A soil microbial community structural-functional index: the
microscopy-based total/active/active fungal/bacterial
(TA/AFB) biovolumes ratio
Donald A. Klein
a,∗, Mark W. Paschke
baDepartment of Microbiology, Colorado State University, Fort Collins, CO 80523-1677, USA bDepartment of Rangeland Ecosystem Science, Colorado State University, Fort Collins, CO 80523, USA
Received 2 June 1999; accepted 17 January 2000
Abstract
In most studies of fungal–bacterial communities in soils, single-value indices such as fumigation–extraction (FE) of microbe-derived organic carbon, measures of specific microbial cell chemical constituents, or activity-related measures have been used. These widely used single value indices, however, do not provide information on the physical structure of the filamentous fungal and bacterial community in a soil. The filamentous fungi, considered as indeterminate organisms, have a variable and changing hyphal network, most of which is devoid of cytoplasm. To meet this need for a direct integrated measure of the physical characteristics of the indeterminate fungi and their associated bacteria, a microscopy-based microbial biovolumes ratios approach is suggested. To provide this information, the total and active biovolumes of both the filamentous fungi and bacteria are assessed by microscopy. To normalize these responses, the ratio of total to active (TA) fungal plus bacterial biovolumes is divided by the ratio of the active fungal to bacterial biovolume (AFB), to yield the total/active/active fungal/bacterial (TA/AFB) biovolumes ratio. This approach has been used to analyze data from recently-cultivated early successional (ES) and uncultivated late successional (LS) sites at a shortgrass steppe of northeastern Colorado, where control plots were compared with those receiving mineral nitrogen amendments, using samples taken during the summer of 1995. The TA/AFB ratio index showed distinct and significant decreases in response to soil disturbance which reflected the decreased hyphal lengths present in these disturbed soils. These changes were not detected by the use of FE-based extractable carbon measurements. The TA/AFB ratio also showed significant positive correlations with indices of plant community development and mineral nitrogen, especially in the plots not amended with N. This TA/AFB ratios index should be able to provide infor-mation on the physical structure of the indeterminate filamentous fungi and associated soil bacteria for use in the assessment of soil quality, health and resiliency. © 2000 Published by Elsevier Science B.V. All rights reserved.
Keywords: Bacteria; Biovolumes ratio; Filamentous fungi; Indeterminate microbes; Microscopy; Nitrogen; Soil health; Soil quality; Soil resilience
∗Corresponding author. Tel.:+1-970-491-6947;
fax:+1-970-491-1815.
E-mail address: [email protected] (D.A. Klein)
1. Introduction
Most studies of fungi and bacteria in soils have been carried out using single value measurements. Such single-value approaches are often based on chloroform-fumigation followed by extraction (FE) of soluble carbon products (Vance et al., 1987) and
the use of conversion factors to provide biomass val-ues. In addition, substrate-induced respiration (SIR) (Anderson and Domsch, 1978; Blagodatskaya and Anderson, 1998) can be used, where biomass values are related to CO2evolution after substrate addition.
Selective antibiotics (Anderson and Domsch, 1973, 1975; Beare et al., 1991; Alphei et al., 1995; Blago-datskaya and Anderson, 1998) also have been used to assess relative contributions of fungi versus bacte-ria to soil respiration. This approach has often been suggested to provide information on the relative fun-gal and bacterial biomass in soils (Lin and Brookes, 1996; Blagodatskaya and Anderson, 1998). Fungal and bacterial chemical markers also can be applied to this problem. Chantigny et al. (1997) monitored fungal-derived glucosamine and bacterial-derived muramic acid to assess fungal–bacterial development in relation to soil aggregation processes. Bardgett and McAlister (1999) have used phospholipid fatty acids (PLFA) to assess fungal and bacterial biomass in control and disturbed pasture soils.
Microbiological aspects of soil quality also can be determined by the use of single value activity measure-ments, whether these involve respiration (Stenberg et al., 1998) or mineralization rates (Smith et al., 1993). In addition, fluorogenic substrates also can provide in-formation related to the fungal soil community. Miller et al. (1998) compared fluorescent substrates related to chitinase and cellulase activities with levels of two fungal-specific molecules, PLFA and ergosterol. These single value types of measurements have been used to assess soil quality (Smith et al., 1993; Doran and Jones, 1996; Doran et al., 1996; Rice et al., 1996; Elliott et al., 1998; Stenberg et al., 1998; Torstensson et al., 1998), soil health (Smith et al., 1993; Doran and Jones, 1996; Doran et al., 1996) and soil resilience (Seybold et al., 1999).
As discussed by Klein et al. (1998), such widely used single-value measurements do not provide information on the structure of the filamentous fungal–bacterial community, particularly related to the total and active biovolumes of these microbes. The fil-amentous fungi are indeterminate microbes (Anderson and Kohn, 1998; Rayner et al., 1999; Worrall, 1999), which are characterized by their variable boundaries, where the hyphal network will be extended into areas where nutrients are available. Cytoplasm can be moved to these new areas of hyphal extension
(Carlisle, 1994), and older hyphae will be abandoned as resources in these soil volumes are depleted.
In this context, it is particularly critical to assess the total and active biovolumes of the filamentous fungi as well as of the bacteria present in a soil. At the present time, filamentous fungal development is best assessed by the use of microscopy (Klein et al., 1998), a technique which can provide information on total hyphal lengths and the portion of the fungal hyphal network which contains active biovolume. Frankland (1975), Söderström (1979), Ingham et al. (1989), Norton and Firestone (1991), Carlisle (1994), Klein et al. (1996) and Thorn (1997) have noted that only a portion of the hyphal lengths will be active. The re-lationship between total and active hyphal lengths in a soil can be influenced by the location of the fungus in relation to energy sources such as the root (Norton and Firestone, 1991), the presence of added nitrogen (Klein et al., 1989), and whether the filamentous fungi are functioning in early versus later successional soils (Klein et al., 1995), as examples. Griffin (1972, p. 17) has emphasized, in this regard, that “Objectively, the length of viable or better of actively growing hyphae of each species, along with the frequency of spores, are necessary to quantitate the fungal population.” In a similar vein, Frankland (1990) has noted that the fungal mycelium should “be specified as living, dead or total.” These critical aspects of filamentous fungal structure will not be documented by the single-value biomass estimates used in most soil assessments. Microscopy-based studies of soil bacteria also have been carried out where various size classes have been measured (Lundgren, 1984). With biovolume infor-mation available, biomass values can be generated using specific conversion factors (Van Veen and Paul, 1979; Jenkinson and Ladd, 1981).
better exploit these more heterogeneous and lignified substrates. This response, as observed by Holland and Coleman (1987), is central to the filamentous fungal growth strategy in soils. The increased allocation of available resources to hyphal extension in such more mature plant–soil systems can result in a decrease in the cytoplasm maintained in the fungal structure, as discussed by Paustian (1985) and Paustian and Schnürer (1987). In disturbed and earlier successional plant communities, in comparison, less-lignified sub-strates are released from these earlier successional plants (Frederick and Klein, 1994). This can result in an increased allocation of carbon to cytoplasm synthe-sis, at the expense of hyphal development, based on concepts presented by Paustian and Schnürer (1987). The filamentous fungi also can be affected by environmental factors which are often observed to decrease the hyphal lengths that occur in a soil. The filamentous fungi are sensitive to physical disturbance (Gupta and Germida, 1988; Dick, 1992; McGonigle and Miller, 1996), mineral nitrogen (Klein et al., 1989; Arnolds, 1991; Berg et al., 1998), pesticides (Ander-son et al., 1981; Duah-Yentumi and John(Ander-son, 1986; Beare et al., 1993), earthworm activity (McLean and Parkinson, 1997; Zhu and Carreiro, 1999), and heavy metals (Nordgren et al., 1983).
To document the total and active biovolumes of fila-mentous fungi and bacteria in the soil, and to provide a normalized index of this filamentous fungal–bacterial structural development, the total/active/active fun-gal/bacterial (TA/AFB) biovolumes ratio method is presented in this communication. This approach has been tested with data from a disturbed and adjacent undisturbed native shortgrass steppe system sam-pled during the summer of 1995, using control and nitrogen-amended subplots. This TA/AFB biovol-umes ratio method has been compared with results for the FE method, as well as with plant community and soil characteristics for these sites.
2. Materials and methods
2.1. Study sites
The study sites used in this experiment have been described by Klein et al. (1998) and by Paschke et al. (2000). The recently disturbed early successional (ES)
site and the uncultivated late successional (LS) site are located in northeastern Colorado at and near the Central Plains Experimental Range, 50 km northeast of Fort Collins, CO. The purpose of the experiment is to assess the effects of soil N availability on the recovery characteristics of the shortgrass steppe after disturbance.
The ES site was last cultivated in 1989. By 1995, it was dominated by exotic weedy annuals. Major species were prickly lettuce (Lactuca scariola L.) and cheatgrass (Bromus tectorum L.). The uncultivated LS site, in comparison, was dominated by native perennial plants. Major species observed included blue grama (Bouteloua gracilis Willd.), buffalo grass (Buchloe
dactyloides (Nutt.) Engl.) and prickly pear (Opuntia polyacantha Haw.).
At each of these sites, the experiment was arranged as a randomized block design. Twelve 10 m×10 m plots with 2 m buffer zones were established at each of the sites in 1993. The plots were arranged in four blocks of three plots each. Control plots received no nitrogen amendments. The nitrogen treatments, which were randomly assigned to one of the three plots in each block, were first applied in the summer of 1993 and continued annually through 1995. The nitrogen plots received ammonium nitrate at a rate of 100 kg N ha−1 per year. The N was hand broadcast in three equal increments annually (April, June and August).
2.2. Field sampling procedures
The individual blocks within plots were sampled us-ing randomly generated coordinates. Two composited samples were taken from each block by combining three replicate 0–10 cm depth samples for each sample. The samples were immediately cooled and samples were sieved using a 2.0 mm mesh screen.
2.3. Fumigation–extraction (FE) analyses
FE extractable organic carbon, without expression as microbial biomass which would require the use of conversion factors. All FE analyses were initiated within 12 h of sample acquisition.
2.4. Microscopic analyses
The microscopic analyses were completed by Soil Food Web, Inc., Corvallis, OR, using procedures de-scribed by Klein et al. (1998). In these analyses, one slide was prepared per soil sample, and three replicate readings of 40 fields were used per soil from each transect. The bacterial biovolume was determined by the use of soil suspensions stained with fluorescein isothiocyanate (FITC) and filtered onto Nucleopore, black stained membrane filters as described by Babiuk and Paul (1970). The corresponding active bacterial biovolume assays were carried out by measuring iodonitrotetrazolium (INT) chloride-responsive bacte-ria as described by Stamatiadis et al. (1990). For the purposes of this intial study, all bacterial cells were assumed to be spherical. The total and active fungal hyphal lengths and hyphal diameter measurements were carried out using agar film soil suspensions with fluorescein diacetate (FDA) and a combination of epifluorescent and phase contrast-differential interfer-ence contrast (DIC) microscopy (Ingham and Klein, 1984a,b; Stamatiadis et al., 1990; Lodge and Ingham, 1991). Hyphal lengths and average hyphal diameters were used in these initial analyses. All microscop-ically determined total and active bacterial values were log transformed before statistical analyses, and all data were expressed on a dry weight basis. The microscopic analyses were completed within 24 h after soil sampling.
2.5. TA/AFB measures of filamentous fungal–bacterial development
The essence of this approach is to estimate the biovolumes in four parts of the microscopically de-termined fungal–bacterial community: (1) the fungal total biovolume (FT); (2) the active fungal biovolume (FA); (3) the bacterial total biovolume (BT) and (4) the active bacterial biovolume (BA). It should be noted that the term ‘total,’ used in this context, represents the maximum values obtained with the microscopic procedures used in this study.
The following equations were used:
FT=π r2×total hyphal length
FA=π r2×total hyphal length×%FDA active BT= 4
3π r
3×total bacteria#
BA= 4
3π r
3
×active bacteria#
The TA/AFB biovolumes ratio was then calculated. As noted in Section 1, the rationale for this approach was the observation that with lower nutrient availabil-ity and more heterogeneous resources the filamentous fungi will allocate more resources to hyphal extension at the expense of cytoplasm synthesis. In addition, as noted by Klein et al. (1998), in more mature systems the active cytoplasm will be more bacterial than fun-gal dominated. In addition, with externally imposed physical and chemical changes (physical disturbance, metals, N additions), the hyphal lengths often will be decreased. The TA/AFB biovolumes ratio value is sug-gested to provide an integrated index of filamentous fungal–bacterial development, which will be increased in more successionally developed soil systems, and which will be decreased, on a comparative basis, when these soils are impacted by externally imposed physi-cal and chemiphysi-cal changes.
2.6. Comparative analyses and statistics
The bacterial (TA/AFB) biovolumes ratio values for the summer and autumn of 1995 were compared with FE-carbon data completed by Klein et al. (1998) and with plant community and soil characteristics for these sites as described by Paschke et al. (2000). The TA/AFB and FE-carbon data were correlated with individual soil sample characteristics using the SAS System CORR procedure (SAS Institute, Cary, NC). The TA/AFB calculations given in Tables 1–4 were based on treatment means to demonstrate the method and therefore do not exactly match the TA/AFB mean values presented in Fig. 1.
3. Results
Table 1
Biovolumes in microscopically determined bacteria and fungi for the summer sampling of an uncultivated late successional (LS) site, northeastern Colorado, 1995, used in TA/AFB calculations to demonstrate methoda
Biovolume Units Soil treatments
None N
FT (Fungi total) Length (cm/g) 5601.715 8,013.25
Diameter (mm) 2.5 2.5
Volume (mm3) 274,978,704 393,356,874
FA (Fungal active) Length (cm g) 52.7 109.78
Volume (mm3) 2,586,937 5,338,915
BT (Bacteria total) log (g−1) 7.95 7.79
Diameter (1.0mm) (0.5236mm3 per cell)
46,665,899 32,284,914
BA (Bacteria active) log (g−1) 7.16 7.18
Volume (mg3) 7,568,322 7,925,007
Calculation
TA=(FT+BT)/(FA+BA) 321,644,603/10,155,259=31.67 425,641,788/13,313,922=31.97
AFB=FA/BA 2,586,937/7,568,322=0.34 5,338,915/7,925,007=0.68
TAB/AFB 31.67/0.34=93.15 31.97/0.68=47.01
aFor statistical analyses see Klein et al. (1998). The TA/AFB calculation procedure and soil nitrogen treatment are described in the text.
Table 2
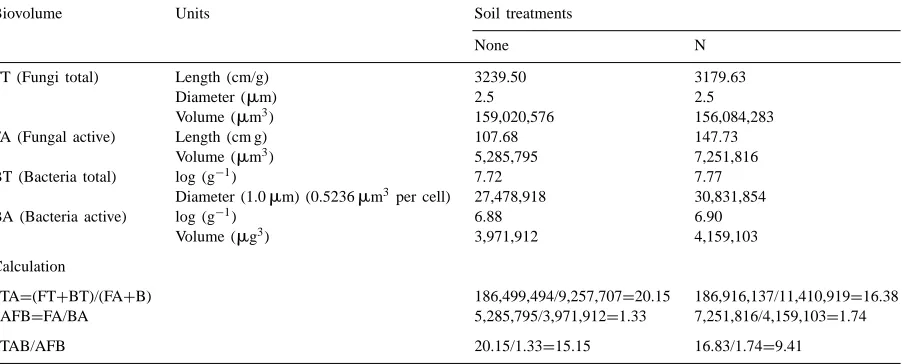
Biovolumes in microscopically determined bacteria and fungi for the summer sampling of early successional (ES) sites, northeastern Colorado, l995, used in TA/AFB calculations to demonstrate methoda
Biovolume Units Soil treatments
None N
FT (Fungi total) Length (cm/g) 3239.50 3179.63
Diameter (mm) 2.5 2.5
Volume (mm3) 159,020,576 156,084,283
FA (Fungal active) Length (cm g) 107.68 147.73
Volume (mm3) 5,285,795 7,251,816
BT (Bacteria total) log (g−1) 7.72 7.77
Diameter (1.0mm) (0.5236mm3 per cell) 27,478,918 30,831,854
BA (Bacteria active) log (g−1) 6.88 6.90
Volume (mg3) 3,971,912 4,159,103
Calculation
TA=(FT+BT)/(FA+B) 186,499,494/9,257,707=20.15 186,916,137/11,410,919=16.38
AFB=FA/BA 5,285,795/3,971,912=1.33 7,251,816/4,159,103=1.74
TAB/AFB 20.15/1.33=15.15 16.83/1.74=9.41
aFor statistical analyses see Klein et al. (1998). The TA/AFB calculation procedure and soil nitrogen treatment are described in the text.
presented in Tables 1–4. These summarize the micro-scopically determined fungal total and fungal active biovolume (hyphal lengths per gram dry weight of soil) and total and active bacterial (log numbers per gram) biovolumes and the calculated TA/AFB values
Table 3
Biovolumes in microscopically determined bacteria and fungi for the autumn sampling of an uncultivated late successional (LS) site, northeastern Colorado, 1995, used in TA/AFB calculations to demonstrate methoda
Biovolume Units Soil treatments
None N
FT (Fungi total) Length (cm/g) 5,288.65 4,404.54
Diameter (mm) 2.0 2.0
Volume (mm3) 166,148,228 138,373,029
FA (Fungal active) Length (cm g) 107.25 124.01
Volume (mm3) 3,369,366 3,895,898
BT (Bacteria total) log (g−1) 8.16 8.17
Diameter (1.0mm) (0.5236mm3per cell) 75,683,226 77,446,115
BA (Bacteria active) log (g−1) 7.54 7.54
Volume (mg3) 18,155,141 18,155,142
Calculation
TA=(FT+BT)/(FA+BA) 241,831,454/21,524,507=11.24 215,819,144/22,051,039=9.79
AFB=FA/BA 3,369,366/18,155,141=0.19 3,895,898/18,155,141=0.21
TAB/AFB 11.24/0.19=59.16 9.79/0.21=46.62
aFor statistical analyses see Klein et al. (1998). The TA/AFB calculation procedure and soil nitrogen treatment are described in the text.
with soil disturbance, from 93.15 to 59.16 which were directly related to a major decrease in the total fungal hyphal lengths.
Similar data for the autumn sampling of the of the uncultivated LS and the ES site, for the unamended and N-treated subplots, are summarized in Tables 3 and 4. The physical disturbance, which caused a halving of
Table 4
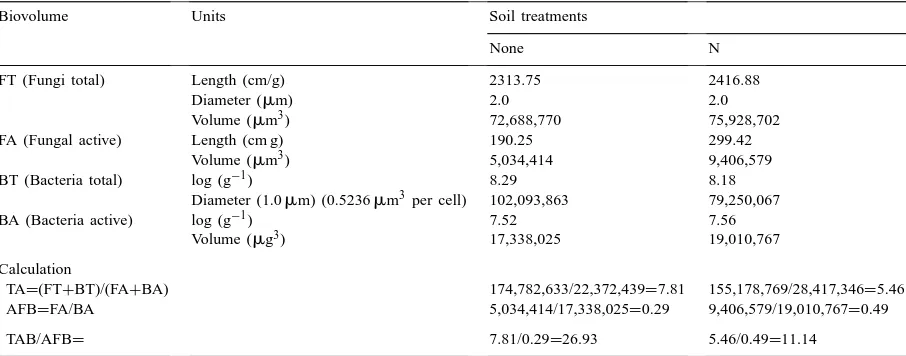
Biovolumes in microscopically determined bacteria and fungi for the autumn sampling of an early successional (ES) site, northeastern Colorado, 1995, northeastern Colorado, 1995, used in TA/AFB calculations to demonstrate methoda
Biovolume Units Soil treatments
None N
FT (Fungi total) Length (cm/g) 2313.75 2416.88
Diameter (mm) 2.0 2.0
Volume (mm3) 72,688,770 75,928,702
FA (Fungal active) Length (cm g) 190.25 299.42
Volume (mm3) 5,034,414 9,406,579
BT (Bacteria total) log (g−1) 8.29 8.18
Diameter (1.0mm) (0.5236mm3per cell) 102,093,863 79,250,067
BA (Bacteria active) log (g−1) 7.52 7.56
Volume (mg3) 17,338,025 19,010,767
Calculation
TA=(FT+BT)/(FA+BA) 174,782,633/22,372,439=7.81 155,178,769/28,417,346=5.46
AFB=FA/BA 5,034,414/17,338,025=0.29 9,406,579/19,010,767=0.49
TAB/AFB= 7.81/0.29=26.93 5.46/0.49=11.14
aFor statistical analyses see Klein et al. (1998). The TA/AFB calculation procedure and soil nitrogen treatment are described in the text.
the fungal lengths on the disturbed site in comparison with the LS site soils, resulted in a distinct decrease in the TA/ABF ratio.
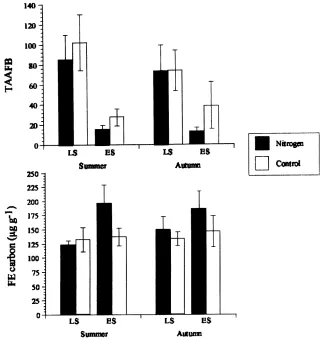
Fig. 1. TA/AFB ratios (top) and fumigation–extraction (FE) carbon (bottom) values for nitrogen-amended and control late successional (LS) and early successional (ES) sites from northeastern Colorado, 1995. See Klein et al. (1998) for FE procedural details. Standard error bars are given.
because: (1) the ratio of TA biovolume decreased in three of four cases and (2) the proportion of the AFB biovolume increased in all cases, when control and N-amended plot treatment means were compared.
The statistical analyses of the TA/AFB biovolumes ratio values, based on the individual soil sample char-acteristics, and of the corresponding FE-carbon val-ues, are summarized in Fig. 1. As noted in the upper panel, the decreased TA/AFB biovolume ratio val-ues observed at the ES sites, in comparison with the higher uncultivated LS site values, indicate that the physical disturbance, which occurred 6 years earlier, was still negatively affecting the TA/AFB biovolume ratio values. The data for the comparative N-treated
plots indicate that the N addition caused only slight further decreases in these TA/AFB values, a trend also observed in Tables 1–4, in comparison with the values observed for the untreated plots. In comparison (lower panel), the FE-carbon values for the various unamended sites did not show significant differences, in spite of the major decreases in total hyphal lengths which had occurred with disturbance. The N-amended soils did show a trend towards increased FE-carbon values which may reflect the higher active fungal plus bacterial biovolume values (and decreased TA/AFB biovolumes ratio values) observed for these sites.
Table 5
TA/AFB ratio and fumigation extraction (FE) carbon correlations with site parameters total plant biomass (TPB), grass biomass (GB), annual grass biomass (AGB), soil carbon (SC), total ion ex-change resin (IER) nitrate (NO3−), ammonium ion (NH4+), and
total IER nitrogen for combined data from combined early succes-sional and late successucces-sional shortgrass steppe sites, northeastern Colorado, 1995a
Site parameter TA/AFB ratio FE-C
Summer Autumn Summer Autumn
TPB 0.72 0.42 −0.33
0.0001 0.0198 0.0655 NS
GB 0.74 0.31 −0.33
<0.0001 0.0853 0.0613 NS
AGB 0.68 0.30 −0.32
<0.0001 0.0973 0.0771 NS
Soil C −0.33
0.0680 NSb NS NS
IER 0.49 0.53 −0.53 −0.50
NO3− 0.0042 0.0022 0.0020 0.0038
IER 0.38 0.46 −0.44
NH4+ 0.0325 0.0089 NS 0.0118
IER 0.45 0.52 −0.42 −0.49
Total N 0.0090 0.0030 0.0165 0.0049
aSee Paschke et al. (2000) for procedural details. Pearson
correlation coefficients are given followed by probability.
bNS=p=>0.10 (N=32).
(2000). The correlations of these results with the TA/AFB and FE-carbon values are presented in Table 5. These results indicate that the TA/AFB biovolumes ratio values showed strong correlations with criti-cal indices of plant community structure (total plant biomass, grass biomass, and annual grass biomass) and mineral nitrogen measurements. The correlations with the FE-carbon were primarily negative and were most evident with the summer sampling. These cor-relations suggest that this TA/AFB biovolumes ratio may be useful as an indicator of plant–soil system development.
4. Discussion
There is a worldwide interest in better understand-ing the structure and function of the soil microbial community, especially in relation to maintaining and
improving soil quality, health and resilience. Single value measurements such as microbial biomass C, specific chemical compounds or microbial activity in-dices have been used in most of these studies. These widely used single-value measurements, however, do not provide information on the physical structure of the filamentous fungal–bacterial community, includ-ing (1) the total biovolume within which the active biovolume is functioning, and (2) the portion of the active biovolume which is fungal versus bacterial.
The occurrence of empty fungal (Newell, 1992; Thorn, 1997) and bacterial (Zweifel and Hagström, 1995) structures emphasizes the need to document total versus active biovolumes of microbes in a soil, as carried out in the present study. Frankland (1974) assessed the portion of the filamentous fungi which represented such empty or ‘ghost’ hyphae and divided the total hyphal length values into ‘live’ and ‘dead’ portions; only 15–37% of the hyphae in these sam-ples were scored as active. Söderström (1979) found that only 2.4–4.3% of observed hyphal lengths from their samples were active, considered to be a ‘minor fraction.’ Frankland et al. (1990) and Stahl and Parkin (1996) also have emphasized that the fungal compo-nent is best considered in terms of its active biovolume; this same point has been made for the bacteria by Macdonald (1980). This is particularly critical as the filamentous fungi are indeterminate organisms (An-derson and Kohn, 1998; Rayner et al., 1999) which also have been characterized as being non-discrete (Klein et al., 1998) or modular (Carlisle, 1994). The TA/AFB biovolumes ratio method, described in this communication, has been evaluated based on the use of this shortgrass steppe site where possible effects of cultivation and nitrogen-amendments on this ratio were evaluated.
become similar to that found in the undisturbed sys-tem. Changes in this ratio also may be able to serve as indicator of responses which will subsequently be observed in slower-responding components of the plant–soil system, such as soil carbon, a process discussed by Anderson (1994).
Nitrogen accretion has been found to have many negative effects on soil microbial communities (Söder-ström et al., 1983; Turner and Newman, 1984; Fred-erick and Klein, 1994; Klein et al., 1996, Berg et al., 1998; Bardgett and McAlister, 1999). The TA/AFB ratios method, based both on the demonstration cal-culations shown in Tables 1–4 and in Fig. 1, indicate that N additions caused slight decreases in this ratio, in comparison with the unamended plot results, which were most evident in the ES soils. This was related to an increase in the active biovolume values in these N-amended soils, which was primarily related to in-creased active fungal biovolume values. The trend towards increased active biovolume and decreased TA/AFB values indicates that this may provide a useful comparative indicator of possible microbial community changes related to nitrogen accretion, as increased nitrogen levels have been suggested to be impacting plant communities and their associated soil microbes on a global basis (Vitousek et al., 1997).
The TA/AFB biovolumes ratio values also showed interesting correlations with plant community char-acteristics on these sites, which were not shown by FE carbon values (Table 5). The strong relationships of the TA/AFB Ratio values to indices of plant com-munity development, including total plant biomass, grass biomass and annual grass biomass, as well as to mineral nitrogen indices determined by ion exchange resin methods, suggest that this measurement may be able to serve as a general index of plant community development. This also may be of potential value for comparing relative plant community-microorganism development in different ecosystems.
The use of ratios, as carried out in the present study, is based on previous investigations where microbial community characteristics have been normalized by use of this approach. Norton and Firestone (1991) used ratios in a study of rhizosphere and non-rhizosphere soil in ponderosa pine microcosms. In their inves-tigation, the ratio of active to total fungal hyphal lengths was used, and up to 40–50% of the fungal hyphae were felt to be active. Staben et al. (1997)
used ratios to evaluate active fungal and bacterial biomass in conservation reserve program (CRP) and wheat-fallow (W-F) soils, where higher ratios were found in CRP than W-F soils. Increases in this ratio also have been observed when no-till and tilled soils have been compared (Beare et al., 1992). Macdonald (1980) used total and active bacterial ratios in a study of catabolism in soil microorganisms, and Bardgett and McAlister (1999) used the ratio of the 18 fungal 2v6-phospholipid fatty acid (PFLA) to 9 bacterial
PFLAs to represent the fungal–bacterial biomass in a soil. As a further application of this approach, Trasar-Cepeda et al. (1998) developed a soil quality index where microbial biomass C, mineralizable N and soil enzyme activities (esterase and urease) were related to total N and C in a series of Galacian soils. Dilly and Munch (1998) used ratios between micro-bial biomass and activity to characterize soil micromicro-bial communities, these ratios were felt to be more useful than microbiological features related to soil weight.
This TA/AFB biovolumes ratio approach is based on the use of microscopy, a methodology which requires patience and intensive, long-term effort for its success-ful application. As discussed by Bottomley (1994), there are a variety of procedural concerns which in-fluence the use of microscopy for the examination of microbial communities in soils. These relate to the need to disrupt the soil matrix to recover the hyphal units in the soil, as well as possible hyphal disrup-tion which can occur in the soil preparadisrup-tion steps. Another concern is that of ‘dwarf’ cells, which may not be enumerated in this procedure. These cells, with biovolumes of less that 0.07mm3(Bakken and Olsen,
1986), considered to dominant in terms of numbers in many environments, may represent 10–20% of the bacterial biovolume. With additional development of these microscopic procedures, it should be possible to more fully assess these portions of the microbial com-munity to improve the robustness of this microscopic approach.
index of filamentous fungal–bacterial community de-velopment and particularly concerning the structural characteristics of the indeterminate filamentous fungi. For these unique organisms, structure and function are inextricably linked, as the filamentous fungal en-tity is both the hyphal volume which is present and the active cytoplasmic biovolume which is able to move within this network. Information concerning these unique characteristics of the filamentous fungi is only obtainable at the present time by direct ob-servational techniques, embodied by the microscopy based TA/AFB biovolumes ratio method.
Based on the experiments which have been carried out at these disturbed and nitrogen-amended short-grass steppe sites, the TA/AFB biovolumes ratio is suggested to provide an easily understood index which will allow the indeterminate nature of the filamentous fungi and the associated bacteria to be characterized. This should provide useful, unique information for studies of soil quality, health and resilience.
Acknowledgements
This study was carried out with support from the USDA-NRICGP under Awards 93-37101-8601 and 97-35101-4317. The assistance of E.F. Redente and T. McLendon with maintenance of the field sites used in these studies is appreciated. J.L. Lowell, C.A Lozupone and S.P. Klein provided valuable comments during the preparation of the manuscript.
References
Alphei, J., Bonkowski, M., Scheu, S., 1995. Application of the selective inhibition method to determine bacterial:fungal ratios in three beechwood soils rich in carbon-optimization of inhibitor concentrations. Biol. Fertil. Soils 19, 173–176.
Anderson, J.B., Kohn, L.M., 1998. Genotyping, gene genealogies and genomics bring fungal population genetics above ground. TREE 13, 444–448.
Anderson, J.P.E., Domsch, K.H., 1973. Quantification of bacterial and fungal contributions to soil respiration. Arch. Mikrobiol. 93, 113–127.
Anderson, J.P.E., Domsch, K.H., 1975. Measurement of bacterial and fungal contributions to respiration of selected agricultural and forest soils. Can. J. Microbiol. 21, 314–322.
Anderson, J.P.E., Domsch, K.H., 1978. A physiological method for the quantitative measurement of microbial biomass in soils. Soil Biol. Biochem. 10, 215–221.
Anderson, J.P.E., Armstrong, R.A., Smith, S.N., 1981. Methods to evaluate pesticide damage to the biomass of the soil microflora. Soil Biol. Biochem. 13, 149–153.
Anderson, T.-H., 1994. Physiological analysis of microbial communities in soil: applications and limitations. In: Ritz, K., Dighton, J., Giller, K.E. (Eds.), Beyond the Biomass. Wiley, Chichester, UK, pp. 67–76.
Arnolds, E., 1991. Decline of ectomycorrhizal fungi in Europe. Agric. Ecosyst. Environ. 35, 209–244.
Babiuk, L.A., Paul, E.A., 1970. The use of fluorescein isothiocyanate in the determination of the bacterial biomass of a grassland soil. Can. J. Microbiol. 16, 57–62.
Bakken, L.R., Olsen, R.A., 1986. Dwarf cells in soil — a result of starvation of ‘normal’ bacteria, or a separate population? In: Proceedings of the IVth International Society of Microbiological Ecology (ISME), pp. 561–566.
Bardgett, R.D., McAlister, E., 1999. The measurement of soil fungal:bacterial biomass ratios as an indicator of ecosystem self-regulation in temperate meadow grasslands. Biol. Fertil. Soils 29, 282–290.
Beare, M.H., Neeley, C.L., Coleman, D.C., Hargrove, W.L., 1991. Characterization of a substrate-induced respiration method for measuring fungal, bacterial and total microbial biomass on plant residues. Agric. Ecosyst. Environ. 34, 65–73.
Beare, M.H., Parmelee, R.W., Hendrix, P.F., Cheng, W., Coleman, D.C., Crossley Jr., D.A., 1992. Microbial and faunal interactions and effects on litter nitrogen and decomposition in agroecosystems. Ecol. Monogr. 62, 569–591.
Beare, M.H., Pohlad, B.R., Wright, D.H., Coleman, D.C., 1993. Residue placement and fungicide effects on fungal communities in conventional and no-tillage soils. Soil Sci. Soc. Am. J. 57, 392–399.
Berg, M.P., Kniese, J.P., Verhoef, H.A., 1998. Dynamics and stratification of bacteria and fungi in the organic layers of a Scots pine forest soil. Biol. Fertil. Soils 22, 313–322. Blagodatskaya, E.V., Anderson, T.-H., 1998. Interactive effects of
pH and substrate quality on the fungal-to-bacterial ratio and QCO2 of microbial communities in forest soils. Soil Biol.
Biochem. 30, 1269–1274.
Bottomley, P.J., 1994. Light microscopic methods for studying soil microorganisms. In: Weaver, R.W. (Ed.), Methods of Soil Analysis. Part 2. Microbial and Biochemical Properties. SSSA Book Series, No. 5, Soil Society of America, Madison, WI, pp. 81–105.
Brussaard, L., Behan Pelletier, V.M., Bignell, D.E., Brown, V.K., Didden, W., Folgarait, P., Fragoso, C., Freckman, D.W., Gupta, V.V.S.R., Hattori, T., Hawksworth, D.L., Klopatek, C., Lavelle, P., Malloch, D.W., Rusek, J., Söderström, B., Tiedje, J.M., Virginia, R.A., 1997. Biodiversity and ecosystem functioning in soil. Ambio 26, 563–570.
Carlisle, M.J., 1994. The success of the hypha and mycelium. In: Gow, N.A.R., Gadd, G.M. (Eds.), The Growing Fungus. Chapman & Hall, London, UK, pp. 3–19.
Dick, R.P., 1992. A review: long-term effects of agricultural systems on soil biochemical and microbial parameters. Agric. Ecosyst. Environ. 40, 25–36.
Dilly, O., Munch, J.-C., 1998. Ratios between estimates of microbial biomass content and microbial activity in soils. Biol. Fertil. Soils 27, 374–379.
Doran, J.W., Jones, A.J., 1996. Methods for Assessing Soil Quality. Soil Science Society of America, Madison, WI.
Doran, J.W., Sarrantonio, M., Liebig, M.A., 1996. Soil health and sustainability. Adv. Agron. 56, 1–54.
Duah-Yentumi, S., Johnson, D.B., 1986. Changes in soil microflora in response to repeated applications of some pesticides. Soil Biol. Biochem. 18, 629–635.
Elliott, L.F., Lynch, J.M., Papendick, R.I., 1998. The microbial component of soil quality. In: Stotzky, G. Bollag, J.-M. (Eds.), Soil Biochemistry, Vol. 9. Marcel Dekker, New York, pp. 1–21. Frankland, J.C., 1974. Importance of phase-contrast microscopy for estimation of total fungal biomass by the agar-film technique. Soil Biol. Biochem. 6, 409–410.
Frankland, J.C., 1975. Estimation of live fungal biomass. Soil Biol. Biochem. 7, 339–340.
Frankland, J.C., 1990. Ecological methods of observing and quantifying soil fungi. Trans. Mycol. Soc. Jpn. 31, 89–101. Frankland, J.C., Dighton, J., Boddy, L., 1990. Methods for studying
fungi in soil and forest litter. Methods Microbiol. 22, 343–404. Frederick, B.A., Klein, D.A., 1994. Nitrogen effects on rhizosphere processes of range grasses from different successional seres. Plant Soil 161, 241–250.
Griffin, D.M., 1972. Ecology of Soil Fungi. Syracuse University Press, Syracuse, New York.
Gupta, V.V.S.R., Germida, J.J., 1988. Distribution of microbial biomass and its activity in different soil aggregate size classes as affected by cultivation. Soil Biol. Biochem. 20, 777–786. Holland, E.A., Coleman, D.C., 1987. Litter placement effects on
microbial and organic matter dynamics in an agroecosystem. Ecology 68, 425–433.
Horwath, W.R., Paul, E.A., 1994. Microbial biomass. In: Weaver, R.W. (Ed.), Methods of Soil Analysis. Part 2. Microbiological and Biochemical Properties. SSSA Book Series, No. 5. Soil Science Society of America, Madison, WI, pp. 753–773. Ingham, E.R., Klein, D.A., 1984a. Soil fungi: relationships between
hyphal activity and staining with fluorescein diacetate. Soil Biol. Biochem. 16, 273–278.
Ingham, E.R., Klein, D.A., 1984b. Soil fungi: measurement of hyphal length. Soil Biol. Biochem. 16, 279–280.
Ingham, E.R., Coleman, D.C., Moore, J.C., 1989. Analysis of food-web structure and function in a shortgrass prairie, a mountain meadow, and a lodgepole pine forest. Biol. Fertil. Soils 8, 29–37.
Jenkinson, D.S., Ladd, J.N., 1981. Microbial biomass in soil: measurement and turnover. In: Paul, E.A., Ladd, J.N. (Eds.), Soil Biochemistry, Vol. 5. Marcel Dekker, New York, pp. 415–471.
Klein, D.A., Frederick, B.A., Redente, E.F., 1989. Fertilizer effects on microbial communities and organic matter in the rhizosphere of Sitanion hystrix and Agropyron smithii. Arid Soil Res. Rehab. 3, 397–404.
Klein, D.A., McLendon, T., Paschke, M.W., Redente, E.F., 1995. Saprophytic fungal–bacterial growth pattern variations in successional communities of a semi-arid steppe ecosystem. Biol. Fertil. Soils 19, 253–256.
Klein, D.A., McLendon, T., Paschke, M.W., Redente, E.F., 1996. Nitrogen availability and fungal–bacterial responses in successional semi-arid steppe soils. Arid Soil Res. Rehab. 10, 321–332.
Klein, D.A., Paschke, M.W., Redente, E.F., 1998. Assessment of fungal–bacterial development in a successional shortgrass steppe by direct integration of chloroform-fumigation extraction (FE) and microscopically derived data. Soil Biol. Biochem. 30, 573–581.
Lin, Q., Brookes, P.C., 1996. Comparison of methods to measure microbial biomass in unamended, ryegrass-amended and fumigated soils. Soil Biol. Biochem. 28, 939–945. Lodge, D.J., Ingham, E.R., 1991. A comparison of agar film
techniques for estimating fungal biovolumes in litter and soil. Agric. Ecosyst. Environ. 34, 131–144.
Lundgren, B., 1984. Size classification of soil bacteria: effects on microscopically-estimated biovolumes. Soil Biol. Biochem. 16, 283–284.
Lynch, J.M., 1984. Interactions between biological processes, cultivation and soil structure. Plant Soil 76, 307–318. Macdonald, R.M., 1980. Cytochemical demonstration of
catabolism in soil microorganisms. Soil Biol. Biochem. 12, 419–423.
McGonigle, T.P., Miller, M.H., 1996. Development of fungi below ground in association with plants growing in disturbed and undisturbed soils. Soil Biol. Biochem. 28, 263–269.
McLean, M.A., Parkinson, D., 1997. Changes in structure, organic matter and microbial activity in pine forest soil following the introduction of Dendrobaena octaedra (oligochaeta, lumbricidae). Soil Biol. Biochem. 29, 537–540.
Miller, M., Palojärvi, A., Rangger, A., Reeslev, M., Kjøller, A., 1998. The use of fluorogenic substrates to measure fungal presence and activity in soil. Appl. Environ. Microbiol. 64, 613–617.
Newell, S.Y., 1992. Estimating fungal biomass and productivity in decomposing litter. In: Carroll, G.C., Wicklow, D.T. (Eds.), The Fungal Community. Its Organization and Role in the Ecosystem, 2nd Edition. Marcel Dekker, New York, pp. 521–561. Nordgren, A., Bååth, E., Söderström, B., 1983. Microfungi and
microbial activity along a heavy metal gradient. Appl. Environ. Microbiol. 45, 1829–1837.
Norton, J.M., Firestone, M.K., 1991. Metabolic status of bacteria and fungi in the rhizosphere of ponderosa pine seedlings. Appl. Environ. Microbiol. 57, 1161–1167.
Paschke, M.W., McLendon, T., Redente, E.F., 2000. Nitrogen availability and old-field succession in a shortgrass steppe. Ecosystems 3, 144–158.
Paustian, K., Schnürer, J., 1987. Fungal growth response to carbon and nitrogen limitation: a theoretical model. Soil Biol. Biochem. 19, 613–620.
Rayner, A.D.M., Beeching, J.R., Crowe, J.D., Watkins, Z.R., 1999. Defining individual fungal boundaries. In: Worrall, J.J. (Ed.), Structure and Dynamics of Fungal Populations. Kluwer Academic Publishers, Boston, pp. 19–42.
Rice, C.W., Moorman, T.B., Beare, M., 1996. Role of microbial biomass carbon and nitrogen in soil quality. In: Doran, J.W., Jones, A.J. (Eds.), Methods for Assessing Soil Quality. Soil Science Society of America, Madison, WI, pp. 203–215.
Seybold, C.A., Herrick, J.E., Brejda, J.J., 1999. Soil resilience: a fundamental component of soil quality. Soil Sci. 164, 224– 234.
Smith, J.L., Papendick, R.I., Bezdicek, D.F., Lynch, J.M., 1993. Soil organic matter dynamics and crop residue management. In: Metting Jr., F.B. (Ed.), Soil Microbial Ecology: Applications in Agricultural and Environmental Management. Marcel Dekker, New York, pp. 65–94.
Snyder, J.D., Trofymow, T.A., 1984. A rapid accurate wet oxidation diffusion procedure for determining organic and organic carbon in plant and soil samples. Comm. Soil Plant Analysis 15, 587– 597.
Söderström, B.E., 1979. Seasonal fluctuations of active fungal biomass in horizons of a podzolized pine-forest soil in central Sweden. Soil Biol. Biochem. 11, 149–154.
Söderström, B.E., Bååth, E., Lundgren, B., 1983. Decrease in soil microbial activity and biomasses owing to nitrogen amendments. Can. J. Microbiol. 29, 1500–1506.
Staben, M.L., Bezdicek, D.F., Smith, J.L., Fauci, M.F., 1997. Assessment of soil quality in conservation reserve program and wheat-fallow soils. Soil Sci. Soc. Am. J. 61, 124–130. Stahl, P.D., Parkin, T.B., 1996. Relationship of soil ergosterol
concentration and fungal biomass. Soil Biol. Biochem. 28, 847– 855.
Stamatiadis, S., Doran, J.W., Ingham, E.R., 1990. Use of staining and inhibitors to separate fungal and bacterial activity in soil. Soil Biol. Biochem. 22, 81–88.
Stenberg, B., Pell, M., Torstensson, L., 1998. Integrated evaluation of variation in biological chemical and physical soil properties. Ambio 27, 9–15.
Tate, K.R., Ross, D.J., Feltham, C.W., 1988. A direct extraction method to estimate soil microbial C: effects of experimental variables and some different calibration procedures. Soil Biol. Biochem. 20, 329–335.
Thorn, G., 1997. The fungi in soil. In: van Elsas, J.D., Trevors, J.T., Ellington, E.M.H. (Eds.), Modern Soil Microbiology. Marcel Dekker, New York, pp. 63–127.
Torstensson, L., Pell, M., Stenberg, B., 1998. Need of a strategy for evaluation of arable soil quality. Ambio 27, 4–8. Trasar-Cepeda, C., Leirós, C., Gil-Sotres, F., Seoane, S., 1998.
Towards a biochemical quality index for soils: an expression relating several biological and biochemical properties. Biol. Fertil. Soils 26, 100–106.
Turner, S.M., Newman, E.I., 1984. Fungal abundance on Lolium perenne roots: influence of nitrogen and phosphorus. Trans. Br. Mycol. Soc. 82, 315–322.
Van Veen, J.A., Paul, E.A., 1979. Conversion of biovolume measurements of soil organisms, grown under various moisture tensions, to biomass and their nutrient content. Appl. Environ. Microbiol. 37, 686–692.
Vance, E.D., Brookes, P.C., Jenkinson, D.S., 1987. An extraction method for measuring soil microbial biomass C. Soil Biol. Biochem. 19, 703–707.
Vitousek, P.M., Aber, J.D., Howarth, R.W., Likens, G.E., Matson, P.A., Schindler, D.W., Schlesinger, W.H., Tilman, G.D., 1997. Human alteration of the global nitrogen cycle: causes and consequences. Ecol. Appl. 7, 737–750.
Worrall, J.J., 1999. Brief introduction to fungi. In: Worrall, J.J. (Ed.), Structure and Dynamics of Fungal Populations. Kluwer Academic Publishers, Boston, pp. 1–18.
Zhu, W.-X., Carreiro, M.M., 1999. Chemoautotrophic nitrification in acidic forest soils along an urban-to-rural transect. Soil Biol. Biochem. 31, 1091–1100.