In vitro and post vitro inoculation of micropropagated
Rhododendrons with ericoid mycorrhizal fungi
Jan Jansa
1, Miroslav Vosátka
∗Institute of Botany, Academy of Sciences of the Czech Republic, CZ-252 43 Pruhonice, Czech Republic
Received 31 May 1999; received in revised form 25 November 1999; accepted 23 March 2000
Abstract
Isolation of more than 200 strains of endophytic fungi from the roots of several host plants belonging to orderEricales
(Vaccinium,Calluna,Rhododendron,Empetrum, etc.) was followed by a successful attempt to verify ericoid mycorrhiza status of some of these fungal isolates under axenic conditions. In two screening experiments, the most efficient ericoid mycorrhiza fungal strains were found beneficial for the growth of micropropagated Rhododendron plants when inoculated post vitro after transplantation to peat-based substrate. No negative influence on the growth of host plants has been observed for any inoculated isolate, while about 10% of tested strains exhibited positive effects on the growth of Rhododendron microcuttings grown in peat-based media. © 2000 Elsevier Science B.V. All rights reserved.
Keywords:Ericoid mycorrhiza; Ericaceae; Horticulture; Micropropagation
1. Introduction
Ericoid mycorrhiza (ERM) belong, together with orchidaceous and arbuscular mycorrhiza types, to the group of endotrophic mycorrhizal associations. Eri-coid mycorrhizas are associations between ascomyce-tous (or rarely hyphomyceascomyce-tous) fungi and plant species belonging to the familiesEricaceae,Epacridaceaeand Empetraceae(Smith and Read, 1997). Also arbutoid and monotropoid mycorrhiza can be found within the familyEricaceae but the most common ericoid my-corrhiza are found in genera such asCalluna,Erica,
∗Corresponding author. Tel./fax:+42-2-67750022.
E-mail addresses:[email protected] (J. Jansa), [email protected] (M. Vos´atka)
1Present address: IPW-ETH Zürich, Eschikon 33, Postfach 185,
CH-8315 Lindau (ZH), Switzerland. Tel.:+41-52-3549216; fax:+41-52-3549119.
Rhododendron,Vaccinium, andEmpetrum(Smith and Read, 1997).
The ERM is characterized by considerably uniform structure, similar to those in arbuscular mycorrhizas, but usually more delicate (Peterson et al., 1980; All-away and Ashford, 1996). A range of differently sep-tated hyphae (simple septal pores of ascomycete type as well as dolipore septated fungi of basidiomyce-tous type can occur) were found inside the corti-cal cells of ericaceous plants (Bonfante-Fasolo and Gianinazzi-Pearson, 1979; Bonfante-Fasolo, 1980). The hyphae of ERM fungi penetrate a single layer of cortical cells of the roots and fill them with intracel-lular hyphal coils. Cortical cells of ericaceous plants never form structures like root-hairs, well described in other plant families.
The ericaceous plants occur in most climatically and edaphically stressed environments particularly when soil acidity becomes extreme and the rate of
nutrient mineralization is low. The function of the ERM fungi is, most probably, to cover nutrient de-mands of the plant under such stress conditions (Harley, 1969). The ERM fungi in acidic heath soils produce external mycelium that is supposed to have an active function in obtaining mineral nutrients: en-zymatic release of nitrogen from predominant organic compounds, that would otherwise be unavailable for the roots. Most remarkable is a high C:N ratio in these soils, which is overcome due to the activity of enzymes produced by the ERM fungi. The ericoid mycorrhizal plants showed access to nitrogen sources that are almost inaccessible for nonmycorrhizal plants (Pearson and Read, 1975; Kerley and Read, 1998). En-zymes hydrolyzing different carbon polymers (oligo-and polysaccharides, including cellulose (oligo-and pectins) were described from pure cultures of the ERM fungi (Pearson and Read, 1975; Perotto et al., 1993; Varma and Bonfante, 1994). Effective uptake of nitrogen by the ERM fungi from different sources in peat-based soils was described — both free ammonia and nitrate fixing pathways are more efficient for fungi compared to the plants, especially under acidic conditions (Pear-son and Read, 1975). Organic polymers-bounded nitrogen was utilized by the ERM fungi especially un-der low pH (Leake and Read, 1990a) and chitinolytic activity of the fungi was proved as well (Leake and Read, 1990b). Increase in phosphate uptake of erica-ceous host plants due to ERM fungi was described by Read and Stribley (1973), mainly due to solubilization of ferric or aluminium phytates (Mitchell and Read, 1981). Moreover, a phosphodiesterase attacking nu-cleic acid bound phosphate was described from fungal cultures by Leake and Miles (1996). A high affinity of the ERM fungi for iron is probably important as maintained by production of siderophores (Schuler and Haselwandter, 1988). Some of the ericoid my-corrhizal host plants (Calluna and Vaccinium) were reported for high tolerance to different environmen-tal stresses. This makes these ERM fungi practically interesting for their potential to enhance plant fitness under unfavourable conditions (Bradley et al., 1982; Burt et al., 1986; Yang and Goulart, 1997). Attempts were made to find a proper fungal strain to decrease losses in propagation of Rhododendrons at commer-cial level, which may reach up to 10% at weaning stage (Lemoine et al., 1992). This work showed com-plexity and probable strain-to-strain specificity of
plant and fungi with respect to positive plant growth reaction, which may depend also on substrate type.
The ERM fungi are characterized by a very slow development — first apparent colonization struc-tures were observable after 3 weeks in g-irradiated
reinoculated soil and after 4 weeks in horticultural soil, collected under Rhododendrons (Duddridge and Read, 1982). This documents possible role of other microorganisms, which may slow down the development of the fungus to a certain extent. The breakdown of some of the mycorrhizal structures was evident after 8 or 11 weeks in irradiated or unsterile soil, respectively. The breakdown process starts by structural desintegration of plant organelles and cells, followed by a loss of integrity of the fungal struc-tures (Duddridge and Read, 1982). This means that the fungus at least in part of its life span plays a role of a sapro-parasitic partner. Simply septated fungi, supposed to be symbiotic ones, frequently observed within cortical cells, can be isolated from roots, but they very scarcely form spores in culture, which hin-ders their classification. They are divided into two groups — slow growing, dark-coloured and usually sterile mycelia (McNabb, 1961; Pearson and Read, 1973a; Singh, 1974), and a group of Oidiodendron sp. observed mainly in isolations fromCallunaor Vac-cinium(Couture et al., 1983; Dalpe, 1986; Douglas et al., 1989). Considerable genetic diversity was found amongst isolates that were superficially very similar, on both biochemical-isozymes (Hutton et al., 1994) and molecular basis (Perotto et al., 1995), as well as classical microbiological techniques (Hambleton and Currah, 1997). A question remains as to whether such diversity observed in root-associated fungi has any impact upon performance of plants grown under con-trolled conditions (Smith and Read, 1997). A demand to extend the research observation beyond classical Calluna–Vaccinium–Rhododendron model to include more taxonomically diverse host and fungal partners has arisen in recent time (Straker, 1996).
2. Material and methods
2.1. Isolation of ericoid mycorrhizal fungi
The roots of different ericaceous plants were sampled in nature from a range of habitats (from Rhododendrons cultivated in botanical gardens to Vaccinium grown in high altitude mountain area). The roots were stained to confirm presence of fun-gal structures according to the following protocol: maceration of the roots in 10% KOH in autoclave at 121◦C for 1 h, cleaning with 5% hydrogen peroxide for 15 min, washing under tap water, acidifying with 1% HCl and staining for 2 h in 0.1% Trypan Blue in lactoglycerol (lactic acid:glycerol:water — 1:1:1) at 80◦C and leaving the roots in the staining solution at room temperature overnight. After this, the roots were destained in water for 24 h and observed under compound microscope at the magnification of 400×. The technique for isolation of the ERM fungi from root samples was modified from Pearson and Read (1973a). The root samples were washed with tap water and surface sterilized by six times repeatedly shaking in sterile tap water (to protect plasmolysis of the fungal hyphae, which usually occurs while using distilled water). This was followed by sterilization step with active chlorine. The roots were submerged in 5% solution of SAVO bleaching solution (10% NaOCl) for 3 min, then the roots were transferred into 5% hydrogen peroxide after washing the previous solution out, followed by another 5 min in 5% SAVO bleaching solution. All the sterilization solutions used contained a drop of detergent (BRIJ 35) to support appropriate wetting of the root surface. Roots were cut under sterile conditions using scissors into pieces approximately 5 mm long and they were put onto the surface of the isolation medium containing strepto-mycin, to prevent the growth of bacteria. Sixteen root pieces were equally distributed over the surface of 9 cm Petri dishes. Root segments were cultivated for 3 weeks on both nutrient (1% malt extract) and water agar at pH 4.5 (according to Leake and Miles, 1996) at room temperature (25◦
C) in dark.
2.2. Cultivation and maintenance of ericoid mycorrhizal fungi
Particular isolates of the ERM fungi growing from the roots were transferred on the specific medium
after Dalpe (1990) and on malt extract agar (MEA). The cultures of isolated ERM fungi strains were main-tained on the medium in both Petri dishes and vials with skewed agar plates. The vials were closed with a Parafilm foil, sealed over the stopper, to ensure good viability of the isolates cultures (up to 1 year) under storage at 4◦C. For preparing inocula of ERM fungi, liquid cultures were used. Ten 9 cm Petri dishes con-taining 25 ml of cultivation media (4% malt extract, pH 4.5) were used for each isolate. The cultures were left to grow for 4 weeks in dark at 20◦C. Then the mycelia were collected on a paper filter, homogenized by blending at high speed by Waring blender in 200 ml sterile water for 5 s and diluted in sterile water to final volume of 500 ml.
2.3. Inoculation in vitro
Experiment 1: Azalea cv. AK 504 were grown in vitro on modified 0.5% agar medium after Anderson (1984) without phytohormones, amended with 2 g of charcoal per 1l of media, pH 5.2. Re-establishment of mycorrhiza was performed in sterile 200 ml Erlen-meyer flasks, filled with 30 ml of Lignocel substrate, enriched with 12 g Osmocote per 1 l. Inoculation was made using mycelium plugs from vial cultures af-ter 3 weeks acclimation period following transfer of plantlets from nutrient medium. Ten strains of the ERM fungi were used for inoculation and the plants were grown for 12 weeks. For evaluation of presence of mycorrhizal structures, plant roots were processed as described above.
2.4. Inoculation experiments, post vitro
the root system of the seedlings was dipped into the mycelial suspension before planting into inoculated container. Each experimental container represented a unit infected with one of the 12 selected ERM fun-gal isolates; 15 plants were grown in each container. Mortality, biomass of shoots and roots, leaf area
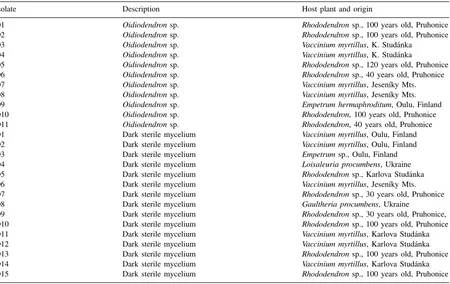
Fig. 1. (a) Intraradical colonization ofCalluna vulgarisfrom natural habitat (Jesen´ıky Mts.) by an ericoid mycorrhizal fungus (bar represents 10mm). (b) Detail of hyphal coils of an ericoid mycorrhizal fungus within cortical cells of a root ofRhododendronsp. from natural habitat (Jesen´ıky Mts.) (bar represents 10mm).
were evaluated after 12 weeks of cultivation in the greenhouse.
2.5. Statistical evaluation of the data
One-way ANOVA was used for evaluation of the effect of different fungal isolates on plant growth. G-test was used only to estimate significant differences in mortality rates. The statistical evaluation was per-formed using SOLO statistical package (BMDP Soft-ware, Los Angeles, CA, 1991).G-test was calculated using CoStat package.
3. Results
Almost all roots of ericoid plants collected from the natural habitats were found to have some kind of in-timate hyphal association within cortical cells of their roots (Fig. 1a and b). Mycorrhizal colonization was well developed in the whole range of habitats and alti-tudes (from 400 to 1200 m above sea level). High col-onization rate of roots sampled in nature was detected (different cultivars of Rhododendron sp., Vaccinium
Table 1
Isolates of ericoid mycorrhizal fungi used for experimental work and their origin
Isolate Description Host plant and origin
O1 Oidiodendronsp. Rhododendronsp., 100 years old, Pruhonice O2 Oidiodendronsp. Rhododendronsp., 100 years old, Pruhonice O3 Oidiodendronsp. Vaccinium myrtillus, K. Stud´anka
O4 Oidiodendronsp. Vaccinium myrtillus, K. Stud´anka
O5 Oidiodendronsp. Rhododendronsp., 120 years old, Pruhonice O6 Oidiodendronsp. Rhododendronsp., 40 years old, Pruhonice O7 Oidiodendronsp. Vaccinium myrtillus, Jesen´ıky Mts. O8 Oidiodendronsp. Vaccinium myrtillus, Jesen´ıky Mts. O9 Oidiodendronsp. Empetrum hermaphroditum, Oulu, Finland O10 Oidiodendronsp. Rhododendron, 100 years old, Pruhonice O11 Oidiodendronsp. Rhododendron, 40 years old, Pruhonice
D1 Dark sterile mycelium Vaccinium myrtillus, Oulu, Finland
D2 Dark sterile mycelium Vaccinium myrtillus, Oulu, Finland
D3 Dark sterile mycelium Empetrumsp., Oulu, Finland
D4 Dark sterile mycelium Loisaleuria procumbens, Ukraine
D5 Dark sterile mycelium Rhododendronsp., Karlova Stud´anka
D6 Dark sterile mycelium Vaccinium myrtillus, Jesen´ıky Mts.
D7 Dark sterile mycelium Rhododendronsp., 30 years old, Pruhonice
D8 Dark sterile mycelium Gaultheria procumbens, Ukraine
D9 Dark sterile mycelium Rhododendronsp., 30 years old, Pruhonice,
D10 Dark sterile mycelium Rhododendronsp., 100 years old, Pruhonice
D11 Dark sterile mycelium Vaccinium myrtillus, Karlova Stud´anka
D12 Dark sterile mycelium Vaccinium myrtillus, Karlova Stud´anka
D13 Dark sterile mycelium Rhododendronsp., 100 years old, Pruhonice
D14 Dark sterile mycelium Vaccinium myrtillus, Karlova Stud´anka
D15 Dark sterile mycelium Rhododendronsp., 100 years old, Pruhonice
myrtillus,Calluna vulgaris, Gaultheria procumbens, andEmpetrum hermaphroditum).
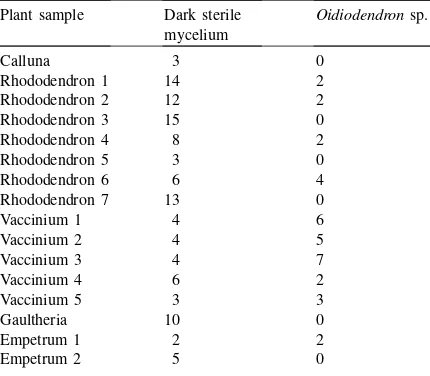
More than 200 strains of endophytic fungi belong-ing toOidiodendron sp. and dark sterile (ascomyce-tous) mycelia were isolated from the roots of different host plants (Table 1). Satisfactory growth was obtained on MEA medium, of slowly growing, dark and sterile mycelia, revealed to develop from hyphal coils inside cortical cells and were supposed to be the symbiotic fungi. During their growth, they were usually masked by rapidly growing saprophytes.
Using our isolation technique, a trend was observed in distribution of symbionts amongst different host plants, a higher number ofOidiodendronsp. were iso-lated fromVacciniumplants than from Rhododendrons (Table 2). Majority of root-endophytic fungal popu-lations by other plants make fungi belonging to dark sterile mycelium group.
Table 2
Numbers of morphologically different colonies of fungal endo-phytes obtained by isolation from root samples ofCalluna vul-garis, differentRhododendrons, Vaccinium myrtillus,Gaultheria procumbensand Empetrum hermaphroditumcollected in natural habitats
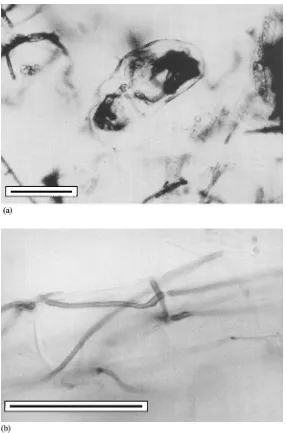
into the system developed structures typical of ericoid mycorrhizas (Fig. 2a and b). However, the majority of fungi appeared to grow too fast in relation to the plants and after harvest, inhibitory effect of fungal growth on root development was observed. There appeared to be a competition for mineral sources, as the plants were showing symptoms of deficiencies. In prolonged cultivation, after another 4 weeks, the plants were overgrown by fungi. Not enough roots for evaluation of infection were obtained, therefore only presence–absence of mycorrhizal structures were recorded (Table 3).
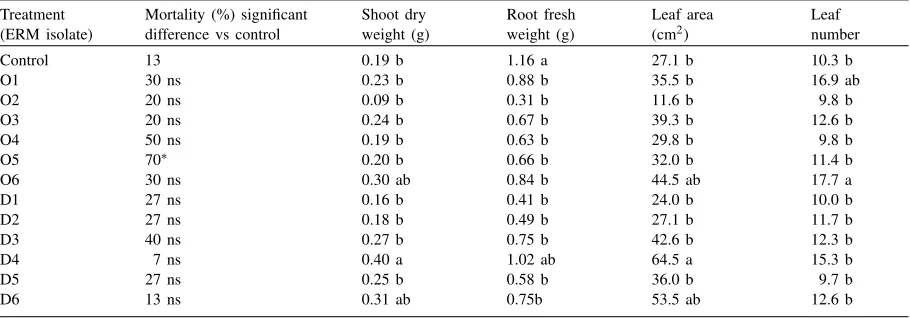
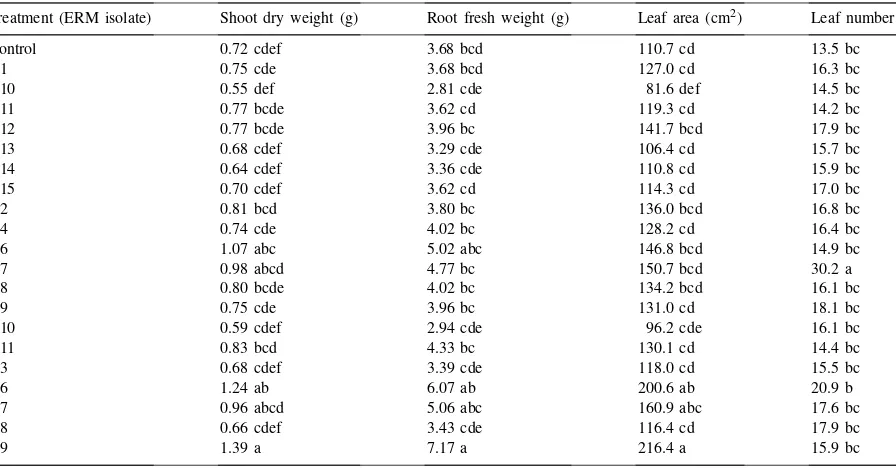
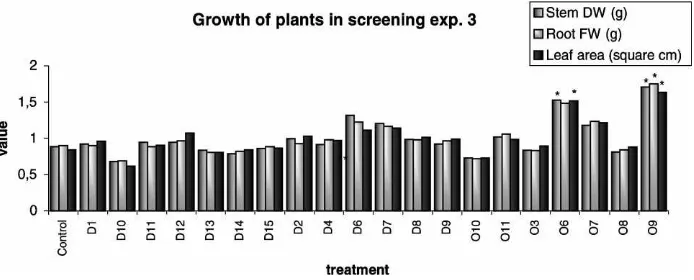
Post vitro mycorrhization of Rhododendrons in ex-periment 2 showed that there was only one isolate (D4) significantly stimulating plant growth. However, there were no inhibitiory effects of any inoculated fungus (Table 4, Fig. 3). Similarly, in experiment 3, two (O6, O9) of 20 strains of ERM showed consistent positive effects on plant growth (Table 5, Fig. 4). Root biomass was generally reduced by inoculation in experiment 2, but such an effect was not observed in experiment 3, probably due to prolonged cultivation time. Most apparent stimulation of plant development due to in-oculation was observed using isolate O6 in both post
vitro experiments (significant leaf number increase in experiment 2, significant stem dry weight and leaf area stimulation in experiment 3). Increase in some of the parameters describing plant growth were also observed for fungal isolate D6 in both experiments.
4. Discussion
The abundance of ERM fungi in the roots of the ma-jority of plants sampled indicates the wide ecological importance of the symbiosis (Haselwandter, 1979). Isolated strains of ericoid mycorrhizal fungi were proved to be true endophytic symbionts and forming ericoid mycorrhizas with tested plants. Soil-water agar, used for axenic cultures establishment by Pear-son and Read (1973a), and Read and Stribley (1973) has the advantage of slowing growth of the fungus, while rich substrates, as that used in our work, have the advantage of supporting growth of the plant for a longer time. During longer periods, a better devel-opment of mycorrhizal structures may be achieved. On the other hand, the cultivation substrate should be probably first inoculated with some bacteria that would diminish the amount of easily available nutri-ents which are released during sterilization process (autoclaving), causing a too fast growth of the fungus relative to the development of the plant. However, this makes the system less convenient for assessing the influence of the fungus on the plant growth, as its complexity increases. On the other hand, using this system we may get near to the real state, compared to using extremely poor environment of water or weak soil-water agar (which are the generally used ones for testing mycorrhization capabilities of fungal strains in vitro, Pearson and Read, 1973a,b). Rich-substrate technique, described in this work, may also be readily exploited in biotechnological application in indus-trial production, just before ex vitro transplantation of plantlets (which have to be kept on rich media to ensure good survival after).
Fig. 2. (a) Detail of hyphal coil in the cortical cell of Azalearoot inoculated in vitro with an ericoid mycorrhizal fungus (isolate D5) isolated fromRhododendronsp. from natural habitat (Jesen´ıky Mts.) (bar represents 10mm). (b) Detail of intraradical hyphae — pelotons of an ericoid mycorrhizal fungus (isolate D5) within root ofAzaleainoculated in vitro with an ericoid mycorrhizal fungus isolated from
Rhododendronsp. from natural habitat (Jesen´ıky Mts.) (bar represents 10mm).
detoxifying role and the role of host nutrition (Smith and Read, 1997). In spite of these complications, steam sterilized sand or soil mixtures are the most fre-quently used substrates for glasshouse experiments, being reliable concerning the growth response of plants to inoculation (Stribley and Read, 1976).
Table 3
Mycorrhizal status of Azaleas, in vitro inoculated with 10 different fungal endophytes isolated from the roots of ericoid plants [experiment 1]
Treatment (ERM isolate) Description Mycorrhizal status
Control No colonization, no intracellular structures –a
D1 Heavy colonization by thick hyphae +b
D2 Thick hyphae, roots covered by hyphae, intracellular pelotons +b
D4 Typical dark mycelium colonizing roots, pelotons! ++c
D5 Superficial hyphal mantle, hyphae penetrating into inner cell layers +b
D7 Fine, dark mycelium ++c
D8 Superficial hyphal mantle, dark pelotons inside cells ++c
O4 Nonspecific intracellular colonization, fine mycelium to massive intracellular infection, no pelotons
+b
O6 Bright mycelium, heavy colonization ++c
O7 Fine intracellular colonization, pelotons +b
O8 Nonspecific hyphae ?d
aNo mycorrhiza. bMycorrhizal fungus.
cMycorrhizal fungus of high infectivity. dUncertain mycorrhizal status.
Table 4
The effect of different isolates of ericoid mycorrhizal fungi on the mortality and growth of Rhododendrons inoculated post vitro (G-test including Williams correction at 5%)a [experiment 2]
Treatment (ERM isolate)
Mortality (%) significant difference vs control
Shoot dry weight (g)
Root fresh weight (g)
Leaf area (cm2)
Leaf number
Control 13 0.19 b 1.16 a 27.1 b 10.3 b
O1 30 ns 0.23 b 0.88 b 35.5 b 16.9 ab
O2 20 ns 0.09 b 0.31 b 11.6 b 9.8 b
O3 20 ns 0.24 b 0.67 b 39.3 b 12.6 b
O4 50 ns 0.19 b 0.63 b 29.8 b 9.8 b
O5 70∗ 0.20 b 0.66 b 32.0 b 11.4 b
O6 30 ns 0.30 ab 0.84 b 44.5 ab 17.7 a
D1 27 ns 0.16 b 0.41 b 24.0 b 10.0 b
D2 27 ns 0.18 b 0.49 b 27.1 b 11.7 b
D3 40 ns 0.27 b 0.75 b 42.6 b 12.3 b
D4 7 ns 0.40 a 1.02 ab 64.5 a 15.3 b
D5 27 ns 0.25 b 0.58 b 36.0 b 9.7 b
D6 13 ns 0.31 ab 0.75b 53.5 ab 12.6 b
∗Treatment is significantly different from control. aDuncan’s Multiple range testp
<0.05, means followed by the same letter are not significantly different within one column.
also reached by Lemoine et al. (1992), who reported growth stimulation by Rhododendron microcuttings due to inoculation with symbiotic fungus up to 200% of the growth of the control plants, together with decreased heterogeneity of inoculated plants. Con-trary to our findings, a positive influence of the ERM fungi on survival of the host plants was observed by Nieuwdorp (1969) and Lemoine et al. (1992). Such discrepancy may be caused by different host plants and experimental conditions, even if close species
Fig. 3. The effect of different isolates of ericoid mycorrhizal fungi on the growth of Rhododendrons inoculated post vitro, (normalized data from experiment 2 — normalization by division by the mean value), (*) significant increase (5% level) due to inoculation with the fungus, compared to the control.
4 weeks, respectively. Also a significant shift in root architecture due to ERM fungi corresponding to the level of endogenous auxins was described by Berta et al. (1988). That may be an indication of the cause of some of the reported morphological and physiological effects. Positive influence of the presence ofPezizella ericaewithin the roots ofVaccinium corymbosumon
Table 5
The effect of different isolates of ericoid mycorrhizal fungi on the growth of Rhododendrons inoculated post vitro (means followed by the same letter are not significantly different within one column according to Duncan’s Multiple range testp<0.05) [experiment 3] Treatment (ERM isolate) Shoot dry weight (g) Root fresh weight (g) Leaf area (cm2) Leaf number
Control 0.72 cdef 3.68 bcd 110.7 cd 13.5 bc
D1 0.75 cde 3.68 bcd 127.0 cd 16.3 bc
D10 0.55 def 2.81 cde 81.6 def 14.5 bc
D11 0.77 bcde 3.62 cd 119.3 cd 14.2 bc
D12 0.77 bcde 3.96 bc 141.7 bcd 17.9 bc
D13 0.68 cdef 3.29 cde 106.4 cd 15.7 bc
D14 0.64 cdef 3.36 cde 110.8 cd 15.9 bc
D15 0.70 cdef 3.62 cd 114.3 cd 17.0 bc
D2 0.81 bcd 3.80 bc 136.0 bcd 16.8 bc
D4 0.74 cde 4.02 bc 128.2 cd 16.4 bc
D6 1.07 abc 5.02 abc 146.8 bcd 14.9 bc
D7 0.98 abcd 4.77 bc 150.7 bcd 30.2 a
D8 0.80 bcde 4.02 bc 134.2 bcd 16.1 bc
D9 0.75 cde 3.96 bc 131.0 cd 18.1 bc
O10 0.59 cdef 2.94 cde 96.2 cde 16.1 bc
O11 0.83 bcd 4.33 bc 130.1 cd 14.4 bc
O3 0.68 cdef 3.39 cde 118.0 cd 15.5 bc
O6 1.24 ab 6.07 ab 200.6 ab 20.9 b
O7 0.96 abcd 5.06 abc 160.9 abc 17.6 bc
O8 0.66 cdef 3.43 cde 116.4 cd 17.9 bc
O9 1.39 a 7.17 a 216.4 a 15.9 bc
the growth and nutrient uptake by the host plants was also described by Powell (1982), and for Calluna vulgarisby Strandberg and Johansson (1999).
Fig. 4. The effect of different isolates of ericoid mycorrhizal fungi on the growth of Rhododendrons inoculated post vitro (normalized data from experiment 3 — normalization by division by the mean value), (*) significant increase (5% level) due to inoculation with the fungus, compared to the control.
Such a behaviour was hypothesized previously (Smith and Read, 1997), but only a little information was available till now (Douglas et al., 1989). Very specific enzymatic apparatus of the ERM fungi was described, releasing nutrients only from a certain spectra of source molecules, that is supposed to vary, depending on isolates and certain conditions (Bajwa and Read, 1986). That may explain, why some ERM fungal iso-lates may not demonstrate their beneficial effects on the growth of the host, according to the conditions, under which they occur. We have described beneficial influence of inoculation with ERM fungi only in about 10–15% of isolates, which corresponds to the results of Gianinazzi-Pearson (pers. commun.). However, since some of the ERM fungi strains were proved not to stimulate plant growth, selection for appropriate stimulatory strains for practical inoculation is essen-tial. Not regarding the principles of functioning of the ericoid mycorrhiza, the consistent improvement of the growth of ericaceous plants cultivated under artificial conditions described in this work may contribute to horticultural practices. The selection of isolates with highest stimulative capabilities in a given environ-ment is needed (especially regarding substrate type and pH as well as host cultivar identity), as already proposed by Lemoine et al. (1992).
Further research on selected strains should continue to find appropriate doses, survival potential of my-corrhiza in the substrate and the most efficient way of inoculum introduction into the substrate. That could
lead to exploitation of these fungi for inoculation of ericaceous plants in horticultural practices.
Acknowledgements
We would like to express many thanks to Mrs. Jana Rouckova for her outstanding technical assistance, as well as to other members of Mycorrhizal Group in the Institute of Botany, Pruhonice for their kind help and to Ministry of Education, Youth and Sports of the Czech Republic for financing cooperative grant ME 256 and to the colleagues from University of Ljubl-jana, Slovenia for valuable cooperation.
References
Allaway, W.G., Ashford, A.E., 1996. Structure of the hair-roots in
Lysinema ciliatumR. Br. and its implications for their water relations. Ann. Bot. 77, 383–388.
Anderson, W.C., 1984. A revised tissue culture medium for shoot multiplication of Rhododendron. J. Am. Soc. Hort. Sci. 109, 343–347.
Bajwa, R., Read, D.J., 1986. Utilization of mineral and amino N sources by the ericoid mycorrhizal endophyteHymenoscyphus ericaeand by mycorrhizal and non-mycorrhizal seedlings of
Vaccinium. Trans. Br. Mycol. Soc. 87, 269–277.
Berta, G., Gianinazzi-Pearson, V., Gay, G., Torri, G., 1988. Morphogenetic effects of endomycorrhiza formation on the root system ofCalluna vulgaris(L.) Hull. Symbiosis 5, 33–44. Bonfante-Fasolo, P., 1980. Occurrence of a basidiomycete in living
cells of mycorrhizal hair roots ofCalluna vulgaris. Trans. Br. Mycol. Soc. 75, 320–325.
Bonfante-Fasolo, P., Gianinazzi-Pearson, V., 1979. Ultrastructural aspects of endomycorrhiza in the Ericaceae. I. Naturally infected hair roots of Calluna vulgaris L Hull. New Phytol. 83, 739–744.
Bradley, R., Burt, A.J., Read, D.J., 1982. The biology of mycorrhiza in the Ericaceae. VIII. The role of mycorrhizal infection in heavy metal resistance. New Phytol. 91, 197–201. Burt, A.J., Hashem, A.R., Shaw, G., Read, D.J., 1986. Comparative analysis of metal tolerance in ericoid and ectomycorrhizal fungi. In: Gianinazzi-Pearson, V., Gianinazzi, S. (Eds.), Physiological and Genetical Aspects of Mycorrhizae. INRA, Paris, France, pp. 683–687.
Couture, M., Fortin, J.A., Daple, Y., 1983.Oidiodendron griseum
(Robak): an endophyte of ericoid mycorrhizas in Vaccinium
spp. New Phytol. 95, 375–380.
Dalpe, Y., 1986. Axenic synthesis of ericoid mycorrhiza in
Vaccinium angustifoliumAit. by Oidiodendronspecies. New Phytol. 103, 391–396.
Dalpe, Y., 1990.Endogone pisiformis: growth, morphology and culture preservation. Can. J. Bot. 68, 910–915.
Douglas, G.C., Heslin, M.C., Reid, C., 1989. Isolation of
Oidiodendron maius from Rhododendron and ultrastructural characterization of synthesized mycorrhizas. Can. J. Bot. 67, 2206–2212.
Duddridge, J.A., Read, D.J., 1982. Ultrastructural analysis of the development of mycorrhizas inRhododendron ponticum. Can. J. Bot. 60, 2345–2356.
Freisleben, R., 1935. Wietere Untersuchungen über die Mycotrophie der Ericaceen. Jahrbuch Wissenschaft. Bot. 82, 413–459.
Hambleton, S., Currah, R.S., 1997. Fungal endophytes from the roots of alpine and borealEricaceae. Can. J. Bot. 75, 1570– 1581.
Harley, J., 1969. The Biology of Mycorrhiza. Leonard Hill, London, UK.
Haselwandter, K., 1979. Mycorrhizal status of ericaceous plants in alpine and subalpine areas. New Phytol. 83, 427–431. Hutton, B.J., Dixon, K.W., Sivasithamparam, K., 1994. Ericoid
endophytes of Western Australian heaths (Epacridaceae). New Phytol. 127, 557–566.
Kerley, S.J., Read, D.J., 1998. The biology of mycorrhiza in the
Ericaceae. XX. Plant and mycorrhizal necromass as nitrogenous substrates for the ericoid mycorrhizal fungusHymenoscyphus ericaeand its host. New Phytol. 139, 353–360.
Koch, R., 1912. Complete Works, Vol. I. George Thieme, Leipzig, pp. 650–660.
Leake, J.R., Miles, W., 1996. Phosphodiesters as mycorrhizas. I. Phosphodiesterase production and the utilization of DNA as a phosphorus source by the ericoid mycorrhizal fungus
Hymenoscyphus ericae. New Phytol. 132, 435–444.
Leake, J.R., Read, D.J., 1990a. Proteinase activity in mycorrhizal fungi. I. The effect of extracellular pH on the production
and activity of proteinase by ericoid endophytes from soils of contrasted pH. New Phytol. 115, 243–250.
Leake, J.R., Read, D.J., 1990b. Chitin as a nitrogen source for mycorrhizal fungi. Mycol. Res. 94, 993–995.
Lemoine, M.C., Gianinazzi, S., Gianinazzi-Pearson, V., 1992. Application of endomycorrhizae to commercial production of
Rhododendronmicroplants. Agronomie 12, 881–885. McNabb, R.F.R., 1961. Mycorrhiza in New Zealand Ericales. Aust.
J. Bot. 9, 57–61.
Mitchell, D.T., Read, D.J., 1981. Utilization of inorganic and organic phosphates by the mycorrhizal endophytes ofVaccinium macrocarponandRhododendron ponticum. Trans. Br. Mycol. Soc. 76, 255–260.
Nieuwdorp, P.J., 1969. Some investigations on the mycorrhiza of
Calluna,EricaandVaccinium. Acta Bot. Neerl. 18, 180–196. Pearson, V., Read, D.J., 1973a. The biology of mycorrhiza in the
Ericaceae. I. The isolation of the endophyte and synthesis of mycorrhizas in aseptic culture. New Phytol. 72, 371–379. Pearson, V., Read, D.J., 1973b. The biology of mycorrhiza in the
Ericaceae. II. The transport of carbon and phosphorus by the endophyte and the mycorrhiza. New Phytol. 72, 1325–1331. Pearson, V., Read, D.J., 1975. The physiology of the mycorrhizal
endophyte of Calluna vulgaris. Trans. Br. Mycol. Soc. 64, 1–7.
Perotto, S., Bettini, V., Bonfante, P., 1993. Evidence of two polygalactouronases produced by a mycorrhizal ericoid fungus during its saprophytic growth. FEMS Microbiol. Lett. 114, 85– 92.
Perotto, S., Perotto, R., Faccio, A., Schubert, A., Varma, A., Bonfante, P., 1995. Ericoid mycorrhizal fungi: cellular and molecular bases of their interactions with the host plant. Can. J. Bot. 73 (1), 557–568.
Peterson, T.A., Müller, W.C., Englander, L., 1980. Anatomy and ultrastructure of a Rhododendron root-fungus association. Can. J. Bot. 59, 2421–2433.
Powell, C.L., 1982. The effect of the ericoid mycorrhizal fungus
Pezizella ericae (Read) on the growth and nutrition of seedlings of blueberry (Vaccinium corymbosum L.) fertilizer, root infection. J. Am. Soc. Hort. Sci. 107, 1012–1015. Read, D.J., Stribley, D.P., 1973. Effect of mycorrhizal infection on
nitrogen and phosphorus nutrition of ericaceous plants. Nature 244, 81.
Schuler, R., Haselwandter, K., 1988. Hydroxamate siderophore pro-duction by ericoid mycorrhizal fungi. J. Plant Nutr. 11, 907– 913.
Singh, K.G., 1974. Mycorrhiza in Ericaceae with particular reference to Calluna vulgaris. Svensk Botanisk Tidskrift 68, 1–16.
Smith, S.E., Read, D.J., 1997. Mycorrhizal Symbiosis. Academic Press, Cambridge, 605 pp.
Straker, C.J., 1996. Ericoid mycorrhiza: ecological and host specificity. Mycorrhiza 6, 215–225.
Strandberg, M., Johansson, M., 1999. Uptake of nutrients in Calluna vulgaris seed plants grown with and without mycorrhiza. For. Ecol. Mgmt. 114, 129–135.
Stribley, D.P., Read, D.J., 1974. The biology of mycorrhiza in the
of15N from labelled soil byVaccinium macrocarponAit. New Phytol. 73, 1149–1155.
Stribley, D.P., Read, D.J., 1976. The biology of mycorrhiza in the Ericaceae. VI. The effects of mycorrhizal infection and concentration of ammonium nitrogen on growth of cranberry (Vaccinium macrocarponAit.) in sand culture. New Phytol. 77, 63–72.
Stribley, D.P., Read, D.J., Hunt, R., 1975. The biology of myco-rrhiza in theEricaceae. V. The effect of mycorrhizal infection, soil type. New Phytol. 75, 119–130.
Varma, A.K., Bonfante, P., 1994. Utilisation of cell-wall related carbohydrates by ericoid mycorrhizal endophytes. Symbiosis 16, 301–313.
Xiao, G., Berch, S.M., 1995. The ability of known ericoid mycorrhizal fungi to form mycorrhizae withGaultheria shallon. Mycologia 87, 467–470.