Supplementary Material Tables for:
MMFF VII. Characterization of MMFF94, MMFF94s, and Other Widely Available
Force Fields for Conformational Energies and for Intermolecular-Interaction
Energies and Geometries.
THOMAS A. HALGREN
Molecular Systems, Merck Research Laboratories, P.O. Box 2000, Rahway, NJ 07065
Contents:
Comparisons of ab initio "MP4SDQ/TZP" and calculated force-field results for 147 conformational energies:
•
Table SM-I
Detailed comparisons of calculated force-field intermolecular interaction energies and geometries to scaled quantum mechanical results:
•
MMFF94 (Table SM-II)
•
MMFF94s (Table SM-III)
•
CFF95 (Table SM-IV)
•
CVFF (Table SM-V)
•
MSI CHARMm (Table SM-VI)
•
CHARMM 22 (Table SM-VII)
•
AMBER* (Table SM-VIII)
•
OPLS* (Table SM-IX)
•
MM2* (Table SM-X)
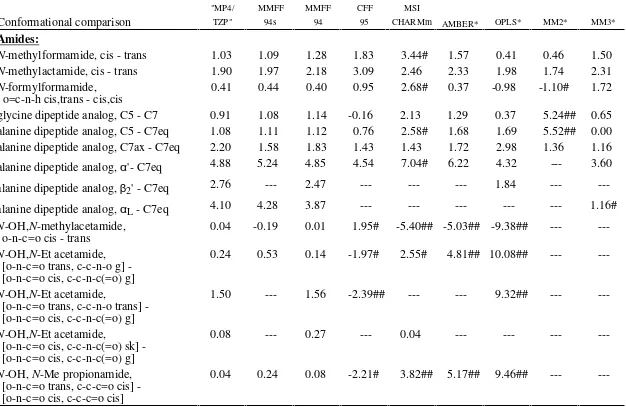
TABLE SM-I.
________________________________________________________________________________________
Comparison of Ab Initio "MP4SDQ/TZP" and Empirical Force Field Conformational Energies (kcal/mol)
Conformational comparison
"MP4/
TZP"
MMFF
94s
MMFF
94
CFF
95
MSI
CHARMm AMBER* OPLS* MM2* MM3*
Amides:
N
-methylformamide, cis - trans
1.03
1.09
1.28
1.83
3.44#
1.57
0.41
0.46
1.50
N
-methylactamide, cis - trans
1.90
1.97
2.18
3.09
2.46
2.33
1.98
1.74
2.31
N
-formylformamide,
o=c-n-h cis,trans - cis,cis
0.41
0.44
0.40
0.95
2.68#
0.37
-0.98
-1.10#
1.72
glycine dipeptide analog, C5 - C7
0.91
1.08
1.14
-0.16
2.13
1.29
0.37
5.24##
0.65
alanine dipeptide analog, C5 - C7eq
1.08
1.11
1.12
0.76
2.58#
1.68
1.69
5.52##
0.00
alanine dipeptide analog, C7ax - C7eq
2.20
1.58
1.83
1.43
1.43
1.72
2.98
1.36
1.16
alanine dipeptide analog, α'- C7eq
4.88
5.24
4.85
4.54
7.04#
6.22
4.32
---
3.60
alanine dipeptide analog, β
2' - C7eq
2.76
---
2.47
---
---
---
1.84
---
---alanine dipeptide analog, α
L- C7eq
4.10
4.28
3.87
---
---
---
---
---
1.16#
N
-OH,
N
-methylacetamide,
o-n-c=o cis - trans
0.04
-0.19
0.01
1.95#
-5.40## -5.03##
-9.38## ---
---N
-OH,
N
-Et acetamide,
[onc=o trans, ccno g]
[o-n-c=o cis, c-c-n-c(=o) g]
0.24
0.53
0.14
-1.97#
2.55#
4.81## 10.08## ---
---N
-OH,
N
-Et acetamide,
[onc=o trans, ccno trans]
[o-n-c=o cis, c-c-n-c(=o) g]
1.50
---
1.56
-2.39## ---
---
9.32## ---
---N
-OH,
N
-Et acetamide,
[onc=o cis, ccnc(=o) sk]
[o-n-c=o cis, c-c-n-c(=o) g]
0.08
---
0.27
---
0.04
---
---
---
---N
-OH,
N
-Me propionamide,
[onc=o trans, ccc=o cis]
[o-n-c=o cis, c-c-c=o cis]
[image:2.792.76.702.128.535.2]---Conformational comparison
"MP4/
TZP"
MMFF
94s
MMFF
94
CFF
95
MSI
CHARMm AMBER* OPLS* MM2* MM3*
N
-OH,
N
-Me propionamide,
[onc=o trans, ccc=o sk]
[o-n-c=o cis, c-c-c=o cis]
0.96
1.24
1.07
-2.41##
3.42#
5.17## 11.29## ---
---N
-OH,
N
-Me propionamide,
[onc=o cis, ccc=o sk]
[o-n-c=o cis, c-c-c=o cis]
0.72
1.24
1.09
0.26
0.26
---
2.19
---
---glycine dipeptide, C5 - C7
1.66
1.40
1.62
1.08
2.55
-1.20#
-0.70#
5.65##
1.25
alanine dipeptide, C5 - C7eq
1.64
1.42
1.68
1.79
2.93
-0.69#
0.44
6.05##
0.56
alanine dipeptide, C7ax - C7eq
2.20
1.84
2.26
1.17
1.84
2.19
2.95
1.53
1.34
alanine dipeptide, α' - C7eq
5.35
5.66
5.47
5.34
7.30#
---
5.48
---
4.26
alanine dipeptide, β
2- C7eq
3.20
---
3.06
---
---
---
---
---
---alanine dipeptide, α
L- C7eq
4.25
4.74
4.75
---
---
---
---
---
---Carboxylic Acids:
formic acid, trans - cis
4.79
4.89
4.89
4.48
5.97
6.55#
7.64#
11.38##
4.89
acetic acid, trans - cis
5.86
5.87
5.87
6.05
4.76
7.23
6.67
13.66##
5.70
propanoic acid, c-c-c=o sk - cis
0.85
0.84
0.84
0.90
0.80
-0.22
-0.10
0.52
0.71
gyloxylic acid,
[o=cc=o trans, o=coh cis]
[o=c-c=o trans, o=c-o-h trans]
0.35
1.91#
1.91#
2.94#
0.89
1.07
5.08## -5.68##
-0.49
glycolic acid, o=c-c-o sk - cis
1.64
0.80
0.80
2.57
-2.76## -0.71#
-3.50##
0.94
2.88
propenoic acid, c=c-c=o trans - cis
0.21
0.25
0.25
0.70
-0.39
-0.03
-0.14
-0.05
-1.17
oxalic acid,
[o=cc=o trans, o=coh cis,cis]
[o=c-c=o trans, o=c-o-h trans,trans]
2.22
0.97
0.97
6.22##
7.46##
0.01#
9.84## -8.85##
-1.74##
oxalic acid,
[o=cc=o trans, o=coh cis,trans]
[o=c-c=o trans, o=c-o-h trans,trans]
Conformational comparison
"MP4/
TZP"
MMFF
94s
MMFF
94
CFF
95
MSI
CHARMm AMBER* OPLS* MM2* MM3*
pyruvic acid,
[o=cc=o cis, o=coh cis]
[o=c-c=o trans, o=c-o-h trans]
1.67
2.42
2.42
2.97
2.48
1.52
4.42#
-4.13##
-0.16#
Esters:
methyl formate, o=c-o-c trans - cis
5.65
5.28
5.28
5.09
5.54
1.34##
0.87##
5.61
2.18##
methyl acetate, o=c-o-c trans - cis
8.21
8.27
8.27
8.67
9.09
7.42
7.47
6.68#
7.85
vinyl formate, c=c-o-c cis - trans
2.79
2.83
2.83
---
4.79#
---
---
2.84
---ethyl formate, c-o-c-c g - a
0.34
0.44
0.44
0.67
---
0.99
0.69
0.27
0.23
isopropyl formate, c-c-o-c g,g - g,a
2.43
2.43
2.43
2.16
5.58##
2.65
2.84
0.74#
1.60
phenyl acetate, o=c-o-c cis - trans
4.30
4.50
4.50
2.58#
1.32#
2.06#
4.93
8.80##
1.28##
methyl glycolate, o=c-c-o sk - cis
1.48
2.43
2.43
2.40
-3.25##
0.35
-0.03#
2.22
2.84
Congujated systems:
1,3-butadiene, g - s-trans
2.39
2.46
2.46
3.39
2.39
2.45
2.80
2.74
1.72
2-methyl-1,3-butadiene, g - s-trans
2.20
2.12
2.12
2.79
1.95
3.29
1.85
2.49
1.63
2-methyl-but-1-ene-3-one,
c=c-c=o cis - trans
2.08
1.90
1.90
-0.42#
2.46
-0.57#
1.37
1.66
1.95
2-methylpropenamide, c=c-c=o cis - sk
1.05
1.04
1.00
-0.42
6.08## -3.13##
0.16
2.90#
1.04
propenamide, c=c-c=o sk - cis
0.61
0.57
0.60
0.31
-3.02## -1.77#
-2.26#
-0.84
-0.79
but-1-ene-3-one, c=c-c=o cis - trans
0.43
0.28
0.28
0.69
0.58
0.67
1.43
0.50
0.71
acrolein, c=c-c=o cis - trans
2.03
2.05
2.05
1.59
2.47
2.03
1.69
1.64
1.98
2-methylpropenal, c=c-c=o cis - trans
3.14
3.15
3.15
-0.07##
5.50#
1.40#
0.64#
3.25
3.46
2-methylpropenoic acid,
c=c-c=o cis - trans
0.60
0.62
0.62
-0.45
0.71
-0.52
0.38
-1.18#
1.30
1,3-pentadiene,
[c=cc=c g, cc=cc trans]
[c=c-c=c s-trans, c-c=c-c trans]
Conformational comparison
"MP4/
TZP"
MMFF
94s
MMFF
94
CFF
95
MSI
CHARMm AMBER* OPLS* MM2* MM3*
1,3-pentadiene,
[strans, cc=cc cis]
[s-trans, c-c=c-c trans]
1.27
2.11
2.11
2.15
0.70
1.29
1.97
2.23
1.12
Aldehydes and ketones:
propionaldehyde, c-c-c=o sk - cis
0.83
0.53
0.53
1.09
0.32
1.65
-0.46
0.90
1.10
2-butanone c-c-c=o sk - cis
0.98
0.83
0.83
1.35
0.19
1.73
-0.04
1.57
1.61
methyl ispropyl ketone,
o=c-c(ch3)2-h cis - trans
0.69
0.89
0.89
1.24
0.87
1.65
0.40
1.36
1.53
butyraldehyde, c-c-c-c g - a
0.14
0.11
0.11
0.87
0.89
1.27
1.08
0.84
0.91
but-3-enal,
[c=ccc sk, ccc=o cis]
[c=c-c-c sk+, c-c-c=o sk+]
0.20
-0.05
-0.05
---
1.16
-0.96
-0.11
0.95
2.11#
but-3-enal,
[c=ccc sk, ccc=o sk+]
[c=c-c-c sk+, c-c-c=o sk+]
0.29
0.98
0.98
0.65
0.71
0.51
0.62
0.07
0.51
3-methyl-but-3-enal,
[c=ccc sk, ccc=o cis]
[c=c-c-c sk, c-c-c=o sk]
0.43
0.72
0.72
0.06
1.34
0.08
1.31
1.25
2.68#
isobutyraldehyde, h-c(=0)-c-h a - g
0.37
0.56
0.56
0.97
0.04
1.58
-0.49
0.99
1.16
2-formylpropanal, o-c-c-c(=o) a - g
0.18
0.14
0.14
0.01
-3.85## -2.60#
-1.94#
0.25
-0.33
4-oxobutanal (o=c-c-c, c-c-c=o cis, cis),
c-c-c-c s-trans - g
0.70
0.77
0.77
-1.34#
-0.98#
2.14
0.03
-0.24
-1.37#
2,3-butanedione, c-c-c-c g - s-trans
5.40
---
---
3.51#
4.66
---
---
7.12#
2.88#
Halides:
1,2-difluoroethane, a - g
0.58
0.63
0.63
-1.57#
1.05
-1.16#
-0.93#
0.01
0.04
1,2-dicloroethane, g - a
1.29
1.24
1.24
1.22
1.47
1.13
0.87
1.13
1.95
Conformational comparison
"MP4/
TZP"
MMFF
94s
MMFF
94
CFF
95
MSI
CHARMm AMBER* OPLS* MM2* MM3*
1-chloropropane, g - a
0.03
0.00
0.00
1.03
0.15
0.07
0.39
0.23
0.29
Imines, guanidines, and amidines
formamidine (
N
puckered),
h-n=c-n cis - trans
2.03
2.03
2.27
1.62
2.08
1.78
4.23#
-0.96#
-0.68#
N
-methylformamidine (
N
puckered),
n-c=n-c cis - trans
2.00
2.00
2.12
2.67
-3.12##
3.82#
---
-0.09#
1.03
butadiene schiff base,
[c=cc=n scis, hn=cc trans]
[c=c-c=n s-trans, h-n=c-c cis]
1.73
1.94
1.94
6.58##
2.63
1.96
1.89
2.60
1.99
Ketals, acetals, and hemiacetals:
2-methoxytetrahydropyran,
[eq, me-o-c-c a] - [ax, me-o-c-c a]
1.30
1.81
1.81
1.88
2.37
0.97
1.70
2.27
1.63
2,4-dioxapentane,
[coco g, ococ a]
[c-o-c-o g+, o-c-o-c g+]
2.44
2.11
2.11
2.54
3.37
2.95
1.93
2.99
2.26
2,5-dimethyl-1,3-dioxane, 5-ax - 5-eq
0.63
0.50
0.50
0.04
-0.65
-0.67
0.41
0.69
0.75
methoxymethanol,
[coco g+, ocoh g]
[c-o-c-o g+, o-c-o-h g+]
2.25
2.97
2.97
1.96
-3.98##
2.34
2.86
2.84
2.94
Cations:
N
-proplyamine cation, c-c-c-n g - a
0.07
0.03
0.03
0.29
-0.32
-0.65
0.34
0.99
1.12
ethylguanidine cation, c-c-n=c g - a
0.62
0.38
0.38
---
-1.37#
-0.18
0.33
0.11
---Amines:
Conformational comparison
"MP4/
TZP"
MMFF
94s
MMFF
94
CFF
95
MSI
CHARMm AMBER* OPLS* MM2* MM3*
isopropylamine,
[
C
1, hcnlp g]
[
C
s, h-c-n-lp a]
0.50
0.45
0.45
0.16
-0.07
0.23
0.00
0.15
0.22
cyclohexylamine, ax - eq
0.69
0.67
0.67
0.83
1.78
-0.74
0.62
1.38
1.23
piperidine, n-h ax - eq
0.78
0.90
0.90
0.05
0.13
0.14
-0.04
0.31
0.31
N
-methylpiperidine, ax - eq
3.58
3.28
3.28
3.25
2.70
1.26#
2.32
2.53
2.31
ethylamine, c-c-n-lp a - g
0.08
0.44
0.44
0.20
0.22
0.26
0.00
0.14
0.12
3-aminopropene,
[c=ccn cis, ccnlp g]
[c=c-c-n sk, c-c-n-lp g]
0.50
0.51
0.51
2.44#
0.00
-0.84
-1.07#
0.71
1.82
3-aminopropene,
[c=ccn sk, ccnlp a]
[c=c-c-n sk, c-c-n-lp g]
0.15
0.65
0.65
-0.17
-0.21
-0.18
0.68
-0.30
-0.08
2-methyl,3-aminopropene,
[c=ccn cis, ccnlp g]
[c=c-c-n sk, c-c-n-lp g]
0.67
0.61
0.61
1.19
0.47
0.32
-0.93#
1.03
1.75
ethylenediamine,
[nccn a, ccnlp g+, g+]
[n-c-c-n g+, c-c-n-lp g+, g+]
1.39
1.45
1.45
0.84
1.48
12.94## 21.84##
1.20
0.59
methylethylamine
N
-oxide, c-n-c-c g - a
1.38
1.41
1.41
-0.73#
3.62#
1.23
---
---
---methylethylhydroxylamine, c-n-c-c g - a
1.69
2.25
2.25
1.63
1.76
1.17
1.61
1.20
---ethylamine
N
-oxide, o-n-c-c a - g
0.68
0.73
0.73
-1.61#
1.61
0.68
---
---
---ethylhydroxylamine, o-n-c-c a - g
0.20
0.05
0.05
-0.57
2.98#
0.55
-0.28
-0.05
---Alcohols:
ethanol, a - g
0.06
-0.18
-0.18
-0.37
-0.23
-0.36
0.00
-0.60
-0.41
n
-propanol,
[ccco g, ccoh g+]
[c-c-c-o a, c-c-o-h g]
Conformational comparison
"MP4/
TZP"
MMFF
94s
MMFF
94
CFF
95
MSI
CHARMm AMBER* OPLS* MM2* MM3*
n
-propanol,
[ccco g+, ccoh g+]
[c-c-c-o a, c-c-o-h g]
0.05
0.24
0.24
0.01
-0.43
-0.62
0.28
0.36
0.35
n
-propanol,
[ccco a, ccoh a]
[c-c-c-o a, c-c-o-h g]
0.20
-0.17
-0.17
-0.49
-0.16
-0.30
0.00
-0.60
-0.42
n
-propanol,
[ccco g, ccoh a]
[c-c-c-o a, c-c-o-h g]
0.10
0.12
0.12
-0.23
-0.29
-0.81
0.28
-0.30
-0.06
isopropanol, h-c-o-h a - g
0.20
0.17
0.17
0.40
0.02
0.25
0.00
0.61
0.68
cyclopentanol, eq
C
s- ax
C
11.11
0.82
0.82
-0.27
---
-0.36
-0.07
0.40
0.89
cyclopentanol, ax
C
s- ax
C
11.05
0.59
0.59
-0.33
-0.94#
---
0.00
0.82
1.35
cyclopentanol, eq
C
1- ax
C
11.14
0.47
0.47
-0.53#
-0.62#
0.70
-0.07
-0.23
-0.15
cyclohexanol, eq
C
s- eq
C
10.18
0.20
0.20
0.38
0.06
0.15
0.00
0.62
0.77
cyclohexanol, ax
C
s- eq
C
11.14
1.01
1.01
-0.49#
0.58
-0.87#
0.50
1.46
2.43
cylohexanol, ax
C
1- eq
C
10.33
0.32
0.32
0.24
0.40
-0.57
0.50
0.58
0.74
vinyl alcohol, c=c-o-h trans - sk
1.43
1.43
1.43
1.58
0.66
-0.81#
-0.69#
1.75
-0.09#
benzyl alcohol, h-o-c-c a - g
1.13
1.59
1.59
1.38
2.97#
0.81
1.23
1.22
1.33
propen-3-ol,
[c=cco sk, ccoh a]
[c=c-c-o sk, c-c-o-h g]
1.24
1.10
1.10
0.82
2.43
1.07
0.19
1.20
1.12
propen-3-ol,
[c=cco cis, ccoh a]
[c=c-c-o sk, c-c-o-h g]
1.11
1.13
1.13
3.39#
0.49
-0.08
-1.06#
0.83
2.76#
2-me-propen-3-ol,
[c=cco c, ccoh a]
[c=c-c-o s, c-c-o-h a]
0.38
0.47
0.47
0.94
0.39
-0.71
-1.57#
-0.10
1.53
Conformational comparison
"MP4/
TZP"
MMFF
94s
MMFF
94
CFF
95
MSI
CHARMm AMBER* OPLS* MM2* MM3*
sec-butanol, ag/ag - ga/ag
0.48
0.47
0.47
0.57
1.02
0.93
0.71
0.53
0.49
sec-butanol, ag/ga - ga/ag
0.29
0.46
0.46
0.74
1.06
0.85
0.71
0.53
0.50
sec-butanol, ag/gg - ga/ag
0.56
0.59
0.59
1.15
0.87
1.07
0.71
1.14
1.14
sec-butanol, gg/ag - ga/ag
0.75
0.72
0.72
0.87
0.79
0.40
1.00
0.82
0.98
sec-butanol, gg/ga - ga/ag
0.57
0.66
0.66
0.68
0.43
0.20
1.00
0.90
0.97
sec-butaone, gg/gg - ga/ag
1.13
1.14
1.14
0.56
1.01
0.33
1.00
1.54
2.06
1,2-ethanediol,
[hocc a, occo a, ccoh a]
[h-o-c-c g-, o-c-c-o g+, c-c-o-h a]
2.71
2.91
2.91
0.70#
2.45
5.69#
17.30##
2.30
0.80#
1,2-ethanediol,
[hocc g, occo g+, ccoh g+]
[h-o-c-c g-, o-c-c-o g+, c-c-o-h a]
0.49
1.15
1.15
1.45
0.79
0.29
---
1.47
---1,2-ethanediol,
[hocc g+, occo g, ccoh g+]
[h-o-c-c g-, o-c-c-o g+, c-c-o-h a]
1.41
1.58
1.58
1.92
1.17
0.37
---
1.50
---Ethers:
methyl ethyl ether, g - a
1.41
1.50
1.50
1.46
1.53
1.79
1.85
1.74
1.48
methyl vinyl ether, c=c-o-c skew - cis
2.27
2.22
2.22
-3.10##
2.10
-2.90##
-3.94##
1.07
0.60#
diethyl ether,
[ccoc a, cocc g]
[c-c-o-c a, c-o-c-c a]
1.48
1.52
1.52
1.37
1.55
1.83
1.84
1.77
1.49
methoxycyclohexane, eq
C
s- ax
C
12.10
1.95
1.95
2.19
1.56
2.76
2.08
1.53
1.80
methoxycyclohexane, eq
C
1- ax
C
10.01
-0.41
-0.41
0.11
-0.70
0.34
-0.37
-0.56
-0.76
methyl isopropyl ether, h-c-o-ch3 a - g
1.91
1.88
1.88
2.10
1.91
2.25
2.20
1.73
2.12
Alkanes:
Conformational comparison
"MP4/
TZP"
MMFF
94s
MMFF
94
CFF
95
MSI
CHARMm AMBER* OPLS* MM2* MM3*
cyclohexane, twist-boat - chair
6.14
5.93
5.93
7.08
6.72
5.79
6.77
5.36
5.76
methylcyclohexane, ax - eq
1.69
1.37
1.37
1.84
1.80
0.90
1.78
1.78
1.77
2,3-dimethylbutane, h-c2-c3-h g - a
0.04
-0.23
-0.23
0.13
0.40
-0.22
-0.48
0.15
0.38
cyclooctane, D4d crown - Cs boat-chair
2.00
1.44
1.44
1.21
0.45#
0.83
-1.67##
0.96
1.12
cyclooctane,
C
2twist-boat-chair -
C
sboat-chair
1.71
1.99
1.99
2.30
1.88
1.77
1.20
1.66
1.85
cyclooctane,
S
4-
C
sboat-chair
2.97
3.27
3.27
0.16#
4.48#
3.63
6.51##
3.12
2.62
methylcyclobutane, ax - eq
0.77
0.78
0.78
1.21
0.06
0.17
0.63
0.58
0.92
cyclononane,
C
2[144] -
D
3[333]
1.97
1.41
1.41
1.02
0.37#
0.53
-1.02#
0.77
1.15
cyclononane,
C
2[225] -
D
3[333]
0.99
1.21
1.21
-1.63#
1.12
1.07
0.80
0.75
0.84
cyclononane,
C
1[234] -
D
3[333]
3.65
3.81
3.81
2.03#
3.45
3.66
4.31
3.16
3.04
Alkenes:
1-butene, c=c-c-c cis - sk
0.26
0.26
0.26
0.72
0.46
0.66
1.00
0.49
0.68
1-pentene (c=c-c-c skew),
c-c-c-c g - a
0.61
0.51
0.51
0.90
-0.10
0.11
0.60
0.78
0.42
2-methyl-1-butene, c=c-c-c skew - cis
0.02
-0.03
-0.03
-0.07
-0.93
-0.97
-0.98
-0.06
-0.38
1,4-pentadiene,
c=c-c-c sk+,sk- -
sk-,sk-0.33
0.43
0.43
0.33
0.19
0.44
0.25
0.07
0.27
2-butene, cis - trans
1.27
1.35
1.35
1.43
1.61
1.55
2.49
1.43
1.02
2-pentene,
[cc=cc cis, ccc=c sk]
[c-c=c-c trans, c-c-c=c sk]
1.31
1.33
1.33
1.35
1.61
1.43
2.39
1.29
0.99
Thiols, sulfides, and disulfides:
Conformational comparison
"MP4/
TZP"
MMFF
94s
MMFF
94
CFF
95
MSI
CHARMm AMBER* OPLS* MM2* MM3*
1-propanethiol,
[sccc g, hscc g+]
[s-c-c-c a, h-s-c-c g]
0.30
0.37
0.37
1.59
-0.84
1.51
0.48
0.84
1.04
1-propanethiol,
[sccc g, hscc a]
[s-c-c-c a, h-s-c-c g]
1.23
0.95
0.95
2.26
-1.65#
1.53
0.48
0.92
0.65
1,2-ethanedithiol,
[all anti]
[h-s-c-c a,g+, s-c-c-s g-]
0.86
1.17
1.17
0.15
-0.95#
6.92##
5.17#
-0.92#
-1.71#
1,2-ethanedithiol,
[hscc a,a, sccs g]
[h-s-c-c a,g+, s-c-c-s g-]
1.77
2.24
2.24
1.71
0.26#
1.62
5.51##
0.68
0.34
methyl propyl sulfide,
[cscc g, sccc g]
[c-s-c-c g, s-c-c-c a]
0.09
0.05
0.05
1.56
-0.08
1.21
0.12
0.58
0.61
methyl propyl sulfide,
[cscc a, sccc g]
[c-s-c-c g, s-c-c-c a]
0.66
0.60
0.60
-0.03
0.18
0.94
-0.24
0.24
0.51
methyl propyl sulfide,
[cscc a, sccc a]
[c-s-c-c g, s-c-c-c a]
0.25
0.33
0.33
-1.66#
0.23
-0.24
-0.43
-0.39
-0.18
a
For the OH14 conformers (sec-butanol), in the conformational designations "wx/yz", "w" indicates the conformation of the c-c-c-o
angle, "x" that of the c-c-c-c angle,"y" that of the h-o-c-ch
2angle, and "z" that of the h-o-c-ch
3angle.
# Denotes a conformational enregy that differs from the reference "MP4SDQ/TZP" value by > 1.5 kcal/mol.
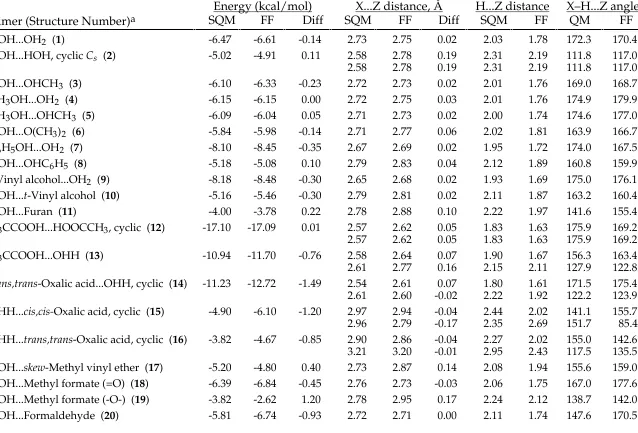
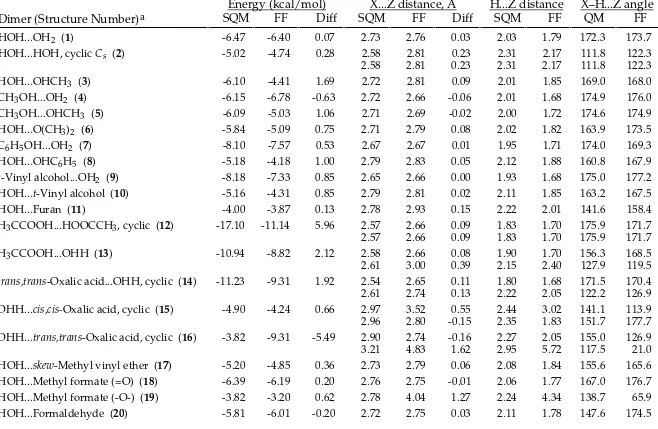
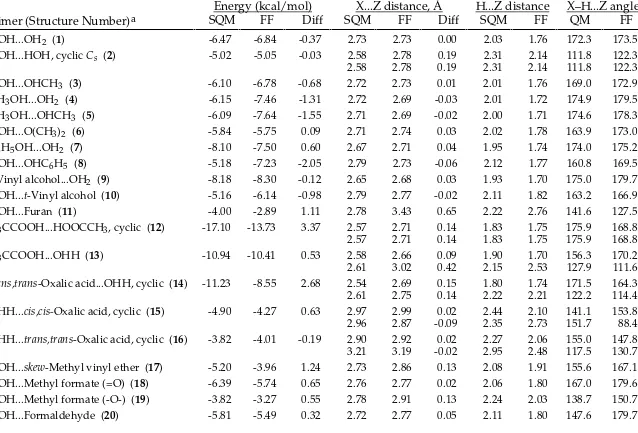
TABLE SM-II.
_________________________________________________________________________________
MMFF94 vs. Scaled HF/-6-31G* Intermolecular-Interaction Energies and Distances.
Energy (kcal/mol)
X...Z distance, Å
H...Z distance
X–H...Z angle
Dimer (Structure Number)
aSQM
FF
Diff SQM
FF
Diff SQM
FF
QM
FF
HOH...OH2 (1) -6.47 -6.61 -0.14 2.73 2.75 0.02 2.03 1.78 172.3 170.4
HOH...HOH, cyclic Cs (2) -5.02 -4.91 0.11 2.58 2.78 0.19 2.31 2.19 111.8 117.0
2.58 2.78 0.19 2.31 2.19 111.8 117.0
HOH...OHCH3 (3) -6.10 -6.33 -0.23 2.72 2.73 0.02 2.01 1.76 169.0 168.7
CH3OH...OH2 (4) -6.15 -6.15 0.00 2.72 2.75 0.03 2.01 1.76 174.9 179.9
CH3OH...OHCH3 (5) -6.09 -6.04 0.05 2.71 2.73 0.02 2.00 1.74 174.6 177.0
HOH...O(CH3)2 (6) -5.84 -5.98 -0.14 2.71 2.77 0.06 2.02 1.81 163.9 166.7
C6H5OH...OH2 (7) -8.10 -8.45 -0.35 2.67 2.69 0.02 1.95 1.72 174.0 167.5
HOH...OHC6H5 (8) -5.18 -5.08 0.10 2.79 2.83 0.04 2.12 1.89 160.8 159.9
t-Vinyl alcohol...OH2 (9) -8.18 -8.48 -0.30 2.65 2.68 0.02 1.93 1.69 175.0 176.1 HOH...t-Vinyl alcohol (10) -5.16 -5.46 -0.30 2.79 2.81 0.02 2.11 1.87 163.2 160.4
HOH...Furan (11) -4.00 -3.78 0.22 2.78 2.88 0.10 2.22 1.97 141.6 155.4
H3CCOOH...HOOCCH3, cyclic (12) -17.10 -17.09 0.01 2.57 2.62 0.05 1.83 1.63 175.9 169.2
2.57 2.62 0.05 1.83 1.63 175.9 169.2
H3CCOOH...OHH (13) -10.94 -11.70 -0.76 2.58 2.64 0.07 1.90 1.67 156.3 163.4
2.61 2.77 0.16 2.15 2.11 127.9 122.8
trans,trans-Oxalic acid...OHH, cyclic (14) -11.23 -12.72 -1.49 2.54 2.61 0.07 1.80 1.61 171.5 175.4
2.61 2.60 -0.02 2.22 1.92 122.2 123.9
OHH...cis,cis-Oxalic acid, cyclic (15) -4.90 -6.10 -1.20 2.97 2.94 -0.04 2.44 2.02 141.1 155.7
2.96 2.79 -0.17 2.35 2.69 151.7 85.4
OHH...trans,trans-Oxalic acid, cyclic (16) -3.82 -4.67 -0.85 2.90 2.86 -0.04 2.27 2.02 155.0 142.6
3.21 3.20 -0.01 2.95 2.43 117.5 135.5
HOH...skew-Methyl vinyl ether (17) -5.20 -4.80 0.40 2.73 2.87 0.14 2.08 1.94 155.6 159.0 HOH...Methyl formate (=O) (18) -6.39 -6.84 -0.45 2.76 2.73 -0.03 2.06 1.75 167.0 177.6 HOH...Methyl formate (-O-) (19) -3.82 -2.62 1.20 2.78 2.95 0.17 2.24 2.12 138.7 142.0
HOH...Acetone (21) -6.87 -7.06 -0.19 2.74 2.71 -0.03 2.06 1.73 163.1 173.1
t-NMA...OH2 (22) -5.97 -6.86 -0.89 2.87 2.84 -0.03 2.12 1.82 178.3 176.9
HOH...t-NMA (23) -8.03 -8.19 -0.16 2.70 2.71 0.00 2.00 1.73 167.6 174.2
Formamide dimer, cyclic (24) -14.78 -12.26 2.52 2.76 2.81 0.06 2.00 1.79 171.6 173.4
2.76 2.81 0.06 2.00 1.79 171.5 173.4
Formamide dimer, parallel (25) -8.12 -7.69 0.43 2.80 2.76 -0.03 2.09 1.74 156.7 174.4
t-NMA dimer, antiparallel (26) -7.70 -9.08 -1.38 2.83 2.76 -0.07 2.08 1.76 171.8 165.3
t-NMA antiparallel stacked (27) -3.86 -5.62 -1.76 3.65 3.61 -0.05 3.88 3.49 87.9 88.5
3.67 3.61 -0.06 3.88 3.49 88.9 88.4
HOH...N-Methylformamide (28) -7.90 -8.07 -0.17 2.71 2.71 0.00 2.01 1.73 164.7 174.8 N-Methylformamide...OH2 (29) -6.05 -6.94 -0.89 2.84 2.82 -0.01 2.09 1.80 173.8 175.4
t-N-OH,N-Meacetamide...OH2 (30) -8.21 -7.99 0.22 2.62 2.70 0.08 1.94 1.72 159.2 175.8 HOH...t-N-OH,N-Methylacetamide (31) -4.10 -3.95 0.15 2.82 2.85 0.03 2.12 1.87 168.7 175.8 H2NH...HNH2, cyclic C2h (32) -3.51 -3.02 0.49 3.01 3.11 0.10 2.60 2.40 123.9 125.4
3.01 3.11 0.10 2.61 2.40 123.8 125.4
H2NH...NH3, linear Cs (33) -3.38 -3.67 -0.29 3.15 3.03 -0.12 2.42 2.00 179.0 178.1
3.15 3.03 -0.12 3.64 3.36 69.8 62.7
HOH...NH3 (34) -7.22 -6.83 0.39 2.80 2.80 0.00 2.09 1.82 176.4 173.9
HOH...NH2CH3 (35) -7.07 -6.70 0.37 2.80 2.82 0.02 2.09 1.84 175.9 173.1
Imidazole...OH2 (36) -7.00 -7.91 -0.91 2.79 2.82 0.04 2.03 1.81 178.2 175.6
HOH...Imidazole (37) -7.77 -7.93 -0.16 2.73 2.75 0.01 2.10 1.81 151.2 158.6
Indole...OH2 (38) -6.33 -6.51 -0.18 2.81 2.86 0.05 2.07 1.84 168.3 179.7
Pyrrole...OH2 (39) -5.89 -6.28 -0.39 2.82 2.86 0.04 2.07 1.84 180.0 180.0
HOH...Pyridine (40) -6.63 -7.10 -0.47 2.77 2.79 0.02 2.12 1.85 155.8 158.9
Formamidine...H2O, cyclic (41) -11.03 -10.65 0.38 2.81 2.97 0.15 2.20 2.09 143.3 142.8
2.70 2.76 0.06 2.06 1.80 151.4 162.0
HOH...Formaldehydeimine (42) -6.74 -7.95 -1.21 2.76 2.74 -0.02 2.11 1.77 154.0 167.6
Guanidine...OHH (43) -7.96 -7.47 0.49 2.88 2.90 0.02 2.25 1.98 145.4 148.1
2.77 2.94 0.18 2.21 2.11 141.1 141.1
Aniline...OH2 (45) -4.62 -4.86 -0.24 2.94 2.87 -0.06 2.20 1.85 173.2 172.8
HSH...OH2 (46) -2.93 -2.91 0.02 3.33 3.38 0.05 2.29 2.03 175.3 172.8
HOH...S(CH3)2 (47) -3.56 -3.48 0.08 3.12 3.35 0.23 3.13 2.38 97.7 170.8
3.12 3.35 0.23 3.13 3.62 97.7 66.4
OHH...CH3SSCH3, cyclic (48) -3.78 -3.22 0.56 3.38 3.55 0.17 2.84 2.58 147.0 174.6
HOH...Thiophene (49) -2.66 -2.71 -0.05 3.59 3.69 0.10 3.50 3.03 108.4 126.8
3.59 3.69 0.10 3.33 3.34 121.0 103.6
HOH...SHC6H5 (50) -2.20 -3.45 -1.25 4.56 4.37 -0.19 4.27 3.55 132.4 144.2
HSH...SH2 (51) -0.96 -1.25 -0.29 4.15 4.02 -0.13 3.19 2.70 175.5 167.3
HOH...SH2 (52) -2.27 -2.00 0.27 3.47 3.49 0.02 2.83 2.55 175.5 163.2
CH3COOH...NH3, bidentate (53) -11.94 -11.58 0.36 2.63 2.66 0.03 1.90 1.66 168.3 174.3
2.89 3.02 0.12 2.53 2.43 119.0 115.2
HOH...PyridineN-oxide (54) -10.69 -10.61 0.08 2.63 2.64 0.01 1.94 1.65 161.0 178.6 MethylethylamineN-oxide...OHH( 55) -15.48 -14.87 0.61 2.49 2.59 0.10 1.83 1.66 148.9 154.6
2.64 2.74 0.11 2.17 2.10 124.2 117.6
Methylethylhydroxylamine...OH2 (56) -7.41 -7.36 0.05 2.66 2.79 0.13 2.11 1.94 138.5 143.2
2.66 2.88 0.22 2.18 2.10 130.2 135.1
HOH...FCH3 (59) -4.87 -4.48 0.39 2.70 2.66 -0.04 2.06 1.69 153.9 172.7
HOH...Chloropropane (58) -3.09 -2.84 0.25 3.34 3.35 0.01 2.82 2.45 143.5 153.9
H2NH...O(CH3)2 (59) -3.12 -3.43 -0.31 3.00 3.00 0.00 2.32 1.97 155.9 173.9
Methylammonium...OH2 (60) -19.30 -21.17 -1.87 2.70 2.62 -0.08 1.79 1.57 173.8 176.7
Guanidinium...OHH (61) -18.48 -19.02 -0.54 2.85 2.78 -0.07 2.08 1.87 147.7 146.8
2.85 2.78 -0.07 2.08 1.87 147.7 146.8
Imidazolium...OH2 (62) -16.95 -17.60 -0.65 2.71 2.62 -0.08 1.81 1.59 174.5 173.4 Formamidinium...OH2 (63) -16.98 -18.75 -1.77 2.71 2.61 -0.10 1.91 1.57 148.7 177.2 Formamidinium...OH2, cyclic C2v (64) -19.59 -19.71 -0.12 2.83 2.78 -0.05 2.09 1.89 142.6 143.6
2.83 2.78 -0.05 2.09 1.89 142.6 143.6
Formaldehydeiminium...OH2 (65) -19.98 -21.65 -1.67 2.66 2.65 -0.01 1.78 1.61 163.4 176.6 OHH...(-)O2CCH3, bidentate (66) -21.85 -22.62 -0.77 2.77 2.74 -0.03 2.06 1.87 142.9 145.7
2.78 2.74 -0.03 2.08 1.87 141.8 145.7
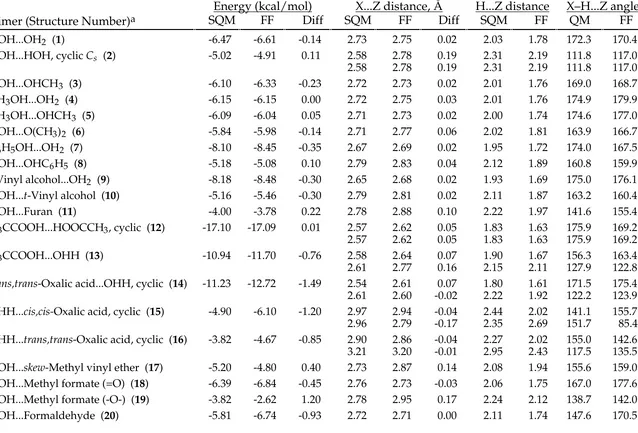
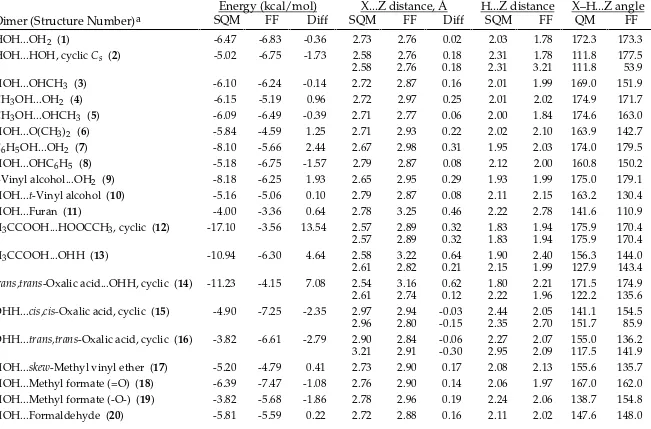
TABLE SM-III.
_________________________________________________________________________________
MMFF94s vs. Scaled HF/-6-31G* Intermolecular-Interaction Energies and Distances.
Energy (kcal/mol)
X...Z distance, Å
H...Z distance
X–H...Z angle
Dimer (Structure Number)
aSQM
FF
Diff SQM
FF
Diff SQM
FF
QM
FF
HOH...OH2 (1) -6.47 -6.61 -0.14 2.73 2.75 0.02 2.03 1.78 172.3 170.4
HOH...HOH, cyclic Cs (2) -5.02 -4.91 0.11 2.58 2.78 0.19 2.31 2.19 111.8 117.0
2.58 2.78 0.19 2.31 2.19 111.8 117.0
HOH...OHCH3 (3) -6.10 -6.33 -0.23 2.72 2.73 0.02 2.01 1.76 169.0 168.7
CH3OH...OH2 (4) -6.15 -6.15 0.00 2.72 2.75 0.03 2.01 1.76 174.9 179.9
CH3OH...OHCH3 (5) -6.09 -6.04 0.05 2.71 2.73 0.02 2.00 1.74 174.6 177.0
HOH...O(CH3)2 (6) -5.84 -5.98 -0.14 2.71 2.77 0.06 2.02 1.81 163.9 166.7
C6H5OH...OH2 (7) -8.10 -8.45 -0.35 2.67 2.69 0.02 1.95 1.72 174.0 167.5
HOH...OHC6H5 (8) -5.18 -5.08 0.10 2.79 2.83 0.04 2.12 1.89 160.8 159.9
t-Vinyl alcohol...OH2 (9) -8.18 -8.48 -0.30 2.65 2.68 0.02 1.93 1.69 175.0 176.1 HOH...t-Vinyl alcohol (10) -5.16 -5.46 -0.30 2.79 2.81 0.02 2.11 1.87 163.2 160.4
HOH...Furan (11) -4.00 -3.78 0.22 2.78 2.88 0.10 2.22 1.97 141.6 155.4
H3CCOOH...HOOCCH3, cyclic (12) -17.10 -17.09 0.01 2.57 2.62 0.05 1.83 1.63 175.9 169.2
2.57 2.62 0.05 1.83 1.63 175.9 169.2
H3CCOOH...OHH (13) -10.94 -11.70 -0.76 2.58 2.64 0.07 1.90 1.67 156.3 163.4
2.61 2.77 0.16 2.15 2.11 127.9 122.8
trans,trans-Oxalic acid...OHH, cyclic (14) -11.23 -12.72 -1.49 2.54 2.61 0.07 1.80 1.61 171.5 175.4
2.61 2.60 -0.02 2.22 1.92 122.2 123.9
OHH...cis,cis-Oxalic acid, cyclic (15) -4.90 -6.10 -1.20 2.97 2.94 -0.04 2.44 2.02 141.1 155.7
2.96 2.79 -0.17 2.35 2.69 151.7 85.4
OHH...trans,trans-Oxalic acid, cyclic (16) -3.82 -4.67 -0.85 2.90 2.86 -0.04 2.27 2.02 155.0 142.6
3.21 3.20 -0.01 2.95 2.43 117.5 135.5
HOH...skew-Methyl vinyl ether (17) -5.20 -4.80 0.40 2.73 2.87 0.14 2.08 1.94 155.6 159.0 HOH...Methyl formate (=O) (18) -6.39 -6.84 -0.45 2.76 2.73 -0.03 2.06 1.75 167.0 177.6 HOH...Methyl formate (-O-) (19) -3.82 -2.62 1.20 2.78 2.95 0.17 2.24 2.12 138.7 142.0
HOH...Acetone (21) -6.87 -7.06 -0.19 2.74 2.71 -0.03 2.06 1.73 163.1 173.1
t-NMA...OH2 (22) -5.97 -6.87 -0.90 2.87 2.84 -0.03 2.12 1.81 178.3 179.9
HOH...t-NMA (23) -8.03 -8.25 -0.22 2.70 2.70 0.00 2.00 1.72 167.6 174.3
Formamide dimer, cyclic (24) -14.78 -12.26 2.52 2.76 2.81 0.06 2.00 1.79 171.6 173.4
2.76 2.81 0.06 2.00 1.79 171.5 173.4
Formamide dimer, parallel (25) -8.12 -7.69 0.43 2.80 2.76 -0.03 2.09 1.74 156.7 174.1
t-NMA dimer, antiparallel (26) -7.70 -9.06 -1.36 2.83 2.76 -0.07 2.08 1.75 171.8 166.8
t-NMA antiparallel stacked (27) -3.86 -5.33 -1.47 3.65 3.64 -0.01 3.88 3.54 87.9 88.0
3.67 3.64 -0.02 3.88 3.54 88.9 88.0
HOH...N-Methylformamide (28) -7.90 -8.14 -0.24 2.71 2.71 0.00 2.01 1.73 164.7 174.9 N-Methylformamide...OH2 (29) -6.05 -6.90 -0.85 2.84 2.83 -0.01 2.09 1.80 173.8 175.5
t-N-OH,N-Meacetamide...OH2 (30) -8.21 -8.30 -0.09 2.62 2.70 0.08 1.94 1.72 159.2 177.0 HOH...t-N-OH,N-Methylacetamide (31) -4.10 -4.26 -0.16 2.82 2.84 0.03 2.12 1.87 168.7 178.2 H2NH...HNH2, cyclic C2h (32) -3.51 -3.02 0.49 3.01 3.11 0.10 2.60 2.40 123.9 125.4
3.01 3.11 0.10 2.61 2.40 123.8 125.4
H2NH...NH3, linear Cs (33) -3.38 -3.67 -0.29 3.15 3.03 -0.12 2.42 2.00 179.0 178.1
3.15 3.03 -0.12 3.64 3.36 69.8 62.7
HOH...NH3 (34) -7.22 -6.83 0.39 2.80 2.80 0.00 2.09 1.82 176.4 173.9
HOH...NH2CH3 (35) -7.07 -6.70 0.37 2.80 2.82 0.02 2.09 1.84 175.9 173.1
Imidazole...OH2 (36) -7.00 -7.91 -0.91 2.79 2.82 0.04 2.03 1.81 178.2 175.6
HOH...Imidazole (37) -7.77 -7.93 -0.16 2.73 2.75 0.01 2.10 1.81 151.2 158.6
Indole...OH2 (38) -6.33 -6.51 -0.18 2.81 2.86 0.05 2.07 1.84 168.3 179.7
Pyrrole...OH2 (39) -5.89 -6.28 -0.39 2.82 2.86 0.04 2.07 1.84 180.0 180.0
HOH...Pyridine (40) -6.63 -7.10 -0.47 2.77 2.79 0.02 2.12 1.85 155.8 158.9
Formamidine...H2O, cyclic (41) -11.03 -10.68 0.35 2.81 2.97 0.16 2.20 2.13 143.3 137.8
2.70 2.77 0.07 2.06 1.80 151.4 167.5
HOH...Formaldehydeimine (42) -6.74 -7.95 -1.21 2.76 2.74 -0.02 2.11 1.77 154.0 167.6
Guanidine...OHH (43) -7.96 -7.02 0.94 2.88 2.88 0.00 2.25 1.88 145.4 165.7
2.77 3.22 0.46 2.21 3.84 141.1 45.0
Aniline...OH2 (45) -4.62 -4.54 0.08 2.94 2.88 -0.06 2.20 1.86 173.2 174.9
HSH...OH2 (46) -2.93 -2.91 0.02 3.33 3.38 0.05 2.29 2.03 175.3 172.8
HOH...S(CH3)2 (47) -3.56 -3.48 0.08 3.12 3.35 0.23 3.13 2.38 97.7 170.8
3.12 3.35 0.23 3.13 3.62 97.7 66.4
OHH...CH3SSCH3, cyclic (48) -3.78 -3.22 0.56 3.38 3.55 0.17 2.84 2.58 147.0 174.6
HOH...Thiophene (49) -2.66 -2.71 -0.05 3.59 3.69 0.10 3.50 3.03 108.4 126.8
3.59 3.69 0.10 3.33 3.34 121.0 103.6
HOH...SHC6H5 (50) -2.20 -3.45 -1.25 4.56 4.37 -0.19 4.27 3.55 132.4 144.2
HSH...SH2 (51) -0.96 -1.25 -0.29 4.15 4.02 -0.13 3.19 2.70 175.5 167.3
HOH...SH2 (52) -2.27 -2.00 0.27 3.47 3.49 0.02 2.83 2.55 175.5 163.2
CH3COOH...NH3, bidentate (53) -11.94 -11.58 0.36 2.63 2.66 0.03 1.90 1.66 168.3 174.3
2.89 3.02 0.12 2.53 2.43 119.0 115.2
HOH...PyridineN-oxide (54) -10.69 -10.61 0.08 2.63 2.64 0.01 1.94 1.65 161.0 178.6 MethylethylamineN-oxide...OHH (55) -15.48 -14.87 0.61 2.49 2.59 0.10 1.83 1.66 148.9 154.6
2.64 2.74 0.11 2.17 2.10 124.2 117.6
Methylethylhydroxylamine...OH2 (56) -7.41 -7.36 0.05 2.66 2.79 0.13 2.11 1.94 138.5 143.2
2.66 2.88 0.22 2.18 2.10 130.2 135.1
HOH...FCH3 (59) -4.87 -4.48 0.39 2.70 2.66 -0.04 2.06 1.69 153.9 172.7
HOH...Chloropropane (58) -3.09 -2.84 0.25 3.34 3.35 0.01 2.82 2.45 143.5 153.9
H2NH...O(CH3)2 (59) -3.12 -3.43 -0.31 3.00 3.00 0.00 2.32 1.97 155.9 173.9
Methylammonium...OH2 (60) -19.30 -21.17 -1.87 2.70 2.62 -0.08 1.79 1.57 173.8 176.7
Guanidinium...OHH (61) -18.48 -19.02 -0.54 2.85 2.78 -0.07 2.08 1.87 147.7 146.8
2.85 2.78 -0.07 2.08 1.87 147.7 146.8
Imidazolium...OH2 (62) -16.95 -17.60 -0.65 2.71 2.62 -0.08 1.81 1.59 174.5 173.4 Formamidinium...OH2 (63) -16.98 -18.75 -1.77 2.71 2.61 -0.10 1.91 1.57 148.7 177.2 Formamidinium...OH2, cyclic C2v (64) -19.59 -19.71 -0.12 2.83 2.78 -0.05 2.09 1.89 142.6 143.6
2.83 2.78 -0.05 2.09 1.89 142.6 143.6
Formaldehydeiminium...OH2 (65) -19.98 -21.65 -1.67 2.66 2.65 -0.01 1.78 1.61 163.4 176.6 OHH...(-)O2CCH3, bidentate (66) -21.85 -22.62 -0.77 2.77 2.74 -0.03 2.06 1.87 142.9 145.7
2.78 2.74 -0.03 2.08 1.87 141.8 145.7
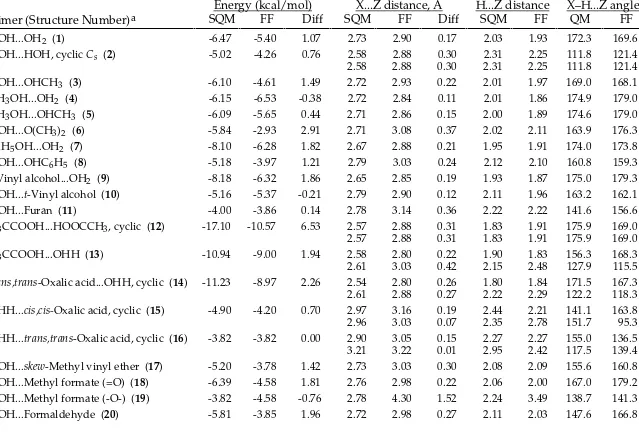
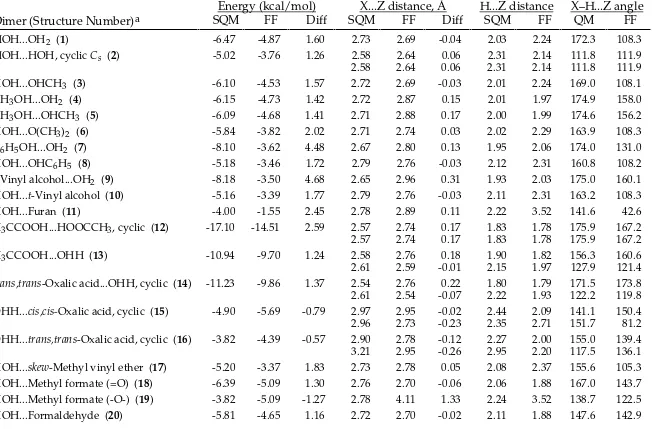
TABLE SM-IV.
_________________________________________________________________________________
CFF95 vs. Scaled HF/-6-31G* Intermolecular-Interaction Energies and Distances.
Energy (kcal/mol)
X...Z distance, Å
H...Z distance
X–H...Z angle
Dimer (Structure Number)
aSQM
FF
Diff SQM
FF
Diff SQM
FF
QM
FF
HOH...OH2 (1) -6.47 -5.40 1.07 2.73 2.90 0.17 2.03 1.93 172.3 169.6
HOH...HOH, cyclic Cs (2) -5.02 -4.26 0.76 2.58 2.88 0.30 2.31 2.25 111.8 121.4
2.58 2.88 0.30 2.31 2.25 111.8 121.4
HOH...OHCH3 (3) -6.10 -4.61 1.49 2.72 2.93 0.22 2.01 1.97 169.0 168.1
CH3OH...OH2 (4) -6.15 -6.53 -0.38 2.72 2.84 0.11 2.01 1.86 174.9 179.0
CH3OH...OHCH3 (5) -6.09 -5.65 0.44 2.71 2.86 0.15 2.00 1.89 174.6 179.0
HOH...O(CH3)2 (6) -5.84 -2.93 2.91 2.71 3.08 0.37 2.02 2.11 163.9 176.3
C6H5OH...OH2 (7) -8.10 -6.28 1.82 2.67 2.88 0.21 1.95 1.91 174.0 173.8
HOH...OHC6H5 (8) -5.18 -3.97 1.21 2.79 3.03 0.24 2.12 2.10 160.8 159.3
t-Vinyl alcohol...OH2 (9) -8.18 -6.32 1.86 2.65 2.85 0.19 1.93 1.87 175.0 179.3 HOH...t-Vinyl alcohol (10) -5.16 -5.37 -0.21 2.79 2.90 0.12 2.11 1.96 163.2 162.1
HOH...Furan (11) -4.00 -3.86 0.14 2.78 3.14 0.36 2.22 2.22 141.6 156.6
H3CCOOH...HOOCCH3, cyclic (12) -17.10 -10.57 6.53 2.57 2.88 0.31 1.83 1.91 175.9 169.0
2.57 2.88 0.31 1.83 1.91 175.9 169.0
H3CCOOH...OHH (13) -10.94 -9.00 1.94 2.58 2.80 0.22 1.90 1.83 156.3 168.3
2.61 3.03 0.42 2.15 2.48 127.9 115.5
trans,trans-Oxalic acid...OHH, cyclic (14) -11.23 -8.97 2.26 2.54 2.80 0.26 1.80 1.84 171.5 167.3
2.61 2.88 0.27 2.22 2.29 122.2 118.3
OHH...cis,cis-Oxalic acid, cyclic (15) -4.90 -4.20 0.70 2.97 3.16 0.19 2.44 2.21 141.1 163.8
2.96 3.03 0.07 2.35 2.78 151.7 95.3
OHH...trans,trans-Oxalic acid, cyclic (16) -3.82 -3.82 0.00 2.90 3.05 0.15 2.27 2.27 155.0 136.5
3.21 3.22 0.01 2.95 2.42 117.5 139.4
HOH...skew-Methyl vinyl ether (17) -5.20 -3.78 1.42 2.73 3.03 0.30 2.08 2.09 155.6 160.8 HOH...Methyl formate (=O) (18) -6.39 -4.58 1.81 2.76 2.98 0.22 2.06 2.00 167.0 179.2 HOH...Methyl formate (-O-) (19) -3.82 -4.58 -0.76 2.78 4.30 1.52 2.24 3.49 138.7 141.3
HOH...Acetone (21) -6.87 -4.13 2.74 2.74 2.99 0.25 2.06 2.01 163.1 179.9
t-NMA...OH2 (22) -5.97 -7.41 -1.44 2.87 2.91 0.04 2.12 1.89 178.3 179.1
HOH...t-NMA (23) -8.03 -5.67 2.36 2.70 2.95 0.25 2.00 1.98 167.6 172.2
Formamide dimer, cyclic (24) -14.78 -10.02 4.76 2.76 2.99 0.23 2.00 1.97 171.6 177.5
2.76 2.99 0.23 2.00 1.97 171.5 177.5
Formamide dimer, parallel (25) -8.12 -6.49 1.63 2.80 2.98 0.19 2.09 2.06 156.7 149.8
t-NMA dimer, antiparallel (26) -7.70 -7.88 -0.18 2.83 2.95 0.12 2.08 1.95 171.8 167.4
t-NMA antiparallel stacked (27) -3.86 -5.28 -1.42 3.65 3.84 0.19 3.88 3.69 87.9 91.1
3.67 3.84 0.17 3.88 3.69 88.9 91.1
HOH...N-Methylformamide (28) -7.90 -5.66 2.23 2.71 2.95 0.24 2.01 1.98 164.7 172.1 N-Methylformamide...OH2 (29) -6.05 -7.43 -1.38 2.84 2.90 0.06 2.09 1.88 173.8 177.0
t-N-OH,N-Meacetamide...OH2 (30) -8.21 -7.50 0.71 2.62 2.82 0.20 1.94 1.88 159.2 160.3 HOH...t-N-OH,N-Methylacetamide (31) -4.10 -8.29 -4.19 2.82 2.82 0.01 2.12 3.49 168.7 40.3 H2NH...HNH2, cyclic C2h (32) -3.51 -1.29 2.22 3.01 3.16 0.15 2.60 2.42 123.9 130.0
3.01 3.16 0.15 2.61 2.42 123.8 130.0
H2NH...NH3, linear Cs (33) -3.38 -1.38 2.00 3.15 3.20 0.05 2.42 2.19 179.0 177.7
3.15 3.20 0.05 3.64 3.25 69.8 78.4
HOH...NH3 (34) -7.22 -3.75 3.47 2.80 2.97 0.18 2.09 2.00 176.4 173.6
HOH...NH2CH3 (35) -7.07 -3.58 3.50 2.80 3.04 0.24 2.09 2.08 175.9 169.3
Imidazole...OH2 (36) -7.00 -7.81 -0.81 2.79 2.85 0.06 2.03 1.82 178.2 178.0
HOH...Imidazole (37) -7.77 -6.05 1.72 2.73 3.14 0.41 2.10 2.25 151.2 150.0
Indole...OH2 (38) -6.33 -6.82 -0.49 2.81 2.88 0.07 2.07 1.84 168.3 175.7
Pyrrole...OH2 (39) -5.89 -6.28 -0.39 2.82 2.89 0.07 2.07 1.85 180.0 180.0
HOH...Pyridine (40) -6.63 -4.25 2.38 2.77 3.20 0.42 2.12 2.32 155.8 148.8
Formamidine...H2O, cyclic (41) -11.03 -8.51 2.52 2.81 2.98 0.17 2.20 2.11 143.3 142.5
2.70 2.90 0.20 2.06 1.92 151.4 171.2
HOH...Formaldehydeimine (42) -6.74 -5.91 0.83 2.76 2.90 0.14 2.11 1.92 154.0 179.6
Guanidine...OHH (43) -7.96 -7.00 0.96 2.88 3.01 0.13 2.25 2.10 145.4 148.2
2.77 2.97 0.20 2.21 2.06 141.1 152.8
Aniline...OH2 (45) -4.62 -5.17 -0.55 2.94 2.93 -0.01 2.20 1.91 173.2 175.0
HSH...OH2 (46) -2.93 -2.19 0.74 3.33 3.58 0.25 2.29 2.84 175.3 113.4
HOH...S(CH3)2 (47) -3.56 -2.05 1.51 3.12 3.28 0.16 3.13 2.30 97.7 178.2
3.12 3.28 0.16 3.13 3.64 97.7 60.6
OHH...CH3SSCH3, cyclic (48) -3.78 -1.71 2.07 3.38 3.47 0.09 2.84 2.50 147.0 172.9
HOH...Thiophene (49) -2.66 -4.36 -1.70 3.59 3.63 0.04 3.50 3.57 108.4 85.7
3.59 3.63 0.04 3.33 2.91 121.0 131.4
HOH...SHC6H5 (50) -2.20 -4.38 -2.18 4.56 4.40 -0.16 4.27 3.77 132.4 125.0
HSH...SH2 (51) -0.96 -1.23 -0.26 4.15 3.77 -0.38 3.19 3.02 175.5 114.4
HOH...SH2 (52) -2.27 -2.75 -0.48 3.47 3.09 -0.38 2.83 2.13 175.5 167.5
CH3COOH...NH3, bidentate (53) -11.94 -6.04 5.90 2.63 2.86 0.23 1.90 1.88 168.3 177.6
2.89 3.27 0.37 2.53 2.74 119.0 112.8
HOH...PyridineN-oxide (54) -10.69 -7.20 3.49 2.63 2.87 0.24 1.94 1.89 161.0 177.3 MethylethylamineN-oxide...OHH( 55) -15.48 -21.09 -5.60 2.49 3.45 0.96 1.83 3.83 148.9 59.9
2.64 2.73 0.09 2.17 1.72 124.2 170.5
Methylethylhydroxylamine...OH2 (56) -7.41 -6.51 0.90 2.66 2.84 0.18 2.11 1.87 138.5 173.0
2.66 3.58 0.92 2.18 3.99 130.2 58.3
HOH...FCH3 (59) -4.87 -1.69 3.17 2.70 3.09 0.39 2.06 2.13 153.9 169.7
HOH...Chloropropane (58) -3.09 -1.09 1.99 3.34 4.43 1.10 2.82 3.81 143.5 125.0
H2NH...O(CH3)2 (59) -3.12 -1.18 1.94 3.00 3.36 0.36 2.32 2.35 155.9 179.8
Methylammonium...OH2 (60) -19.30 -18.81 0.49 2.70 2.74 0.04 1.79 1.74 173.8 167.9
Guanidinium...OHH (61) -18.48 -16.40 2.08 2.85 2.96 0.11 2.08 2.02 147.7 152.4
2.85 2.96 0.11 2.08 2.02 147.7 152.4
Imidazolium...OH2 (62) -16.95 -15.95 1.00 2.71 2.78 0.07 1.81 1.74 174.5 172.4
OHH...(-)O2CCH3, bidentate (66) -21.85 -17.22 4.63 2.77 2.86 0.09 2.06 2.00 142.9 143.9
2.78 2.86 0.08 2.08 2.00 141.8 143.9
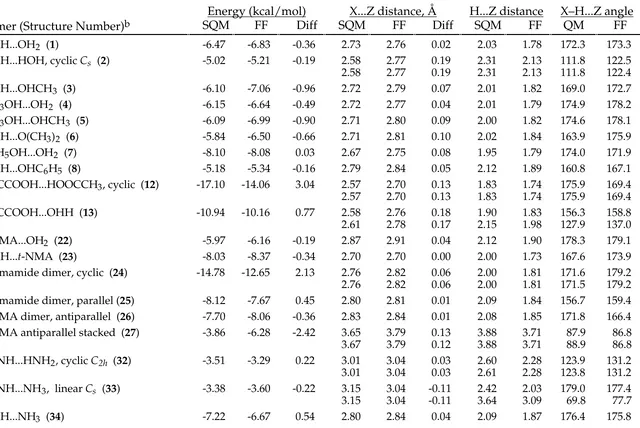
TABLE SM-IV.
_________________________________________________________________________________
CVFF vs. Scaled HF/-6-31G* Intermolecular-Interaction Energies and Distances.
Energy (kcal/mol)
X...Z distance, Å
H...Z distance
X–H...Z angle
Dimer (Structure Number)
aSQM
FF
Diff SQM
FF
Diff SQM
FF
QM
FF
HOH...OH2 (1) -6.47 -6.40 0.07 2.73 2.76 0.03 2.03 1.79 172.3 173.7
HOH...HOH, cyclic Cs (2) -5.02 -4.74 0.28 2.58 2.81 0.23 2.31 2.17 111.8 122.3
2.58 2.81 0.23 2.31 2.17 111.8 122.3
HOH...OHCH3 (3) -6.10 -4.41 1.69 2.72 2.81 0.09 2.01 1.85 169.0 168.0
CH3OH...OH2 (4) -6.15 -6.78 -0.63 2.72 2.66 -0.06 2.01 1.68 174.9 176.0
CH3OH...OHCH3 (5) -6.09 -5.03 1.06 2.71 2.69 -0.02 2.00 1.72 174.6 174.9
HOH...O(CH3)2 (6) -5.84 -5.09 0.75 2.71 2.79 0.08 2.02 1.82 163.9 173.5
C6H5OH...OH2 (7) -8.10 -7.57 0.53 2.67 2.67 0.01 1.95 1.71 174.0 169.3
HOH...OHC6H5 (8) -5.18 -4.18 1.00 2.79 2.83 0.05 2.12 1.88 160.8 167.9
t-Vinyl alcohol...OH2 (9) -8.18 -7.33 0.85 2.65 2.66 0.00 1.93 1.68 175.0 177.2 HOH...t-Vinyl alcohol (10) -5.16 -4.31 0.85 2.79 2.81 0.02 2.11 1.85 163.2 167.5
HOH...Furan (11) -4.00 -3.87 0.13 2.78 2.93 0.15 2.22 2.01 141.6 158.4
H3CCOOH...HOOCCH3, cyclic (12) -17.10 -11.14 5.96 2.57 2.66 0.09 1.83 1.70 175.9 171.7
2.57 2.66 0.09 1.83 1.70 175.9 171.7
H3CCOOH...OHH (13) -10.94 -8.82 2.12 2.58 2.66 0.08 1.90 1.70 156.3 168.5
2.61 3.00 0.39 2.15 2.40 127.9 119.5
trans,trans-Oxalic acid...OHH, cyclic (14) -11.23 -9.31 1.92 2.54 2.65 0.11 1.80 1.68 171.5 170.4
2.61 2.74 0.13 2.22 2.05 122.2 126.9
OHH...cis,cis-Oxalic acid, cyclic (15) -4.90 -4.24 0.66 2.97 3.52 0.55 2.44 3.02 141.1 113.9
2.96 2.80 -0.15 2.35 1.83 151.7 177.7
OHH...trans,trans-Oxalic acid, cyclic (16) -3.82 -9.31 -5.49 2.90 2.74 -0.16 2.27 2.05 155.0 126.9
3.21 4.83 1.62 2.95 5.72 117.5 21.0
HOH...skew-Methyl vinyl ether (17) -5.20 -4.85 0.36 2.73 2.79 0.06 2.08 1.84 155.6 165.6 HOH...Methyl formate (=O) (18) -6.39 -6.19 0.20 2.76 2.75 -0.01 2.06 1.77 167.0 176.7 HOH...Methyl formate (-O-) (19) -3.82 -3.20 0.62 2.78 4.04 1.27 2.24 4.34 138.7 65.9
HOH...Acetone (21) -6.87 -5.30 1.57 2.74 2.76 0.02 2.06 1.79 163.1 179.2
t-NMA...OH2 (22) -5.97 -4.19 1.78 2.87 3.07 0.20 2.12 2.04 178.3 176.8
HOH...t-NMA (23) -8.03 -6.80 1.23 2.70 2.74 0.04 2.00 1.77 167.6 175.6
Formamide dimer, cyclic (24) -14.78 -6.53 8.25 2.76 3.00 0.24 2.00 1.97 171.6 175.9
2.76 3.00 0.24 2.00 1.97 171.5 175.9
Formamide dimer, parallel (25) -8.12 -5.23 2.89 2.80 3.08 0.29 2.09 2.28 156.7 133.4
t-NMA dimer, antiparallel (26) -7.70 -5.69 2.01 2.83 3.02 0.19 2.08 2.00 171.8 168.8
t-NMA antiparallel stacked (27) -3.86 -4.84 -0.98 3.65 4.00 0.35 3.88 3.82 87.9 92.7
3.67 4.00 0.33 3.88 3.82 88.9 92.7
HOH...N-Methylformamide (28) -7.90 -7.18 0.72 2.71 2.73 0.02 2.01 1.76 164.7 172.1 N-Methylformamide...OH2 (29) -6.05 -4.46 1.59 2.84 3.05 0.21 2.09 2.02 173.8 169.3
t-N-OH,N-Meacetamide...OH2 (30) -8.21 -8.40 -0.19 2.62 2.64 0.02 1.94 1.68 159.2 168.0 HOH...t-N-OH,N-Methylacetamide (31) -4.10 -9.06 -4.97 2.82 2.65 -0.17 2.12 3.31 168.7 40.6 H2NH...HNH2, cyclic C2h (32) -3.51 -0.46 3.05 3.01 3.69 0.69 2.60 2.92 123.9 132.8
3.01 3.69 0.69 2.61 2.92 123.8 132.8
H2NH...NH3, linear Cs (33) -3.38 -0.50 2.88 3.15 3.69 0.53 2.42 2.66 179.0 176.0
3.15 3.69 0.53 3.64 3.80 69.8 76.0
HOH...NH3 (34) -7.22 -1.99 5.23 2.80 3.18 0.39 2.09 2.22 176.4 173.1
HOH...NH2CH3 (35) -7.07 -4.05 3.02 2.80 3.07 0.27 2.09 2.10 175.9 178.7
Indole...OH2 (38) -6.33 -2.59 3.74 2.81 3.09 0.28 2.07 2.06 168.3 176.9
Pyrrole...OH2 (39) -5.89 -2.28 3.61 2.82 3.11 0.29 2.07 2.07 180.0 180.0
HOH...Pyridine (40) -6.63 -3.36 3.27 2.77 3.48 0.70 2.12 3.26 155.8 95.0
Formamidine...H2O, cyclic (41) -11.03 -3.49 7.54 2.81 3.21 0.40 2.20 2.28 143.3 150.4
2.70 3.18 0.48 2.06 2.25 151.4 160.8
HOH...Formaldehydeimine (42) -6.74 -3.00 3.74 2.76 3.17 0.41 2.11 2.23 154.0 162.4
Guanidine...OHH (43) -7.96 -5.74 2.22 2.88 3.16 0.28 2.25 2.19 145.4 157.1
2.77 3.16 0.40 2.21 3.80 141.1 42.7
Vinylamine...OH2 (44) -3.80 -1.76 2.03 3.04 3.15 0.11 2.19 2.12 164.2 179.7
Aniline...OH2 (45) -4.62 -2.82 1.80 2.94 3.12 0.18 2.20 2.09 173.2 179.9
HOH...S(CH3)2 (47) -3.56 -1.23 2.33 3.12 3.40 0.29 3.13 3.60 97.7 70.4
3.12 3.40 0.29 3.13 2.46 97.7 166.0
OHH...CH3SSCH3, cyclic (48) -3.78 -1.32 2.46 3.38 3.41 0.03 2.84 2.48 147.0 162.6
HOH...Thiophene (49) -2.66 -3.76 -1.10 3.59 3.49 -0.10 3.50 3.75 108.4 66.7
3.59 3.49 -0.10 3.33 2.79 121.0 129.3
HOH...SHC6H5 (50) -2.20 -3.45 -1.25 4.56 4.56 -0.01 4.27 3.99 132.4 120.4
HSH...SH2 (51) -0.96 -0.46 0.50 4.15 3.51 -0.64 3.19 3.13 175.5 94.8
HOH...SH2 (52) -2.27 -1.54 0.73 3.47 3.04 -0.43 2.83 2.09 175.5 171.7
CH3COOH...NH3, bidentate (53) -11.94 -2.95 8.99 2.63 3.05 0.42 1.90 2.09 168.3 176.2
2.89 3.43 0.53 2.53 2.84 119.0 116.9
HOH...PyridineN-oxide (54) -10.69 -3.53 7.16 2.63 3.82 1.19 1.94 4.26 161.0 57.4
MethylethylamineN-oxide...OHH( 55) -15.48 -16.58 -1.10 2.49 3.50 1.01 1.83 3.85 148.9 62.2
2.64 2.84 0.21 2.17 1.80 124.2 174.1
Methylethylhydroxylamine...OH2 (56) -7.41 -6.99 0.43 2.66 2.80 0.15 2.11 2.03 138.5 135.8
2.66 2.97 0.31 2.18 2.08 130.2 151.5
HOH...FCH3 (59) -4.87 -3.91 0.96 2.70 2.81 0.10 2.06 1.84 153.9 175.5
HOH...Chloropropane (58) -3.09 -3.16 -0.08 3.34 3.09 -0.25 2.82 2.12 143.5 178.1
H2NH...O(CH3)2 (59) -3.12 -1.44 1.68 3.00 3.29 0.29 2.32 2.27 155.9 177.7
Methylammonium...OH2 (60) -19.30 -17.49 1.81 2.70 2.81 0.11 1.79 1.76 173.8 177.2
Guanidinium...OHH (61) -18.48 -5.50 12.98 2.85 3.20 0.34 2.08 3.48 147.7 65.9
2.85 3.53 0.68 2.08 3.76 147.7 69.1
Imidazolium...OH2 (62) -16.95 -13.21 3.74 2.71 2.86 0.16 1.81 1.82 174.5 175.5
Formamidinium...OH2 (63) -16.98 -2.40 14.58 2.71 3.11 0.41 1.91 2.08 148.7 179.3 Formamidinium...OH2, cyclic C2v (64) -19.59 -3.20 16.39 2.83 3.24 0.40 2.09 2.24 142.6 162.5
2.83 3.24 0.40 2.09 2.24 142.6 162.5
OHH...(-)O2CCH3, bidentate (66) -21.85 -22.84 -0.99 2.77 2.69 -0.07 2.06 1.81 142.9 147.2
2.78 2.69 -0.08 2.08 1.81 141.8 147.2
TABLE SM-VI.
_________________________________________________________________________________
MSI_CHARMm vs. Scaled HF/-6-31G* Intermolecular-Interaction Energies and Distances.
Energy (kcal/mol)
X...Z distance, Å
H...Z distance
X–H...Z angle
Dimer (Structure Number)
aSQM
FF
Diff SQM
FF
Diff SQM
FF
QM
FF
HOH...OH2 (1) -6.47 -6.83 -0.36 2.73 2.76 0.02 2.03 1.78 172.3 173.3
HOH...HOH, cyclic Cs (2) -5.02 -6.75 -1.73 2.58 2.76 0.18 2.31 1.78 111.8 177.5
2.58 2.76 0.18 2.31 3.21 111.8 53.9
HOH...OHCH3 (3) -6.10 -6.24 -0.14 2.72 2.87 0.16 2.01 1.99 169.0 151.9
CH3OH...OH2 (4) -6.15 -5.19 0.96 2.72 2.97 0.25 2.01 2.02 174.9 171.7
CH3OH...OHCH3 (5) -6.09 -6.49 -0.39 2.71 2.77 0.06 2.00 1.84 174.6 163.0
HOH...O(CH3)2 (6) -5.84 -4.59 1.25 2.71 2.93 0.22 2.02 2.10 163.9 142.7
C6H5OH...OH2 (7) -8.10 -5.66 2.44 2.67 2.98 0.31 1.95 2.03 174.0 179.5
HOH...OHC6H5 (8) -5.18 -6.75 -1.57 2.79 2.87 0.08 2.12 2.00 160.8 150.2
t-Vinyl alcohol...OH2 (9) -8.18 -6.25 1.93 2.65 2.95 0.29 1.93 1.99 175.0 179.1 HOH...t-Vinyl alcohol (10) -5.16 -5.06 0.10 2.79 2.87 0.08 2.11 2.15 163.2 130.4
HOH...Furan (11) -4.00 -3.36 0.64 2.78 3.25 0.46 2.22 2.78 141.6 110.9
H3CCOOH...HOOCCH3, cyclic (12) -17.10 -3.56 13.54 2.57 2.89 0.32 1.83 1.94 175.9 170.4
2.57 2.89 0.32 1.83 1.94 175.9 170.4
H3CCOOH...OHH (13) -10.94 -6.30 4.64 2.58 3.22 0.64 1.90 2.40 156.3 144.0
2.61 2.82 0.21 2.15 1.99 127.9 143.4
trans,trans-Oxalic acid...OHH, cyclic (14) -11.23 -4.15 7.08 2.54 3.16 0.62 1.80 2.21 171.5 174.9
2.61 2.74 0.12 2.22 1.96 122.2 135.6
OHH...cis,cis-Oxalic acid, cyclic (15) -4.90 -7.25 -2.35 2.97 2.94 -0.03 2.44 2.05 141.1 154.5
2.96 2.80 -0.15 2.35 2.70 151.7 85.9
OHH...trans,trans-Oxalic acid, cyclic (16) -3.82 -6.61 -2.79 2.90 2.84 -0.06 2.27 2.07 155.0 136.2
3.21 2.91 -0.30 2.95 2.09 117.5 141.9
HOH...skew-Methyl vinyl ether (17) -5.20 -4.79 0.41 2.73 2.90 0.17 2.08 2.13 155.6 135.7 HOH...Methyl formate (=O) (18) -6.39 -7.47 -1.08 2.76 2.90 0.14 2.06 1.97 167.0 162.0 HOH...Methyl formate (-O-) (19) -3.82 -5.68 -1.86 2.78 2.96 0.19 2.24 2.06 138.7 154.8
HOH...Acetone (21) -6.87 -6.18 0.70 2.74 2.91 0.17 2.06 1.98 163.1 161.1
t-NMA...OH2 (22) -5.97 -5.20 0.77 2.87 3.11 0.25 2.12 2.11 178.3 179.3
HOH...t-NMA (23) -8.03 -6.87 1.16 2.70 2.90 0.20 2.00 1.99 167.6 158.0
Formamide dimer, cyclic (24) -14.78 -11.68 3.11 2.76 2.91 0.15 2.00 1.90 171.6 176.2
2.76 2.91 0.15 2.00 1.90 171.5 176.2
Formamide dimer, parallel (25) -8.12 -8.38 -0.26 2.80 2.85 0.05 2.09 1.95 156.7 147.1
t-NMA dimer, antiparallel (26) -7.70 -7.83 -0.13 2.83 2.82 -0.01 2.08 1.97 171.8 140.6
t-NMA antiparallel stacked (27) -3.86 -8.08 -4.22 3.65 3.39 -0.26 3.88 3.55 87.9 72.8
3.67 3.39 -0.28 3.88 3.55 88.9 72.8
HOH...N-Methylformamide (28) -7.90 -6.81 1.09 2.71 2.96 0.26 2.01 2.01 164.7 173.0 N-Methylformamide...OH2 (29) -6.05 -5.39 0.66 2.84 3.08 0.24 2.09 2.08 173.8 177.0
t-N-OH,N-Meacetamide...OH2 (30) -8.21 -8.21 0.00 2.62 2.90 0.28 1.94 2.08 159.2 143.6 HOH...t-N-OH,N-Methylacetamide (31) -4.10 -8.21 -4.11 2.82 2.90 0.09 2.12 3.46 168.7 48.2 H2NH...HNH2, cyclic C2h (32) -3.51 -5.60 -2.09 3.01 2.75 -0.26 2.60 2.03 123.9 125.8
3.01 2.75 -0.26 2.61 2.03 123.8 125.8
H2NH...NH3, linear Cs (33) -3.38 -4.52 -1.14 3.15 2.75 -0.40 2.42 2.03 179.0 125.8
3.15 2.75 -0.40 3.64 2.03 69.8 125.8
HOH...NH3 (34) -7.22 -6.39 0.82 2.80 2.96 0.16 2.09 2.02 176.4 162.9
HOH...NH2CH3 (35) -7.07 -2.43 4.65 2.80 3.15 0.36 2.09 2.26 175.9 155.6
Imidazole...OH2 (36) -7.00 -10.83 -3.83 2.79 2.94 0.15 2.03 1.96 178.2 176.0
HOH...Imidazole (37) -7.77 -7.42 0.35 2.73 2.81 0.08 2.10 2.32 151.2 111.4
Indole...OH2 (38) -6.33 -5.31 1.01 2.81 3.04 0.24 2.07 2.07 168.3 168.9
Pyrrole...OH2 (39) -5.89 -8.75 -2.85 2.82 2.97 0.15 2.07 1.98 180.0 180.0
HOH...Pyridine (40) -6.63 -4.56 2.08 2.77 2.99 0.22 2.12 2.31 155.8 127.1
Formamidine...H2O, cyclic (41) -11.03 -3.14 7.90 2.81 3.06 0.24 2.20 2.22 143.3 139.1
2.70 3.26 0.56 2.06 2.59 151.4 126.8
HOH...Formaldehydeimine (42) -6.74 -2.94 3.81 2.76 3.15 0.39 2.11 2.38 154.0 136.8
Guanidine...OHH (43) -7.96 -8.93 -0.97 2.88 2.96 0.08 2.25 2.03 145.4 151.7
2.77 2.97 0.21 2.21 3.60 141.1 43.1
Aniline...OH2 (45) -4.62 -4.17 0.45 2.94 3.01 0.07 2.20 2.41 173.2 117.7
HSH...OH2 (46) -2.93 -2.96 -0.03 3.33 3.08 -0.25 2.29 2.34 175.3 111.0
HOH...S(CH3)2 (47) -3.56 -1.68 1.87 3.12 3.24 0.13 3.13 2.77 97.7 111.2
3.12 3.24 0.13 3.13 2.78 97.7 110.7
OHH...CH3SSCH3, cyclic (48) -3.78 -1.20 2.59 3.38 3.42 0.04 2.84 3.19 147.0 95.4
HOH...Thiophene (49) -2.66 -4.61 -1.95 3.59 3.21 -0.39 3.50 3.13 108.4 86.2
3.59 3.21 -0.39 3.33 2.38 121.0 143.2
HOH...SHC6H5 (50) -2.20 -5.63 -3.43 4.56 3.30 -1.26 4.27 2.45 132.4 146.9
HSH...SH2 (51) -0.96 -1.39 -0.43 4.15 3.22 -0.93 3.19 2.48 175.5 111.7
HOH...SH2 (52) -2.27 -2.31 -0.04 3.47 3.15 -0.32 2.83 2.36 175.5 139.1
CH3COOH...NH3, bidentate (53) -11.94 -5.56 6.37 2.63 2.95 0.33 1.90 2.08 168.3 151.5
2.89 2.86 -0.03 2.53 1.89 119.0 157.9
HOH...PyridineN-oxide (54) -10.69 -11.75 -1.06 2.63 2.78 0.15 1.94 1.96 161.0 141.9 MethylethylamineN-oxide...OHH (55) -15.48 -12.82 2.66 2.49 2.84 0.36 1.83 1.97 148.9 148.0
2.64 2.76 0.12 2.17 1.96 124.2 130.4
Methylethylhydroxylamine...OH2 (56) -7.41 -8.95 -1.54 2.66 2.74 0.08 2.11 2.99 138.5 65.7
2.66 3.10 0.44 2.18 3.20 130.2 75.0
HOH...FCH3 (59) -4.87 -3.52 1.35 2.70 3.08 0.37 2.06 2.23 153.9 146.4
HOH...Chloropropane (58) -3.09 -2.72 0.37 3.34 3.55 0.21 2.82 2.72 143.5 145.4
H2NH...O(CH3)2 (59) -3.12 -3.51 -0.39 3.00 3.02 0.02 2.32 2.01 155.9 178.4
Methylammonium...OH2 (60) -19.30 -20.25 -0.95 2.70 2.76 0.06 1.79 1.88 173.8 140.5
Guanidinium...OHH (61) -18.48 -20.18 -1.70 2.85 2.88 0.03 2.08 1.97 147.7 148.1
2.85 2.88 0.03 2.08 1.97 147.7 148.1
Imidazolium...OH2 (62) -16.95 -17.18 -0.23 2.71 2.88 0.18 1.81 1.94 174.5 159.0 OHH...(-)O2CCH3, bidentate (66) -21.85 -17.23 4.62 2.77 2.93 0.16 2.06 2.11 142.9 140.6
2.78 2.93 0.15 2.08 2.11 141.8 140.6
TABLE SM-VII.
________________________________________________________________________________
CHARMM 22
avs. Scaled HF/-6-31G* Intermolecular-Interaction Energies and Distances.
Energy (kcal/mol)
X...Z distance, Å
H...Z distance
X–H...Z angle
Dimer (Structure Number)
bSQM
FF
Diff SQM
FF
Diff SQM
FF
QM
FF
HOH...OH2 (1) -6.47 -6.83 -0.36 2.73 2.76 0.02 2.03 1.78 172.3 173.3
HOH...HOH, cyclic Cs (2) -5.02 -5.21 -0.19 2.58 2.77 0.19 2.31 2.13 111.8 122.5
2.58 2.77 0.19 2.31 2.13 111.8 122.4
HOH...OHCH3 (3) -6.10 -7.06 -0.96 2.72 2.79 0.07 2.01 1.82 169.0 172.7
CH3OH...OH2 (4) -6.15 -6.64 -0.49 2.72 2.77 0.04 2.01 1.79 174.9 178.2
CH3OH...OHCH3 (5) -6.09 -6.99 -0.90 2.71 2.80 0.09 2.00 1.82 174.6 178.1
HOH...O(CH3)2 (6) -5.84 -6.50 -0.66 2.71 2.81 0.10 2.02 1.84 163.9 175.9
C6H5OH...OH2 (7) -8.10 -8.08 0.03 2.67 2.75 0.08 1.95 1.79 174.0 171.9
HOH...OHC6H5 (8) -5.18 -5.34 -0.16 2.79 2.84 0.05 2.12 1.89 160.8 167.1
H3CCOOH...HOOCCH3, cyclic (12) -17.10 -14.06 3.04 2.57 2.70 0.13 1.83 1.74 175.9 169.4
2.57 2.70 0.13 1.83 1.74 175.9 169.4
H3CCOOH...OHH (13) -10.94 -10.16 0.77 2.58 2.76 0.18 1.90 1.83 156.3 158.8
2.61 2.78 0.17 2.15 1.98 127.9 137.0
t-NMA...OH2 (22) -5.97 -6.16 -0.19 2.87 2.91 0.04 2.12 1.90 178.3 179.1
HOH...t-NMA (23) -8.03 -8.37 -0.34 2.70 2.70 0.00 2.00 1.73 167.6 173.9
Formamide dimer, cyclic (24) -14.78 -12.65 2.13 2.76 2.82 0.06 2.00 1.81 171.6 179.2
2.76 2.82 0.06 2.00 1.81 171.5 179.2
Formamide dimer, parallel (25) -8.12 -7.67 0.45 2.80 2.81 0.01 2.09 1.84 156.7 159.4
t-NMA dimer, antiparallel (26) -7.70 -8.06 -0.36 2.83 2.84 0.01 2.08 1.85 171.8 166.4
t-NMA antiparallel stacked (27) -3.86 -6.28 -2.42 3.65 3.79 0.13 3.88 3.71 87.9 86.8
3.67 3.79 0.12 3.88 3.71 88.9 86.8
H2NH...HNH2, cyclic C2h (32) -3.51 -3.29 0.22 3.01 3.04 0.03 2.60 2.28 123.9 131.2
3.01 3.04 0.03 2.61 2.28 123.8 131.2
H2NH...NH3, linear Cs (33) -3.38 -3.60 -0.22 3.15 3.04 -0.11 2.42 2.03 179.0 177.4
3.15 3.04 -0.11 3.64 3.09 69.8 77.7
HOH...NH2CH3 (35) -7.07 -7.61 -0.54 2.80 2.84 0.05 2.09 1.87 175.9 173.3
Imidazole...OH2 (36) -7.00 -6.85 0.16 2.79 2.87 0.08 2.03 1.85 178.2 176.9
HOH...Imidazole (37) -7.77 -8.44 -0.67 2.73 2.84 0.11 2.10 1.87 151.2 172.8
Indole...OH2 (38) -6.33 -5.07 1.26 2.81 2.89 0.09 2.07 1.90 168.3 178.8
HSH...OH2 (46) -2.93 -3.19 -0.26 3.33 3.31 -0.02 2.29 1.99 175.3 171.6
HOH...S(CH3)2 (47) -3.56 -2.70 0.86 3.12 3.37 0.25 3.13 3.75 97.7 59.5
3.12 3.37 0.25 3.13 2.41 97.7 176.7
OHH...CH3SSCH3, cyclic (48) -3.78 -2.78 1.00 3.38 3.46 0.08 2.84 2.66 147.0 140.5
HSH...SH2 (51) -0.96 -1.66 -0.69 4.15 3.75 -0.40 3.19 2.44 175.5 168.0
HOH...SH2 (52) -2.27 -2.30 -0.03 3.47 3.32 -0.16 2.83 2.37 175.5 167.7
CH3COOH...NH3, bidentate (53) -11.94 -9.23 2.70 2.63 2.81 0.18 1.90 1.86 168.3 168.0
2.89 3.02 0.13 2.53 2.28 119.0 129.6
H2NH...O(CH3)2 (59) -3.12 -3.82 -0.70 3.00 3.02 0.02 2.32 2.01 155.9 175.9
Methylammonium...OH2 (60) -19.30 -19.83 -0.53 2.70 2.75 0.05 1.79 1.69 173.8 177.2
Guanidinium...OHH (61) -18.48 -18.63 -0.15 2.85 2.89 0.04 2.08 1.96 147.7 152.6
2.85 2.89 0.04 2.08 1.96 147.7 152.7
Imidazolium...OH2 (62) -16.95 -16.83 0.12 2.71 2.75 0.04 1.81 1.72 174.5 178.3
OHH...(-)O2CCH3, bidentate (66) -21.85 -24.18 -2.33 2.77 2.67 -0.10 2.06 1.80 142.9 146.4
2.78 2.67 -0.11 2.08 1.80 141.8 146.4
a
See text for a specification of cases in which "cloned" force field parameters were employed.
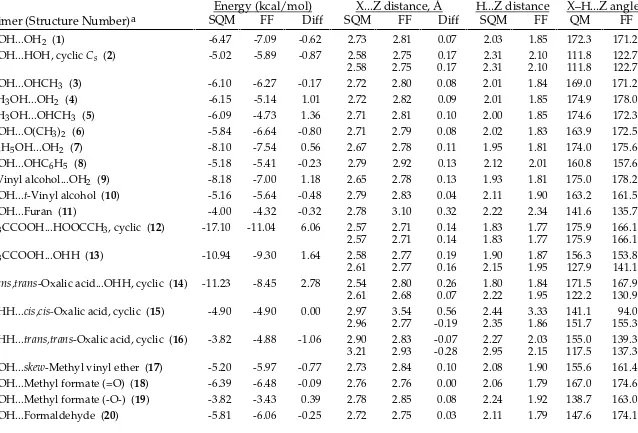
TABLE SM-VIII.
_______________________________________________________________________________
AMBER* vs. Scaled HF/-6-31G* Intermolecular-Interaction Energies and Distances.
Energy (kcal/mol)
X...Z distance, Å
H...Z distance
X–H...Z angle
Dimer (Structure Number)
aSQM
FF
Diff SQM
FF
Diff SQM
FF
QM
FF
HOH...OH2 (1) -6.47 -7.09 -0.62 2.73 2.81 0.07 2.03 1.85 172.3 171.2
HOH...HOH, cyclic Cs (2) -5.02 -5.89 -0.87 2.58 2.75 0.17 2.31 2.10 111.8 122.7
2.58 2.75 0.17 2.31 2.10 111.8 122.7
HOH...OHCH3 (3) -6.10 -6.27 -0.17 2.72 2.80 0.08 2.01 1.84 169.0 171.2
CH3OH...OH2 (4) -6.15 -5.14 1.01 2.72 2.82 0.09 2.01 1.85 174.9 178.0
CH3OH...OHCH3 (5) -6.09 -4.73 1.36 2.71 2.81 0.10 2.00 1.85 174.6 172.3
HOH...O(CH3)2 (6) -5.84 -6.64 -0.80 2.71 2.79 0.08 2.02 1.83 163.9 172.5
C6H5OH...OH2 (7) -8.10 -7.54 0.56 2.67 2.78 0.11 1.95 1.81 174.0 175.6
HOH...OHC6H5 (8) -5.18 -5.41 -0.23 2.79 2.92 0.13 2.12 2.01 160.8 157.6
t-Vinyl alcohol...OH2 (9) -8.18 -7.00 1.18 2.65 2.78 0.13 1.93 1.81 175.0 178.2 HOH...t-Vinyl alcohol (10) -5.16 -5.64 -0.48 2.79 2.83 0.04 2.11 1.90 163.2 161.5
HOH...Furan (11) -4.00 -4.32 -0.32 2.78 3.10 0.32 2.22 2.34 141.6 135.7
H3CCOOH...HOOCCH3, cyclic (12) -17.10 -11.04 6.06 2.57 2.71 0.14 1.83 1.77 175.9 166.1
2.57 2.71 0.14 1.83 1.77 175.9 166.1
H3CCOOH...OHH (13) -10.94 -9.30 1.64 2.58 2.77 0.19 1.90 1.87 156.3 153.8
2.61 2.77 0.16 2.15 1.95 127.9 141.1
trans,trans-Oxalic acid...OHH, cyclic (14) -11.23 -8.45 2.78 2.54 2.80 0.26 1.80 1.84 171.5 167.9
2.61 2.68 0.07 2.22 1.95 122.2 130.9
OHH...cis,cis-Oxalic acid, cyclic (15) -4.90 -4.90 0.00 2.97 3.54 0.56 2.44 3.33 141.1 94.0
2.96 2.77 -0.19 2.35 1.86 151.7 155.3
OHH...trans,trans-Oxalic acid, cyclic (16) -3.82 -4.88 -1.06 2.90 2.83 -0.07 2.27 2.03 155.0 139.3
3.21 2.93 -0.28 2.95 2.15 117.5 137.3
HOH...skew-Methyl vinyl ether (17) -5.20 -5.97 -0.77 2.73 2.84 0.10 2.08 1.90 155.6 161.4 HOH...Methyl formate (=O) (18) -6.39 -6.48 -0.09 2.76 2.76 0.00 2.06 1.79 167.0 174.6 HOH...Methyl formate (-O-) (19) -3.82 -3.43 0.39 2.78 2.85 0.08 2.24 1.92 138.7 163.0
HOH...Acetone (21) -6.87 -6.29 0.58 2.74 2.75 0.01 2.06 1.79 163.1 179.1
t-NMA...OH2 (22) -5.97 -5.57 0.40 2.87 2.90 0.03 2.12 1.88 178.3 177.3
HOH...t-NMA (23) -8.03 -8.75 -0.72 2.70 2.72 0.02 2.00 1.75 167.6 179.4
Formamide dimer, cyclic (24) -14.78 -15.04 -0.26 2.76 2.75 0.00 2.00 1.74 171.6 176.7
2.76 2.75 0.00 2.0