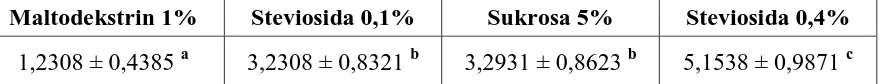POTENCY OF STEVIOSIDE FROM Stevia rebaudiana (Bert.)
demand of natural sweetener. Stevia rebaudiana (Bert.) produces steviol glycosides included stevioside that have properties as natural sweetener. The aims of this study were to investigate extraction method effectiveness based on stevioside content, to compare pre-formulation of stevioside sweetener and sucrose solution, and to investigate hypoglycemic potency of ethanol extract of Stevia leaf. The method used was solvent extraction by maseration and soxhlet extraction followed by decolorization pigment and crystallization of steviol glycoside. Stevioside content analysis based on High Performance Liquid Chromatography (HPLC). Hedonic test was used for comparing sucrose solution and pre-formulation of stevioside sweetener, and glucose tolerance method was used to investigate hypoglycemic test of ethanol extract of stevia leaf. The results showed that maseration extraction method was more effective to extract stevioside than soxhlet extraction and 0.1% pre-formulation stevioside solution had the same sweet taste with 5 % sucrose solution. Concentration 0.7 kg/mg BW of ethanol extract of stevia leaf decreased ± 27% blood glucose level in mice.Key words: stevioside, Stevia rebaudiana (Bert.), natural sweetener
INTRODUCTION
The worldwide demand for high potency sweeteners is increasing and, with blending of different sweeteners becoming a standard practice, the demand for alternatives is expected to increase. The sweet herb, Stevia rebaudiana Bertoni, produces an alternative sweetener with the added advantage that Stevia sweeteners are natural plant products. In addition, the sweet steviol glycosides have functional and sensory properties superior to those of many high potency sweeteners.
Stevia leaves contain diterpene glycosides, namely, stevioside, rebaudiosides A-F, steviolbioside, and dulcoside A (Figure 1), which are responsible for the typical sweet taste. Of the four major diterpene glycoside sweeteners present in Stevia leaves only two, stevioside and rebaudioside A, have had their physical and sensory properties well characterized. Stevioside is between 110 and 270 times sweeter than sucrose. (Brandle et all., 2005).
they are used as dietary supplements. For example, the Japanese and Koreans have used annually in recent years about 200 and 115 tons of stevia extracts, respectively. (Gardana et all., 2003). Extract of the plant Stevia rebaudiana Bertoni have been used for many years in the treatment of diabetes in South America (Gregersen et all., 2004). Therefore, it was considered of interest to investigate extraction method effectiveness based on stevioside content, to compare pre-formulation of stevioside sweetener and sucrose solution, and to investigate hypoglycemic potency of ethanol extract of Stevia leaf.
Figure 1. Chemical structures of the main Stevia rebaudiana (Bert.) sweetener and their aglycon steviol.
MATERIALS AND METHOD
Chemicals. Stevia rebaudiana (Bert.) leafs were taken from Tawangmangu, Karanganyar, Central Java. Ethanol, ether, ethyl acetate, hexane (technical grade), methanol, acetonitrile (LC grade, Merck), Stevioside standard (Wako, Japan, 99.2%).
Sample preparation.
Stevia rebaudiana (Bert.) leafs were cleaned and dried under 50 οC for 24 hours. Dried leafs were pulverized with grinder. Sample was defatted with hexane by soxhlet extraction.
Sample extraction
Maseration Method
Continuous Extraction by soxhlet
100 g defatted sample were extracted by soxhlet with 1000 mL ethanol at 90°C until clear solution gained (Sx).
Both, ethanol solution from Ms and Sx ectraction were concentrated by rotary evaporator. Concentrated solutions were added by water (1:1 v/v) and ready to be declhorophyllated.
Sample Dechlorophyllation (Jumpatong et all., 2006)
Dechlorophyllation was used to decolourise solution especially from chlorophyll pigment. The method used was electrocoagulation. The conditions for electrocoagulation were as follows: two clean aluminium plates, each of 3x15 cm dimensions were used as electrodes. These were spaced 1.5 cm apart and dipped 7 cm into the magnetically-stirred solution containing 0.1% (w/v) NaCl as supporting electrolyte. Direct current (0.9 A, 16.9-31.6 V) from a power supply was then passed via the two electrodes through the solution. After 2 x 4 hours of electrolysis, the mixture in the beaker was filtered. This step will produced yellowish clear solution. Then, solution was re-defatted by grading partition with ether (3 x 100 mL). Aqueous phase was taken and ready to be clarified.
Sample clarification and Crystallization
pH solution was adjusted with 50% citric acid until pH = 3.00. The solution was clarified with caoline and filtered. pH solution was adjusted to 10.5 with CaO and clarified again with caoline. Solution was filtered and adjusted to pH = 7.00 with 50% citric acid. Solution was re-defatted by ether (2 x 100 mL) and partitioned with ethyl acetate (5 x 100 mL). Organic phase was concentrated by rotary evaporator until crystal formed. For maximizing yield, solution was kept in refrigerator for 24 hours.
Crystal analysis
Thin Layer Chromatography (TLC) (Pasquel et all, 2000)
Stevioside identification in crystal was conducted by TLC and compared with standard. Stationary was silica plate 60 F254 and mobile phase was ethyl acetate: ethanol: water (130: mL/min and detected at 217 nm by UV Smart Line Knauer detector.
Spectral Analysis by Spectrophotometer
Pre-formulation
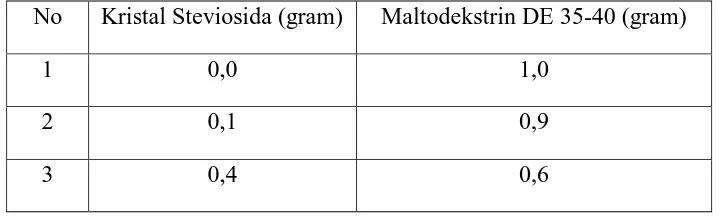
Stevioside crystal was covered with Pharmacoat 606, and added with carrier substance maltodextrine DE 35-40 with ratio as mention in Table 1.
Table 1. Pre-Formulation Stevioside Crystal With Maltodextrine DE 35-40
No Kristal Steviosida (gram) Maltodekstrin DE 35-40 (gram)
1 0,0 1,0
2 0,1 0,9
3 0,4 0,6
Organoleptic Test
Hedonic test method was used to investigate organoleptic receive from panelist. Pre-formulation crystals were dissolved in 100 mL. Hedonic test for sweeteness degree was done by 17 panelists. Six parameters were used for assessment, 6 = sweetest, 5 = very sweet, 4 = sweet, 3 = sweet enough, 2 = not sweet, and 1 = lack of sweet. Treatments were compared with 5% (w/v) sucrose solution.
Hypoglycemic Test
Glucose Tolerance Test (Yulinah et all., 2001)
Male Wistar mice (Biofarma, Yogyakarta) were divided in to five groups, negative control (starch 1%), sucrose, dose 0.3 g/kg bw of ethanol extract, dose 0.7 kg/kg bw of ethanol extract, and positive control saccharine 1 g/kg bw. Before test, mice were fasted for 18 hours, but were still given tap water. Each mice was treated with the treatments according to the groups and after one hour was given 10% glucose solution at dose 2 g/kg bw per oral. Blood glucose level was determined 30, 60, 90, and 120 minutes after glucose intake.
Blood Glucose Level Determination (Yulinah et all., 2001)
RESULT AND DISCUSSION
Extraction and Crystallization
Stevioside extraction was conducted through several steps such as defattitation, extraction, dechlorophyllation, clarification, and crystallization. Two methods were used to extract sample, maseration and soxhlet. According to Moraes and Macido (2001), ethanol could extract stevioside with clearly solution compared to methanol and water. Impurities elimination, such as fat, non-polar substances, must be done for forming stevioside crystal.
Dechlorophyllation was done to eliminate green color from pigment. Green color could affect visualization of crystal and reduce economical value. The result showed that dechlorophyllation by electrocoagulation could reduce 90.99% green color. This result was consistent with Jumpatong et all (2006) that electrocoagulation could eliminate green color more than 90%.
Beside impurities elimination, crystallization was affected by pH of solution. Treatment to adjust pH into extreme condition, for example from acid to base could form crystal (DuBois, 2005). Citric acid was used to eliminate metal, protein, and color impurities to produce better crystal physical characterization (Kumpar and Sampath, 1986). The result showed that maseration method was more effective to produce crystal than soxhlet extraction. Maseration method yields 0.65% crystal, while soxhlet extraction yields 0.45%. Beside on yield quantities, effectiveness was determined based on stevioside content in crystal.
Stevioside Identification and Quantitation
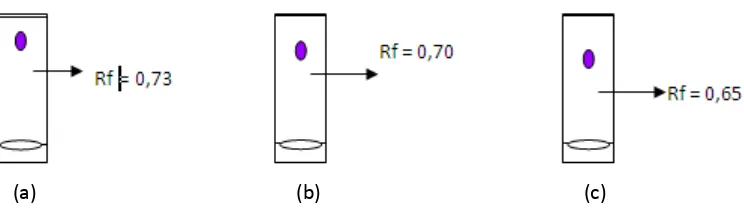
Qualitatively, extract component could be identified by TLC method (Pasquel et all., 2000). TLC profiles of crystal compared to standard were shown in Figure 2.
(a) (b) (c)
Figure 2. TLC profile of (a) standard; (b) crystal of Ms; (c) crystal of Sx
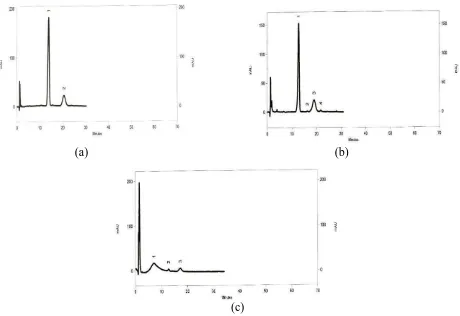
The result showed that Ms crystal had closer Rf (0.70) to standard (0.73) than Sx crystal (0.65). Ms crystal was identified higher similar to standard than Sx crystal. This result was supported by spectra analysis of Ms and Sx crystal compared to standard. The result showed that spectra of Ms crystal was much similar with standard than Sx crystal. The spectra profiles of Ms, Sx, and standard were shown in Figure 3. The identification was continuoued by HPLC method. Chromatogram profile of Ms and Sx crystal compared to standard shown in
profile with standar than Sx crystal. Event, Sx crystal chromatogram showed that stevioside compound was degraded. Identification of stevioside showed that maseration method was more effective than soxhlet to produce stevioside crystal.
Based on relative measured compared to standard by HPLC method, stevioside content in Ms crystal was higher than Sx crystal. Stevioside content in Ms crystal was 34.72% while in Sx crystal was 24.64%. This result showed that maseration method was more efective than soxhlet. Stevioside in Sx crystal was degraded that can be affected by high temperature factor. Soxhlet method used 90 °C to extract stevioside in sample. This step caused stevioside degradation (Pasquel et all., 2000).
(a) (b) (c)
Figure 3. Spectra profiles of (a) standard; (b) Ms crystal; (c) Sx crystal
Pre-formulation Stevioside Crystal
Stevioside crystal was pre-formulated with carier substance maltodextrine DE 35-40. Based on organoleptic result showed that 0.1% stevioside had the same sweet taste with 5% sucrose solution. While, 0.4 % stevioside was much sweeter than 5% sucrose solution. This result still can be optimized by improving pre-formulation with suitable binder and carrier. Beside, improving disolution crystal in water can increase sweet taste of solution and increasing sweeteness value of crystal in solution. The organoleptic results of each pre-formula were shown in Table 2.
Table 2. Organoleptic Test Result
Maltodekstrin 1% Steviosida 0,1% Sukrosa 5% Steviosida 0,4%
1,2308 ± 0,4385 a 3,2308 ± 0,8321 b 3,2931 ± 0,8623 b 5,1538 ± 0,9871 c
(a) (b)
(c)
Figure 4. Chromatogram profiles of (a) standard; (b) Ms crystal; (c) Sx crystal
Hypoglycemic Test
This test was conducted to investigate stevioside potency to reduce blood glucose level (hypoglycemic). This parameter could be used as parameter for antidiabetic agent potency. The result was shown in Table 3., Table 4., and Figure 5.
Table 3. Glucose Tolerance Test Ethanol Extract Stevia Leaf In Mice
Treatments
Glucose level (mg/dL)
Before 30 min 60 min 90 min 120 min
Control 63 120 117 108 109
Saccharine 1 g/kg BW 80 103 112 105 97
Sucrose 1 g/kg BW 95 134 158 176 189
Dose 0,3 g/kg BW 77 130 129 97 101
Dose 0,7 g/kg BW 81 129 119 103 94
Tolbutamid 50 mg/kg BW* 72,4 85,7 99 88,6 48
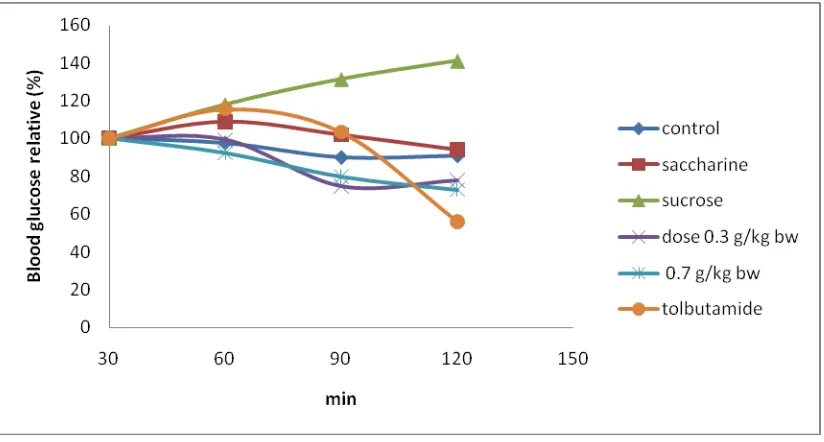
Figure 5 showed that sucrose increased blood glucose level at 120 min. Saccharine did not both increased or decreased blood glucose level. It means that saccharine as artificial sweetener is used only for increasing sweet taste but not for decreasing blood glucose level. Continuous over consumsion of saccharine in long periode can induced cancer cell (Mudjajanto, 2005).
Tabel 4. Blood Glucose Level Relative (%) After Glucose Intake
Treatments
Relatif kadar gula darah (%) setelah penambahan glukosa
30 menit 60 menit 90 menit 120 menit
Control 100 97,5 90 90,83
Saccharine 1 g/kg BW 100 108,74 101,94 94,17
Sucrose 1 g/kg BW 100 117,91 131,34 141,04
Dose 0,3 g/kg BW 100 99,23 74,62 77,69
Dose 0,7 g/kg BW 100 92,25 79,84 72,87
Tolbutamid 50 mg/kg BW * 100 115,52 103,38 56,01 *Source : Yulinah et all., 2001.
Figure 5. Blood Glucose Level Relative (%) After Glucose Intake
suppression of glucagon are the primary cause of antihyperglycemic effect of Stevia rebaudiana (Bert.) in diabetic rats. Stevioside containing in Stevia rebaudiana (Bert.) may indirectly contribute to anti-hyperglycemic action of Stevia rebaudiana (Bert.) in diabetic rats via its effect to potentiate insulin release (Suanarunsawat et all., 2004).
CONCLUSION
The results showed that maseration extraction method was more effective to extract stevioside than soxhlet extraction and 0.1% pre-formulation stevioside solution had the same sweet taste with 5 % sucrose solution. Concentration 0.7 kg/mg BW of ethanol extract of stevia leaf decreased ± 27% blood glucose level in mice. Stevioside is not only potent as natural sweetener but also for antidiabetic agent.
REFERENCE
Brandle, J.E., Starratt, A.N., dan M. Gijzen. 2005. Stevia rebaudiana Its biological, chemical and agricultural properties. http://www.lni.unipi.it/stevia/stevia/stevia0005.htm.
DuBois,G.E. 2005. Steviolmonoside Analogs.
http://www.freepatentsonline.com/4402990.html [140 September 2008]
Gardana, C., Simonetti, P., Canzi, E., Zanchi, R., and Pietta, P. 2003. Metabolism of Stevioside and Rebaudioside A from Stevia rebaudiana Extracts by Human Microflora. J. Agric. Food Chem., 51, 6618-6622
Gregersen, S., Jeppesen, P.B., Holst, J.J., and Hermansen, K. 2004. Antihyperglycemic Effects of Stevioside in Type 2 Diabetic Subjects. Metabolism, Vol 53, No 1: pp 73-76
Jumpatong, K., Phutdhawong., dan Buddhasukh D. 2006. Dechlorophyllation by Electrocoagulation. Molecules. 11 : 156-162
Kumar dan Sampath. 1986. Method for Recovery of Stevioside, United State Patent 4599403.
Moraes, Ĕlida de Paula., Machado, Nádia Regina Camargo Fernandes. 2001. Clarification of
Stevia rebaudiana (Bert.) Bertoni extract by adsorption in modified zeolites. Maringá,
23(6): 1375-1380.
Mudjajanto, E.S. 2005. Keamanan Jajanan Tradisional. http://www.kompas.com/kompas-cetak/0502/17/ilpeng/1563189.htm [12 September 2008]
Pasquel, A., Meireles, M.A.A., Marques, M.O.M., dan A.J. Petenate. (2000). Extraction Of Stevia Glycosides With CO2 + Water, CO2 + Ethanol, AND CO2 + Water+ Ethanol, Braz. J.
Chem. Eng. São Paulo, vol.17 n.3: 438-448.