Summary Bur oak (Quercus macrocarpa Michx.) and chin-quapin oak (Q. muehlenbergii Engl.) leaves were exposed to high temperatures at various photosynthetic photon flux den-sities under laboratory conditions to determine if species-spe-cific responses to these factors were consistent with the distribution of these oaks in gallery forests in the tallgrass prairies of northeastern Kansas, USA. Measurements of the ratio of chlorophyll fluorescence decrease, Rfd, indicated that chinquapin oak maintained greater photosynthetic capacity than bur oak across all tested combinations of irradiance (100, 400, 700 and 1000 µmol m−2 s−1) and temperature (40, 42, 44, 46 and 48 °C). In both oak species, manipulation of leaf temperature to about 47 °C for 45 min in the field led to a 45% decrease in carbon assimilation up to one week after the heat treatment, and to sharp reductions in stomatal conductance. Photosynthetic recovery patterns indicated that bur oak took longer to recover from heat stress than chinquapin oak, sug-gesting that heat stress may be important in determining distri-bution patterns of these oak species. Based on a comparison of the results with data from other forest species, we conclude that the photosynthetic temperature tolerances of bur oak and chin-quapin oaks facilitate their dominance at the western limit of the eastern deciduous forest.
Keywords: chlorophyll fluorescence, heat stress, Quercus mar-crocarpus, Quercus muehlenbergii.
Introduction
High temperatures can affect trees through impacts on growth and reproductive performance (Burton and Bazzaz 1991, Ko-zlowski et al. 1991, Bassow et al. 1994), seasonal photosyn-thetic acclimation (Berry and Bjorkman 1980, Seemann et al. 1984, Jurik et al. 1988, Hamerlynck and Knapp 1994a), distri-bution at several scales (Kozlowski et al. 1991, Jeffree and Jeffree 1994, Vann et al. 1994) and community structure (Bur-ton and Bazzaz 1991, Kozlowski et al. 1991, Kramer 1995). Leaf temperature can rise well above air temperature when solar radiative inputs exceed evaporative and convective heat losses (Campbell 1977, Jones 1992). For this reason, plant
responses to temperature should not be studied independently of irradiances because irradiance partially determines leaf tem-peratures in the field (Berry and Bjorkman 1980).
We have studied the interacting effects of light and tempera-ture on two North American eastern deciduous forest oak species growing at the western limit of their range. Bur oak (Quercus macrocarpa Michx.) and chinquapin oak (Quercus muehlenbergii Engl.) are canopy dominants in gallery forests lining streams dissecting tallgrass prairie (Abrams 1986). Water relations are a strong determinant of local tree distribu-tion in these forests, with bur oak occupying more mesic, closed-canopy gallery forest locations, whereas chinquapin oak establishes in open, xeric upland sites (Abrams 1986, Abrams and Knapp 1986, Bragg et al. 1993). Leaf-level char-acteristics correlate fairly well with distributional patterns, with chinquapin oak having smaller leaves, lower thetic rates and stomatal conductances, and higher photosyn-thetic temperature tolerance than bur oak (Hamerlynck and Knapp 1994a). Although the independent effects of light and high temperature on oak performance in tallgrass prairie gal-lery forests have been intensively studied (Knapp 1992, Hamerlynck and Knapp 1994a and 1994b), the interactive effects of these variables on photosynthesis of bur oak and chinquapin oak in this ecotonal system are unknown.
We manipulated light and temperature under laboratory con-ditions to assess species-specific responses to light and tem-perature, and we also manipulated leaf temperatures of attached entire leaves in the field under full-sun conditions to determine if photosynthetic recovery from heat stress corre-lated with the observed distribution of these species in tallgrass prairie gallery forests (Abrams 1986, Abrams and Knapp 1986, Bragg et al. 1993). Despite much research on the ecological implications of temperature optima in tree species (Chabot and Lewis 1976, Strain et al. 1976, Teskey et al. 1986, Jurik et al. 1988, Ranney and Peet 1994), few studies have been under-taken to assess the importance of photosynthetic recovery after heat stress (Methey and Trabaud 1993, Bassow et al. 1994).
Specifically, we tested four hypotheses. (1) Chinquapin oak has greater temperature tolerance across all irradiances than bur oak, facilitating its establishment in drier, more open
Photosynthetic and stomatal responses to high temperature and light
in two oaks at the western limit of their range
ERIK HAMERLYNCK
1,2and ALAN K. KNAPP
11
Division of Biology, Kansas State University, Ackert Hall, Manhattan, KS 66506-4901, USA
2 Present address: Department of Biological Sciences, University of Nevada Las Vegas, 4505 Maryland Parkway, Box 454004, Las Vegas, NV
89154-4004, USA
Received May 25, 1995
gallery forest locations. There is evidence that chinquapin oak is more drought tolerant than bur oak (Abrams 1986, Abrams and Knapp 1986, Abrams 1990), and that drought tolerance can accompany thermal tolerance and a high light requirement (Berry and Bjorkman 1980, Seemann et al. 1984, Ivanov et al. 1992). (2) Low irradiance (about 100 µmol m−2 s−1) and high temperature cause stress in bur oak and chinaquapin oak. Elevated temperature depresses the photosynthetic light com-pensation point as a result of increased respiration rates rela-tive to photosynthetic rates (Berry and Bjorkman 1980). (3) Chinaquapin oak and bur oak have tolerances to combinations of light and temperature that correlate with seedling microen-vironment conditions. Bur oak seedlings establish in lower light (about 400 µmol m−2 s−1) microsites than chinquapin oak seedlings (700 µmol m−2 s−1) (Bragg et al. 1993). We also postulated that, over the PPFD range 400--700 µmol m−2 s−1, the photosynthetic capacity of bur oak would be depressed more at higher temperature than that of chinquapin oak. (4) Photosynthetic recovery from heat stress is slower in bur oak than in chinquapin oak as a result of the larger leaves and heat-induced reductions of gs in bur oak.
Materials and methods
Study area
Measurements were made from July 7 to September 9, 1994 on trees growing in the Konza Prairie Research Natural Area (KPRNA, 39° N 96°32′ W), a 3487-ha tallgrass prairie pre-serve in the Flint Hills of northeastern Kansas, USA. Warm-season C4 grasses are the dominant vegetation on KPRNA, though extensive gallery forests line streambeds dissecting the prairie (Abrams 1986, Knight et al. 1994). The climate is warm continental, with hot, humid summers, and cold, dry winters (Borchert 1950). Growing season temperatures range from 17 to 43 °C. Tallgrass prairie is characterized by frequent and unpredictable drought (Borchert 1950, Fahnestock and Knapp 1994), and growing season conditions of high, though variable, photosynthetic photon flux densities (PPFD, ranging from 100 to 2100 µmol m−2 s−1, Knapp 1985, Knapp 1993, Fay and Knapp 1993). Compared to mean values over the last 10 years, rainfall during the 1994 growing season was average, and temperatures were slightly lower than average in July but typical for August and September.
Fluorescence and oxygen evolution measurements
Bur oak and chinquapin oak leaves were collected between July 7 and August 8, 1994, at representative locations in gallery forests on KPRNA. Leaves were harvested from 0930 to 1030 h CST, sealed in plastic bags containing moistened paper to maintain turgor, and transported to the laboratory for analy-sis of concurrent photosynthetic oxygen evolution and chloro-phyll fluorescence. Five leaves of each species were exposed for 45 min to 20 treatment combinations of temperature (40, 42, 44, 46, and 48 °C) and PPFD (100, 400, 700, and 1000
µmol m−2 s−1), for a total sample size of 200. Oxygen evolution was measured with a Clark type oxygen electrode (Hansatech Instruments, Ltd., Kings Lynne, UK) in saturating (5%) CO2
conditions provided by a bicarbonate buffer. The experimental leaf temperatures were maintained during the measurements by attaching the electrode housing to a water bath (Isotemp Model 9000, Fisher Scientific, Pittsburgh, PA). Differences in water bath and leaf chamber temperatures were accounted for by correlating water bath temperatures with leaf temperatures measured with a fine wire type copper-constantan thermocou-ple. Chlorophyll fluorescence was measured with a FDP/2 fluorescence probe and TR1 transient recorder (Hansatech Instruments). The ratio of fluorescence decrease, Rfd ((Fp− Ft)/Ft, where Fp = peak fluorescence yield, Ft = steady-state trace fluorescence after illumination), provides an index of photosynthetic capacity (Lichtenthaler 1988, Lichtenthaler and Rinderle 1988), where Rfd > 3.0 indicates unimpaired photosynthetic function, Rfd < 1.0 indicates irreversible photo-synthetic damage, and intermediate values indicate reversible photosynthetic stress (Lichtenthaler 1988). Use of Rfd as an indicator of photosynthetic capacity eliminates the need for accurate estimation of baseline fluorescence, Fo, which is often difficult at PPFDs > 100 µmol m−2 s−1 (Lavorel and Etienne 1977). Before each chlorophyll fluoresence measurement, leaf samples were dark acclimated for 5 min at the measurement temperature, and then exposed for 15 min to red light (λ = 660 nm, half-power bandwidth ± 30 nm) from an LED light source (LH36U, Hansatech Instruments).
Field gas exchange measurements
estimated from the equations of von Caemmerer and Farquhar (1981). System parameters were used to calculate leaf transpi-ration in the cuvette, which was used to estimate instantaneous water use efficiency (WUE, µmol CO2 µmol−1 H2O). Leaf temperature (Tleaf) was measured by a fine wire thermocouple pressed to the underside of the leaf throughout measurement. Statistical analysis
Three-way analysis of variance (Statistix v4.1, Analytical Software, St. Paul, MN) was used to detect differences in Rfd and O2 evolution, using species, temperature, and irradiance as main effects. Additionally, two-tailed t-tests were used to de-termine if mean Rfd departed significantly from critical values of 3.0 and 1.0 (Zar 1974). Field gas exchange measurements were repeated measures, and a split-plot analysis of variance was used, with species as the whole-plot factor and recovery time as the sub-plot factor. A level of 0.05 was considered significant, with means separation by LSD.
Results
Fluorescence and oxygen evolution
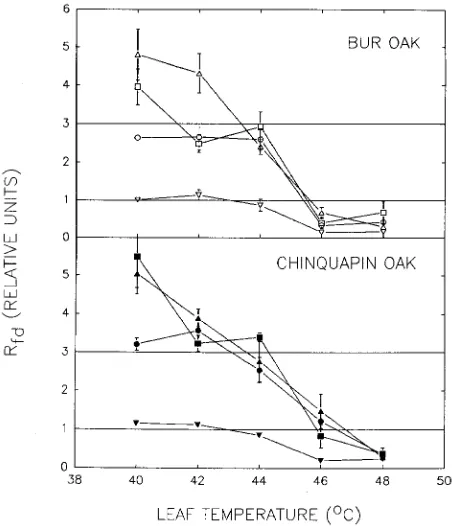
Pooled across all temperature and light treatment combina-tions, chinquapin oak maintained higher Rfd (2.07 ± 0.175 (SE)) than bur oak (1.74 ± 0.155) (F = 12.41, df = 1,160, P <
0.05) (Figure 1). In bur oak, Rfd was reduced below the critical values of 3.0 and 1.0 more often than in chinquapin oak. Over the PPFD range 400--1000 µmol m−2 s−1 and the temperature range 40--44 °C, bur oak had no Rfd values significantly above 3.0, but two values (temperature/light treatments 40/1000 and 44/700) were significantly below 3.0, whereas chinquapin oak had Rfd values greater than 3.0 for four of the light/temperature combinations (treatments 40/400, 42/700, 42/1000 and 44/700) and Rfd values of 3.0 in the other treatments. At 46 °C, Rfd of bur oak was generally below 1.0 at all irradiances except 700 µmol m−2 s−1, whereas chinquapin oak maintained Rfd above or equal to 1.0, except at 100 µmol m−2 s−1. At 48 °C, all Rfd values of both species were below 1.0 indicating perma-nent damage. In both species, Rfd at 100 µmol m−2 s−1 PPFD remained at the damage threshold of 1.0 at leaf temperatures up to 44 °C, and then declined below 1.0 at leaf temperatures above 46 °C. Overall, there were no significant effects of species on the Rfd responses to light and temperature, but the temperature × light interaction was significant (F = 13.25, df = 12,160, P < 0.05), indicating that the Rfd response to tempera-ture was dependent on irradiance.
Because high leaf temperatures are usually accompanied by high radiation loads, we analyzed the responses of Rfd to the most common high temperature × high light combinations (40--48 °C × 700 and 1000 µmol m−2 s−1 PPFD) that occur in the field. At high irradiances, Rfd of chinquapin oak was above or equal to 3.0 until 44 °C, did not significantly decrease below 3.0 until 46 °C, and was below 1.0 at 48 °C (Figure 2). In contrast, Rfd of bur oak was significantly below 3.0 at 44 °C, and was significantly below 1.0 at 46 °C.
Despite the differences in Rfd responses between the species, there were no significant differences between species in
pho-Figure 1. Effect of leaf temperature on ratio of fluorescence decrease (Rfd) in leaves of bur oak and chinquapin oak exposed to 1000 (circle), 700 (upright triangle), 400 (square) or 100 (inverted triangle) µmol m−2 s−1 photosynthetic photon flux density. Critical Rfd values of 3.0 (< 3.0 = stressed) and 1.0 (< 1.0 = permanently damaged) are indicated by horizontal lines. Each point is the mean of five measurements, bars indicate ± 1 SE of the mean.
tosynthetic oxygen evolution in response to combinations of PPFD and high temperature (Figure 3). There was a significant temperature × light interaction on Anet (F = 10.55, df = 12,160, P < 0.05), most likely because the curves converged at 48 °C. Leaves exposed to elevated temperature and low PPFD (100
µmol m−2 s−1) were not significantly above photosynthetic compensation point (Anet = respiration) at 40 °C, and declined to rates well below photosynthetic compensation point at tem-peratures between 42 and 48 °C. At higher PPFDs (400--1000
µmol m−2 s−1), Anet declined as the temperature increased from 40 °C to the photosynthetic compensation point at about 44 °C. Only in chinquapin oak leaves at a PPFD of 1000 µmol m−2 s−1 was Anet significantly above (1.9 ± 0.58 µmol m−2 s−1) the photosynthetic compensation point at 44 °C. At 46 and 48 °C, light effects on Anet were indistinguishable statistically.
Field gas exchange
In the field, bur oak had 15 and 46% significantly higher Anet and gs, respectively, than chinquapin oak (Table 1), and slightly higher Ci (6%, P < 0.05). The Tleaf of bur oak was about 1.5 °C lower than that of chinquapin oak. Both oak species showed broad responses in Anet and gs to leaf temperatures ranging from 26 to 40 °C (data not shown).
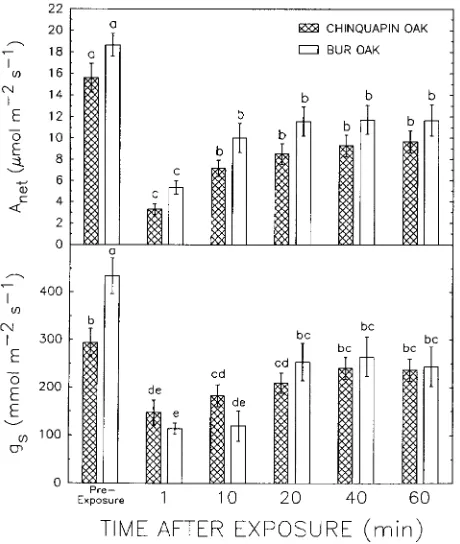
In both species, increasing leaf temperature to 46.7 ± 0.46 °C, about 18 °C above ambient air temperature, at a PPFD
of about 1500 µmol m−2 s−1, led to significant reductions in short-term Anet (Figure 4). The ANOVA showed no significant species main effect on Anet in the leaves sampled, or any significant time × species interaction. Within 1 min of raising leaf temperature to about 47 °C, Anet declined by about 70% from pre-exposure values in both oaks and then increased to new reduced maxima of 11.6 ± 1.40 and 9.3 ± 1.00 µmol m−2 s−1 in bur oak and chinquapin oak, respectively, after 10 min. Stomatal conductance (gs) declined 74% from pre-exposure values in bur oak compared with a 50% reduction in chinqua-pin oak, which led to a significant species × time interaction Figure 3. Effect of leaf temperature on net photosynthetic oxygen
evolution (Anet) of bur oak and chinquapin oak exposed to 1000 (circle), 700 (upright triangle), 400 (square) or 100 (inverted triangle)
µmol m−2 s−1 PPFD. Horizontal line indicates photosynthetic com-pensation point (Anet = respiration). Each point is the mean of five measurements, bars indicate ± 1 SE of the mean.
Table 1. Gas exchange characteristics of field-grown bur oak (Quercus macrocarpa) and chinquapin oak (Q. muehlenbergii) at the Konza Prairie Research Natural Area. Species differences at P < 0.05 are indicated by different letters (one-way ANOVA). Each number is the mean of 30 measurements, with ± 1 SE of the mean in parentheses.
Parameter Bur oak Chinquapin oak
Anet (µmol m−2 s−1) 17.76 (0.549)a 15.48 (0.519)b gs (mmol m−2 s−1) 422.4 (20.85)a 289.5 (11.07)b Ci (µl l−1) 247.0 (3.35)a 233.5 (1.76)b WUE (µmol mmol −1) 1.43 (0.067)a 1.53 (0.081)a Tleaf (°C) 34.6 (0.48)a 36.1 (0.44)b
(F = 4.7, df = 5, 90, P < 0.05). In bur oak and chinquapin oak, gs reached constant post-exposure values after a 20 and 10 min recovery that were 41 and 22% below pre-exposure values, respectively.
Twenty-four h after heat treatment (Figure 5), Anet in bur oak leaves was significantly lower (52%) than in chinquapin oak leaves, and 79% lower than in control bur oak leaves, whereas Anet in chinquapin oak was the same after 24 h as after 1 h, i.e., about 52% of the control value. Stomatal conductance was 84 and 46% of control values in bur oak and chinquapin oak, respectively, after 24 h of recovery. One week following heat stress, both oaks had Anet of about 9.1 ± 1.35 µmol m−2 s−1 compared with rates in control bur oak and chinquapin leaves of 17.1 ± 1.07 and 15.0 ± 0.67 µmol m−2 s−1, respectively. After one week of recovery, gs of bur oak significantly increased (181.3 ± 33.88 µmol m−2 s−1), reaching values similar to chinquapin oak (159.3 ± 28.34 µmol m−2 s−1), both of which were well below gs in control leaves (52% of control in bur oak, 42% in chinquapin oak).
Post heat-stress recovery dynamics are summarized in Fig-ure 6. Overall, the proportional decrease in Anet was similar (about 55%) in both species after one hour of recovery, whereas the decrease in gs was larger in bur oak (about 40%) than in chinquapin oak (about 20%). Twenty-four h after heat treatment, both Anet and gs in bur oak had decreased by 84%
compared with control values, whereas Anet in chinquapin oak remained unchanged during the first 24 h after heat treatment at about 50% of the control value.
Discussion
Fluorescence analyses showed that chinquapin oak may be slightly more thermotolerant than bur oak (Figures 1 and 2), which is consistent with earlier observations (Hamerlynck and Knapp 1994a). Because changes in Rfd result from decreased Fp and increased Ft, which, in turn, reflect changes in photo-chemical (qp) and non-photochemical (qnp) fluorescence quenching (Lichtenthaler 1988, Epron and Dreyer 1990, Methy and Trabaud 1993), and increasing temperature de-creases qp and increases qnp (Georgiva and Yordanov 1994), the reductions in Rfd could be due to PSII instability, limited electron transfer to Q (Armond et al. 1978, Berry and Bjork-man 1980, Naus et al. 1992), changes in thylakoid membranes (McCain et al. 1989, Harding et al. 1990, Kuropatwa et al. 1992), or photoinhibition (Havaux 1993, 1994). The ability of chinquapin oak to maintain Rfd at slightly higher values than bur oak suggests that this species may allocate resources to stabilize photosystem II (Berry and Bjorkman 1980, Kuropatwa et al. 1992, Naus et al. 1992), protect against Figure 5. Long-term recovery kinetics of net photosynthesis (Anet) and
stomatal conductance (gs) in bur oak and chinquapin oak leaves ex-posed for 45 min to leaf temperatures of about 47 °C in the field, and appropriate controls. Bars are means of 10 measurements, error bars indicate ± 1 SE of the mean. Letters differ at P < 0.05 (two-way ANOVA).
photoinhibition (Ghashghaie and Cornic 1994, Havaux 1994), or maintain enzymatic activity at higher temperatures than bur oak (Kobza and Edwards 1987, Ghosh et al. 1989, Simon and Vairinhos 1991,Ranney and Peet 1994).
Thermal tolerance has been linked to photosynthetic re-sponses to drought (Ivanonv et al. 1992) and light (Berry and Bjorkman 1980, Midmore and Prange 1992, but see Armond et al. 1978, Al-Khatib and Paulsen 1989, and Mishra and Singhal 1992). In three Mediterranean oak species with differing drought tolerances, Rfd, qp, and qnp did not respond directly to severe water stress (Epron and Dreyer 1990, Epron et al. 1992, Epron and Dreyer 1993). In our study, the differences in Rfd suggest that photosynthetic responses to high temperature and light may be linked to drought tolerance. Reductions in Rfd of bur oak and chinquapin oak in response to high temperature were similar to other oaks (Methy and Trabaud 1993), but less than in maple and walnut (McCain et al. 1989, Agati et al. 1995). In our study, it is unlikely that differences in thermal tolerance were due to growth conditions, which strongly influ-ence temperature responses (Seemann et al. 1984, Williams et al. 1986, Jones 1992), because leaf samples were taken from locations where the oak species co-occurred.
Low PPFD (100 µmol m−2 s−1) and high temperature re-sulted in stress to both oaks (Figures 1 and 3). Under condi-tions of low irradiance and high temperature, photosynthetic carbon assimilation is reduced by rapid increases in respiration (Berry and Bjorkman 1980, Midmore and Prange 1992). De-creased Rfd at an irradiance of 100 µmol m−2 s−1 was caused by reduced Fp, possibly because of reduced electron transfer to Q from PSII (Lichtenthaler 1988). Although it may seem un-likely that many leaves would experience leaf temperatures exceeding 40 °C at a PPFD of 100 µmol m−2 s−1, inner canopy shade leaves can experience transient periods of both high irradiance and leaf temperature (Pearcy 1990). We have pre-liminary evidence (Hamerlynck, unpublished data) that shade leaves of both oak species have equal, or greater, heat toler-ances than sun leaves. It is possible, therefore, that shade leaf photosynthesis, which contributes significantly to daily and seasonal carbon gain (Kozlowski et al. 1991), could be limited by interactions between temperature and light.
Bragg et al. (1993) suggested that bur oak seedlings estab-lish in lower PPFD microenvironments than chinquapin oak because they are less drought tolerant. At PPFDs between 400 and 700 µmol m−2 s−1 and temperatures from 40 to 44 °C, bur oak Rfd was equal to or below the critical value of 3.0, whereas chinquapin oak had Rfd values significantly above 3.0 at 400
µmol m−2 s−1, and equal to 3.0 at 700 µmol m−2 s−1. These findings support our hypothesis that chinquapin oak is more thermotolerant than bur oak to the light and temperature com-binations likely to prevail in the field. Our observations also provide evidence that bur oak and chinquapin oak seedling establishment is influenced primarily by differences in tem-perature tolerance and irradiance, as has been suggested for other tree species (Pearcy 1990, Küppers and Schneider 1993, Poorter and Oberbauer 1993).
We have no explanation for the lack of a species effect on the responses of O2 evolution to high temperature and light. It
is possible that the O2 evolution response was slower than the fluorescence response (Epron and Dreyer 1990), and that the sampling times were not sufficiently frequent to resolve spe-cific differences. We note that the laboratory measurements were made on evenly heated, isolated tissue and so may differ from whole-leaf responses in the field (Jones 1992). Field measurements with attached leaves at 40 ± 1 °C (data not shown) often showed Anet to be well above the 10.0--12.0 µmol m−2 s−1 range measured at similar temperatures and irradiances in the laboratory. In addition, measurement in saturating CO2 in the O2 electrode housing may shift the photosynthetic tem-perature optimum to higher values and narrow the response, resulting in greater proportional decreases at temperature ex-tremes compared to measurement at atmospheric CO2 concen-trations (Mooney et al. 1978, Berry and Bjorkman 1980).
Heat treatment in the field reduced Anet in both oaks, indicat-ing that damage occurred (Figure 4). In both oaks, stomata closed substantially in response to elevated temperature (Fig-ure 4). Stomatal clos(Fig-ure at high temperat(Fig-ures is well docu-mented, (cf. Roessler and Monson 1985, Samuelson and Teskey 1991, Bassow, et al. 1994, Ranney and Peet 1994), though exceptions occur (Al-Khatib and Paulsen 1989, Du-frene and Saugier 1993). Roessler and Monson (1985) con-cluded that stomata closed because of increased VPD, whereas Anet reductions were temperature dependent. Our results indi-cate stomatal closure in these oaks was more closely coupled to increased temperature than to increased VPD, because gs was reduced in a saturated vapor pressure environment. The concurrent decreases in gs and Anet (Figures 4 and 6) indicate that, in these oaks, gs may rapidly adjust to short-term changes in photosynthetic capacity, as occurs over longer periods (Wong et al. 1979, Chandler and Dale 1993, Hamerlynck and Knapp 1994b). Reduced gs may also indicate damage to the stomatal apparatus. In wheat exposed to similar irradiance and temperatures, gs increased and Anet declined, indicating that high temperature and light mainly affected mesophyll function (Al-Khatib and Paulsen 1989).
Twenty-four h after heat stress, Tleaf was 36--39 °C in both oaks, but only in bur oak were the values of Anet and gs less than after 1 h of recovery. After 7 days of recovery, Anet of bur oak had increased but was still less than the control value. Thus, large leaves and greater proportional reductions in gs after heat stress (Figure 7) could limit bur oak to moist forest sites, whereas small leaves and smaller proportional reductions in gs after heat stress, as well as low gs under favorable conditions, could allow chinquapin oak to establish and maintain popula-tions in exposed dry locapopula-tions. We conclude that reducpopula-tions in gs after heat stress may not affect Tleaf as much in chinquapin oak as in bur oak, thus allowing for more rapid photosynthetic readjustment in chinquapin oak.
In the field, Anet was not depressed below the compensation point after exposure for 45 min at light × temperature combi-nations that reduced Rfd below the damage threshold after only 5 min (Figure 1), which indicates that Rfd and other fluores-cence signals may overestimate the impact of high temperature on intact leaves under field conditions (Schreiber and Berry 1977, Lichtenthaler 1988, Agati et al. 1995). However, the estimated thermal tolerances of these oaks (Figure 1) were similar to desert shrubs (Mooney et al. 1978, Seemann et al. 1984, Midgley and Moll 1993), xeric Mediterranean oaks (Methy and Trabaud 1993), shortgrass prairie species (Monson and Williams 1982, Roessler and Monson 1985), and tropical species (Smillie and Nott 1979, Dufrene and Saugier 1993), although higher than those reported for many North American oak species found further east (Chabot and Lewis 1976, Tingey et al. 1979, Jurik et al. 1988, Loreto and Sharkey 1990), as well as many deciduous broadleaf and conifer species (Teskey et al. 1986, Jurik et al. 1988, Bassman and Zwier 1991, Foster 1992, Ranney and Peet 1994, Vann et al. 1994). The ability to recover over 50% of photosynthetic capacity after extreme heat stress may be crucial for the success of these oaks in tallgrass prairie gallery forests. Even after prolonged exposure to 47 °C, pho-tosynthesis of bur oak and chinquapin oak was equal to or higher than Anet at optimum temperatures (22 to 30 °C) for other oak and hardwood species (Chabot and Lewis 1976, Jurik et al. 1988, Foster 1992). We conclude that the thermal tolerance characteristics of bur oak and chinquapin oak con-tribute to the persistence of these species in deciduous forest extending west into the hot, arid grasslands of the central plains of North America.
Acknowledgments
This research was supported by the NSF Long Term Ecological Re-search program at the Konza Prairie ReRe-search Natural Area (DEB-9011662) and by the Kansas State University Agricultural Experimental Station.
References
Abrams, M.D. 1986. Historical development of gallery forests in northeast Kansas. Vegetatio 65:29--37.
Abrams, M.D. 1990. Adaptations to drought in Quercus species of North America. Tree Physiol. 7:227--238.
Abrams, M.D. and A.K. Knapp. 1986. Seasonal water relations of three gallery forest hardwood species in northeastern Kansas. For. Sci. 32:687--696.
Agati, G., P. Mazzinghi, F. Fusi and I. Ambrosini. 1995. The F685 /F730 chlorophyll fluorescence ratio as a tool in plant physiology: re-sponse to physiological and environmental factors. J. Plant Physiol. 145:228--238.
Al-Khatib, K. and G.M. Paulsen. 1989. Enhancement of thermal injury to photosynthesis in wheat plants and thylakoids by high light intensity. Plant Physiol. 90:1041--1048.
Armond, P.A., U. Schreiber and O. Bjorkman. 1978. Photosynthetic acclimation to temperature in the desert shrub, Larrea divaricata. Plant Physiol. 61:411--415.
Bassman, J.H. and J.C. Zwier. 1991. Gas exchange characteristics of Populus trichocarpa, Populus deltoides and Populus trichocarpa × P. deltoides clones. Tree Physiol. 8:145--159.
Bassow, S.L., D.M. McConnaughay and F.A. Bazzaz. 1994. The response of temperate tree seedlings grown in elevated CO2 to extreme temperature events. Ecol. Appl. 4:593--603.
Berry, J.A. and Bjorkman, O. 1980. Photosynthetic response and adaptation to temperature in higher plants. Annu. Rev. Plant Physiol. 31:491--543.
Borchert, J.R. 1950. The climate of the central North American grass-land. Ann. Assoc. Am. Geog. 15:1--39.
Bragg, W.K., A.K. Knapp and J.M. Briggs. 1993. Comparative water relations of seedlings and adult Quercus species during gallery forest expansion in tallgrass prairie. For. Ecol. Manage. 56:29--41. Burton, P.J. and F.A. Bazzaz. 1991. Tree seedling emergence on
interactive temperature and moisture gradients and in patches of old-field vegetation. Am. J. Bot. 78:131--149.
Campbell, G.S. 1977. An introduction to environmental biophysics. Springer, NY, 159 p.
Chabot, B.F. and A.R. Lewis. 1976. Thermal acclimation of photosyn-thesis in northern red oak. Photosynthetica 10:130--135.
Chandler, J.W. and J.E. Dale. 1993. Photosynthesis and nutrient sup-ply to needles of Sitka spruce (Picea sitchensis (Bong.) Carr.). New Phytol. 125:101--111.
Dufrene, E. and B. Saugier. 1993. Gas exchange of oil palm in relation to light, vapour pressure deficit, temperature, and leaf age. Funct. Ecol. 7:97--104.
Epron, D. and E. Dreyer. 1990. Stomatal and non-stomatal limitation of photosynthesis by leaf water deficits in three oak species: a comparison of gas exchange and chlorophyll a fluorescence data. Ann. Sci. For. 47:435--450.
Epron, D. and E. Dreyer. 1993. Photosynthesis of oak leaves under water stress: maintenance of high photochemical efficiency of pho-tosystem II and the occurrence of non-uniform CO2 assimilation. Tree Physiol. 13:107--117.
Epron, D., E. Dreyer and N. Breda. 1992. Photosynthesis of oak trees (Quercus petraea (Matt.) Liebl.) during drought under field condi-tions: diurnal course of net CO2 assimilation and photochemical efficiency of photosystem II. Plant Cell Environ. 15:809--820. Fahnestock, J.T. and A.K. Knapp. 1994. Plant responses to selective
grazing by bison: interaction between light, herbivory and water stress. Vegetatio 115:123--131.
Fay, P.A. and A.K. Knapp. 1993. Photosynthetic and stomatal re-sponses of Avena sativa (Poaceae) to a variable light environment. Am. J. Bot. 80:1369--1373.
Foster, J.R. 1992. Photosynthesis and water relations of the floodplain tree, boxelder (Acer negundo L.). Tree Physiol. 11:133--149. Georgiva, K. and I. Yordanov. 1994. Temperature dependence of
Ghashghaie, J. and G. Cornic. 1994. Effect of temperature on partion-ing of photosynthetic electron flow between CO2 assimilation and O2 reduction and on the CO2/O2 specificity of Rubisco. J. Plant Physiol. 143:643--650.
Ghosh, S., S. Gepstein, B.R. Glick, J.J. Heikkila and E.B. Dumbroff. 1989. Thermal regulation of phosphenolpyruvate carboxylase and ribulose-1,5-bisphosphate carboxylase in C3 and C4 plants native to hot and temperate climates. Plant Physiol. 90:1298--1304. Hamerlynck, E.P. and A.K. Knapp. 1994a. Leaf-level responses to
light and temperature in two co-occurring Quercus (Fagaceae) species: implications for tree distribution patterns. For. Ecol. Man-age. 68:149--159.
Hamerlynck, E.P. and A.K. Knapp. 1994b. Stomatal responses to variable sunlight in bur oak (Quercus macrocarpa) leaves with different photosynthetic capacities. Int. J. Plant Sci. 155:583--587. Havaux, M. 1993. Characterization of thermal damage to the photo-synthetic electron transport system in potato leaves. Plant Sci. 94:19--23.
Havaux, M. 1994. Temperature-dependent modulation of the photoin-hibition sensitivity of photosystem II in Solanum tuberosum leaves. Plant Cell Physiol. 35:757--766.
Harding, S.A., J.A. Guikema and G.M. Paulsen. 1990. Photosynthetic decline from high temperature stress during maturation of wheat. II. Interaction with source and sink processes. Plant Physiol. 92:654--658.
Ivanov, A.G., A.I. Kitcheva, A.M. Christov and L.P. Popova. 1992. Effects of abscisic acid treatment on the thermostablity of the photosynthetic apparatus in barley chloroplasts. Plant Physiol. 98:1228--1232.
Jeffree, E.P. and C.E. Jeffree. 1994. Temperature and the bio-geographical distributions of species. Funct. Ecol. 8:640--650. Jones, H.G. 1992. Plants and microclimate, 2nd Edn. Cambridge
University Press, 428 p.
Jurik, T.W., J.A. Weber and D.A. Gates. 1988. Effects of temperature and light on photosynthesis of dominant species of a northern hardwood forest. Bot. Gaz. 149:203--208.
Knapp, A.K. 1985. Early season production and microclimate associ-ated with topography in a C4 dominated grassland. Oecol. Plant. 6:337--346.
Knapp, A.K. 1992. Leaf gas exchange in Quercus macrocarpa (Fa-gaceae): rapid stomatal responses to variability in sunlight in a tree growth form. Am. J. Bot. 79:599--604.
Knapp, A.K. 1993. Gas exchange dynamics in C3 and C4 grasses: consequences of differences in stomatal conductance. Ecology 74:113--123.
Knight, C.L, J.M. Briggs and D.M. Nellis. 1994. Expansion of gallery forest in Konza Prairie Research Natural Area, Kansas, USA. Land-scape Ecol. 9:117--125.
Kobza, J. and G.E. Edwards. 1987. Influences of leaf temperature on photosynthetic carbon metabolism in wheat. Plant Physiol. 83:69--74.
Kozlowski, T.T., P.J. Kramer and S.G. Pallardy. 1991. The physiologi-cal ecology of woody plants. Academic Press, NY, 657 p. Kramer, K. 1995. Phenotypic plasticity of the phenology of seven
European tree species in relation to climatic warming. Plant Cell Environ. 18:93--104.
Küppers, M. and H. Schneider. 1993. Leaf gas exchange of beech (Fagus sylvatica L.) seedlings in lightflecks: effects of fleck length and leaf temperature in leaves grown in deep and partial shade. Trees 7:160--168.
Kuropatwa, R., J. Naus, M. Maslan and L. Dvorak. 1992. Basic properties of the chlorophyll fluorescence temperature curve in barley leaves. Photosynthetica 27:129--138.
Lavorel, J. and A.L. Etienne. 1977. In vivo chlorophyll fluorescence. In Primary Processes of Photosynthesis. Ed. J. Barber. Elseiver, Amsterdam, pp 206--268.
Lichtenthaler, H.K. 1988. In vivo chlorophyll fluorescence as a tool for stress detection in plants. In Applications of Chlorophyll Fluo-rescence, Ed. H.K. Lichtenthaler. Kluwer Academic Publishers, Dordrecht, Boston, London, pp 129--142.
Lichtenthaler, H.K. and U. Rinderle. 1988. Chlorophyll fluorescence signatures as a vitality indicator in forest decline research. In Appli-cations of Chlorophyll Fluorescence, Ed. H.K. Lichtenthaler. Kluwer Academic Publishers, Dordrecht, Boston, London, pp 143--149.
Loreto, F. and T.D. Sharkey. 1990. A gas exchange study of photosyn-thesis and isoprene emission in Quercus rubra L. Planta 182:523--531.
McCain, D.C., J. Croxdale and J.L. Markley. 1989. Thermal damage to chloroplast envelope membranes. Plant Physiol. 90:606--609. Methy, M. and L. Trabaud. 1993. Seasonal courses of photosynthetic
activity and sublethal temperature tolerance of Quercus ilex leaves. For. Ecol. Manage. 61:339--348.
Midgley, G.F. and E.J. Moll. 1993. Gas exchange in arid-adapted shrubs: when is efficient water use a disadvantage? S. Afr. J. Bot. 59:491--495.
Midmore, D.J. and R.K. Prange. 1992. Growth responses of two Solanum species to contrasting temperature and irradiance levels: relations to photosynthesis, dark respiration and chlorophyll fluo-rescence. Ann. Bot. 69:13--20.
Mishra, R.K. and G.S. Singhal. 1992. Function of photosynthetic apparatus of intact wheat leaves under high light and heat stress and its relationship with peroxidation of thylakoid lipids. Plant Physiol. 98:1--6.
Monson, R.K. and G.J. Williams III. 1982. A correlation between photosynthetic temperature adaptation and seasonal phenology pat-terns in the shortgrass prairie. Oecologia 54:58--62.
Mooney, H.A., O. Bjorkman and C.J. Collatz. 1978. Photosynthetic acclimation to temperature in the desert shrub, Larrea divaricata. I. Carbon dioxide exchange characteristics of intact leaves. Plant Physiol. 61:406--410.
Naus, J., L. Dvorak, R. Kuropatwa and M. Maslan. 1992. Transitions in the thylakoid membranes of barley leaves studied by the chloro-phyll fluorescence temperature curve. Photosynthetica 27:563--570.
Pearcy, R.W. 1990. Sunflecks and photosynthesis in plant canopies. Annu. Rev. Plant Physiol. Plant Mol. Biol. 41:421--453.
Poorter, L. and S.F. Oberbauer. 1993. Photosynthetic induction re-sponses of two rainforest tree species in relation to light environ-ment. Oecologia 96:193--199.
Ranney, T.G. and M.M. Peet. 1994. Heat tolerance of five taxa of birch (Betula): physiological responses to supraoptimal leaf tempera-tures. J. Am. Soc. Hortic. Sci. 119:243--248.
Roessler, P.G. and R.K. Monson. 1985. Midday depression in net photosynthesis and stomatal conductance in Yucca glauca Oecolo-gia 67:380--387.
Samuelson, L.J. and R.O. Teskey. 1991. Net photosynthesis and leaf conductance of loblolly pine seedlings in 2 and 21% oxygen as influenced by irradiance, temperature and provenance. Tree Physiol. 8:205--211.
Schreiber, U. and J.A. Berry. 1977. Heat induced changes of chloro-phyll fluorescence response in intact leaves correlated with damage of the photosynthetic apparatus. Planta 136:233--238.
Simon, J.P. and F. Vairinhos. 1991. Thermal stability and kinetic properties of NADP+-malate dehydrogenase isomorphs in two populations of the C4 weed species Echinohloa cru-galli (Barnyard grass) from sites of contrasting climates. Physiol. Plant. 83:216--224.
Smillie, R.M. and R. Nott. 1979. Heat injury in leaves of alpine, temperate and tropical plants. Aust. J. Plant Physiol. 6:135--141. Strain, B.R., K.O. Higgenbotham and J.C. Mulroy. 1976. Temperature
preconditioning and photosynthetic capacity of Pinus taeda L. Photosynthetica 10:47--53.
Teskey, R.O., J.A. Fites, L.J. Samuelson and B.C. Bongarten. 1986. Stomatal and nonstomatal limitations to photosynthesis in Pinus taeda under different environmental conditions. Tree Physiol. 2:131--142.
Tingey, D.T., M. Manning, L.C. Grothaus and W.F. Burns. 1979. The influence of light and temperature on isoprene emission rates from live oak. Physiol. Plant. 47:112--118.
Turner, C.L., J.R. Kneisler and A.K. Knapp. 1995. Comparative gas exchange and nitrogen responses of the dominant C4 grass Andro-pogon gerardii and five C3 forbs to fire and topographic position in tallgrass prairie during a wet year. Int. J. Plant Sci. 156:216--226. Vann, D.R., A.H. Johnson and B.B. Casper. 1994. Effect of elevated
temperatures on carbon dioxide exchange in Picea rubens. Tree Physiol. 14:1339--1349.
von Caemmerer, S. and G.D. Farquhar. 1981. Some relationships between the biochemistry of photosynthesis and gas exchange of leaves. Planta 153:367--387.
Williams, G.J. III, R.K. Monson and J.K. Detling. 1986. Growth temperature effects on chlorophyll fluorescence yield in 15 grami-noid species. Am. Mid. Nat. 116:197--201.
Wong, S.C., I.R. Cowan and G.D. Farquhar. 1979. Stomatal conduc-tance correlates with stomatal capacity. Nature 282:424--426. Zar, J.H. 1974. Biostatistical analysis. Prentice-Hall, Inc. Engelwood