Supplementary Material for
ICFF: A New Method to Incorporate Implicit Flexibility into an
Internal Coordinate Force Field
VSEVOLOD KATRITCH, MAXIM TOTROV and RUBEN ABAGYAN
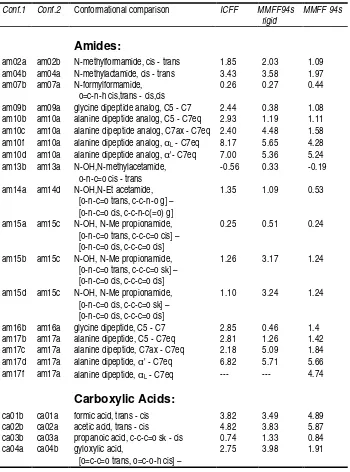
Table SM-I.
Comparison of equilibrium conformational energies (kcal/mol) for ICFF
and MMFF94s rigid models with published MMFF94s results. Initial geometries and
corresponding MMFF94s energies for this molecular set were adopted from the MMFF94
evaluation suite (
http://ccl.osc.edu/cca/data/ff_evaluation_suite/index.shtml
) and
supplementary material to
J. Comp. Chem
. (1999),
20
(7), pp.730-748.
Conf.1 Conf.2 Conformational comparison ICFF MMFF94s rigid
MMFF 94s
Amides:
am02a am02b N-methylformamide, cis - trans 1.85 2.03 1.09
am04b am04a N-methylactamide, cis - trans 3.43 3.58 1.97
am07b am07a N-formylformamide, o=c-n-h cis,trans - cis,cis am13b am13a N-OH,N-methylacetamide,
o-n-c=o cis - trans
-0.56 0.33 -0.19
am14a am14d N-OH,N-Et acetamide, [o-n-c=o trans, c-c-n-o g] – [o-n-c=o cis, c-c-n-c(=o) g]
1.35 1.09 0.53
am15a am15c N-OH, N-Me propionamide, [o-n-c=o trans, c-c-c=o cis] – [o-n-c=o cis, c-c-c=o cis]
0.25 0.51 0.24
am15b am15c N-OH, N-Me propionamide, [o-n-c=o trans, c-c-c=o sk] – [o-n-c=o cis, c-c-c=o cis]
1.26 3.17 1.24
am15d am15c N-OH, N-Me propionamide, [o-n-c=o cis, c-c-c=o sk] –
ca04a ca04b gyloxylic acid,
[o=c-c=o trans, o=c-o-h cis] –
[o=c-c=o trans, o=c-o-h trans]
ca05b ca05a glycolic acid, o=c-c-o sk - cis 0.86 0.26 0.8
ca07a ca07b propenoic acid, c=c-c=o trans – cis 0.39 0.07 0.25 ca08c ca08a oxalic acid,
[o=c-c=o trans, o=c-o-h cis,trans] – [o=c-c=o trans, o=c-o-h trans,trans]
3.01 4.23 1.9
ca09b ca09a pyruvic acid,
[o=c-c=o cis, o=c-o-h cis] – cj03a cj03b 2-methyl-but-1-ene-3-one,
c=c-c=o cis - trans cj09b cj09a 2-methylpropenoic acid,
c=c-c=o cis – trans
0.69 1.03 0.62
cj13b cj13a 1,3-pentadiene,
[c=c-c=c g, c-c=c-c trans] – [c=c-c=c s-trans, c-c=c-c trans]
1.93 2.70 2.46
cj13c cj13a 1,3-pentadiene, [s-trans, c-c=c-c cis] –
co06b co06a methyl ispropyl ketone, o=c-c(ch3)2-h cis - trans
0.85 1.55 0.89
co07b co07a butyraldehyde, c-c-c-c g - a 0.12 0.20 0.11
co08a co08c but-3-enal,
[c=c-c-c sk, c-c-c=o cis] – [c=c-c-c sk+, c-c-c=o sk+]
0.41 0.00 -0.05
co08b co08c but-3-enal,
[c=c-c-c sk-, c-c-c=o sk+] – [c=c-c-c sk+, c-c-c=o sk+]
1.00 0.99 0.98
co09a co09b 3-methyl-but-3-enal, [c=c-c-c sk, c-c-c=o cis] – [c=c-c-c sk, c-c-c=o sk]
co10b co10a isobutyraldehyde, h-c(=0)-c-h a - g 0.42 1.30 0.56 co12a co12b 2-formylpropanal, o-c-c-c(=o) a - g 0.30 0.44 0.14 co13a co13b 4-oxobutanal (o=c-c-c, c-c-c=o cis, cis),
c-c-c-c s-trans - g
im01a im01b formamidine (N puckered), h-n=c-n cis - trans
1.32 1.54 2.03
im04a im04b N-methylformamidine (N puckered), n-c=n-c cis - trans
1.35 1.09 2.
im07b im07a butadiene schiff base,
[c=c-c=n s-cis, h-n=c-c trans] – [c=c-c=n s-trans, h-n=c-c cis]
1.70 1.83 1.94
Ketals, acetals, and
hemiacetals:
kt03b kt03a 2,4-dioxapentane, [c-o-c-o g, o-c-o-c a] – [c-o-c-o g+, o-c-o-c g+]
2.23 2.42 2.11
kt04b kt04a 2,5-dimethyl-1,3-dioxane, 5-ax - 5-eq 0.35 -0.01 0.5 kt05b kt05a methoxymethanol,
[c-o-c-o g+, o-c-o-h g-] –
nh03a nh03b isopropylamine, [C1, h-c-n-lp g] –
[Cs, h-c-n-lp a]
0.51 0.30 0.45
nh11b nh11a ethylamine, c-c-n-lp a - g 0.52 0.29 0.44
nh16c nh16a 3-aminopropene, [c=c-c-n sk, c-c-n-lp a] – [c=c-c-n sk, c-c-n-lp g]
0.47 0.28 0.65
nh16b nh16a 3-aminopropene, [c=c-c-n cis, c-c-n-lp g] – [c=c-c-n sk, c-c-n-lp g]
-0.36 -0.62 0.51
nh17b nh17a 2-methyl,3-aminopropene, [c=c-c-n cis, c-c-n-lp g] – [c=c-c-n sk, c-c-n-lp g]
-0.25 -0.23 0.61
nh18a nh18b ethylenediamine,
[n-c-c-n a, c-c-n-lp g+, g+] – [n-c-c-n g+, c-c-n-lp g+, g+]
1.00 2.05 1.45
nh21b nh21a methylethylhydroxylamine, c-n-c-c g - a 2.58 2.91 2.25
nh22b nh22a ethylamine N-oxide, o-n-c-c a - g 0.74 0.96 0.73
nh23b nh23a ethylhydroxylamine, o-n-c-c a - g -0.01 0.52 0.05
Alcohols:
oh02a oh02b ethanol, a - g 0.23 -0.03 -0.18
oh03b oh03a n-propanol,
[c-c-c-o g-, c-c-o-h g+] – [c-c-c-o a, c-c-o-h g]
0.50 0.71 0.58
oh03c oh03a n-propanol,
[c-c-c-o g+, c-c-o-h g+] – [c-c-c-o a, c-c-o-h g]
0.20 0.17 0.24
oh03d oh03a n-propanol,
[c-c-c-o a, c-c-o-h a] – [c-c-c-o a, c-c-o-h g]
-0.23 0.07 -0.17
oh03e oh03a n-propanol,
[c-c-c-o g, c-c-o-h a] –
oh12a oh12c propen-3-ol,
[c=c-c-o sk, c-c-o-h a] – [c=c-c-o sk, c-c-o-h g]
1.09 1.75 1.1
oh12b oh12c propen-3-ol,
[c=c-c-o cis, c-c-o-h a] – [c=c-c-o sk, c-c-o-h g]
0.62 0.63 1.13
oh13b oh13a 2-me-propen-3-ol, [c=c-c-o c, c-c-o-h a] –
oh15a oh15b 1,2-ethanediol,
[h-o-c-c a, o-c-c-o a, c-c-o-h a] – [h-o-c-c g-, o-c-c-o g+, c-c-o-h a]
2.64 3.46 2.91
oh15c oh15b 1,2-ethanediol,
[h-o-c-c g-, o-c-c-o g+, c-c-o-h g+] – [h-o-c-c g-, o-c-c-o g+, c-c-o-h a]
1.13 0.74 1.15
oh15d oh15b 1,2-ethanediol,
[h-o-c-c g+, o-c-c-o g-, c-c-o-h g+] – or03b or03a diethyl ether,
[c-c-o-c a, c-o-c-c g] –
[c-c-o-c a, c-o-c-c a]
re04b re04a 1-pentene (c=c-c-c skew), c-c-c-c g - a
re10b re10a 2-pentene,
[c-c=c-c cis, c-c-c=c sk] – sr09b sr09a 1-propanethiol,
[s-c-c-c g-, h-s-c-c g+] - [s-c-c-c a, h-s-c-c g]
0.41 0.52 0.37
sr09c sr09a 1-propanethiol, [s-c-c-c g, h-s-c-c a] – [s-c-c-c a, h-s-c-c g]
0.80 0.96 0.95
sr11a sr11c 1,2-ethanedithiol, [all anti] –
[h-s-c-c a,g+, s-c-c-s g-]
1.03 2.38 1.17
sr11b sr11c 1,2-ethanedithiol, [h-s-c-c a,a, s-c-c-s g] – [h-s-c-c a,g+, s-c-c-s g-]
2.13 2.38 2.24
sr12b sr12a methyl propyl sulfide, [c-s-c-c g-, s-c-c-c g-] – [c-s-c-c g, s-c-c-c a]
0.12 0.25 0.05
sr12c sr12a methyl propyl sulfide, [c-s-c-c a, s-c-c-c g] – [c-s-c-c g, s-c-c-c a]
0.48 0.64 0.6
sr12d sr12a methyl propyl sulfide, [c-s-c-c a, s-c-c-c a] – [c-s-c-c g, s-c-c-c a]
0.38 0.50 0.33