of heavy metals. In: Hemphill, D.D. (Ed.), Trace Substances in Environmental Health — XIII University of Missouri, Columbia.
Roth-Holzapfel, M., 1990. Multi-element analysis of invertebrate animals in a forest ecosystem (Picea abies L.). In: Lieth, H., Markert, B. (Eds.), Element Concentration Cadasters in Ecosystems, VCH Verlagsgesellschaft, Weinheim, Germany, pp. 281–295.
Steinnes, E., 1980. Atmospheric deposition of heavy metals in Norway studied by the analysis of moss samples using neutron activation analysis and atomic absorption spectroscopy. J. Radio-anal. Chem. 58, 387–391.
Steubing, L., Grobecker, K.-H., Kurfürst, U., 1980. Zeeman-Atomabsorption zur Bestimmung von Schwermetallen in Pflanzen. Staub-Reinhalt. Luft. 40 (12), 537–540.
Wardlaw, A.C., 1985. Practical Statistics for Experimental Biologists. A Wiley–Interscience Publication, Chichester, Great Britain.