Purification and characterization of the 26S proteasome from
cultured rice (
Oryza sati
6
a
) cells
Yuki Yanagawa
a,b, Akiko Ohhashi
a, Yasuko Murakami
c, Yasushi Saeki
d,
Hideyoshi Yokosawa
d, Keiji Tanaka
e, Junji Hashimoto
b,f, Takahide Sato
a,
Hiroki Nakagawa
a,*
aDepartment of Bioproduction Science,Faculty of Horticulture,Chiba Uni6ersity,Matsudo,Chiba271-8510,Japan bCREST(Core Research for E6olutional Science and Technology)of Japan Science and Technology Corporation(JST),Chiyoda-ku,
Tokyo101-0062,Japan
cDepartment of Biochemistry2,The Jikei Uni6ersity School of Medicine,Nishishinbashi,Minato-ku,Tokyo105-8461,Japan dDepartment of Biochemistry,Graduate School of Pharmaceutical Sciences,Hokkaido Uni6ersity,Kita-ku,Sapporo060-0812,Japan
eThe Tokyo Metropolitan Institute of Medical Science,Honkomagome,Bunkyo-ku,Tokyo113-8613,Japan fNational Institute of Agrobiological Resources,Kannondai,Tsukuba,Ibaraki305-8602,Japan
Received 30 April 1999; received in revised form 12 July 1999; accepted 13 July 1999
Abstract
The 26S proteasome was purified from cultured rice cells to near homogeneity by ultracentrifugation for 5 h at 85,000×g, chromatography on Biogel A-1.5m, and glycerol density-gradient centrifugation analysis. The purified enzyme had two distinct forms, termed 26Sa- and 26Sb-type proteasomes, with different electrophoretic mobilities by nondenaturing polyacrylamide gel electrophoresis. It consisted of multiple polypeptides with molecular masses of 25 – 35 and 42 – 120 kDa, which presumably corresponded to those of the 20S proteasome and an associated PA700 regulatory complex, respectively. The rice 26S proteasome resembled, but was not identical to, one from other sources in their subunit composition and immunochemical reactivity. Intriguingly, both rice and spinach 26S proteasomes could not degrade rat ornithine decarboxylase in the presence of antizyme and ATP, unlike the rat 26S proteasome, implying the existence of functional differences between mammalian and plant 26S proteasomes. © 1999 Published by Elsevier Science Ireland Ltd. All rights reserved.
Keywords:26S Proteasome; 20S Proteasome; Rice (Oryza sati6aL.)
www.elsevier.com/locate/plantsci
1. Introduction
The 26S proteasome (multicatalytic endopepti-dase complex EC 3.4.99.46) is a large multisubunit complex composed of a core proteinase, known as the 20S proteasome, and a pair of symmetrically disposed PA700 (also named 19S complex) regula-tory particles [1 – 3]. It is known to catalyze the
ATP-dependent degradation of ubiquitinated
proteins and is involved in a variety of important cellular processes [4,5]. Proteasomes in higher plants have been characterized both structurally
and immunologically [6 – 10]. The 20S proteasomes have been purified from dicotyledonous plants such as spinach leaves [7] and dry pea seeds [8], and monocotyledonous plants such as wheat leaves [9]. The biochemical characteristics of proteasomes isolated from these three plant sources are remarkably similar to those of the proteasomes found in other eukaryotes. The 26S proteasome was recently isolated from spinach leaves [10]. By electron microscopy, this 26S proteasome from spinach was found to be
struc-turally similar to those of Xenopus [11], rat [12]
and yeast [13]. However, 26S proteasome from only spinach was purified in higher plants, al-though the genes that encode many of the 26S
* Corresponding author. Tel./fax: +81-47-308-8862.
E-mail address: [email protected] (H. Nakagawa)
proteasome subunits, including all of the 20S proteasome, have been reported [14 – 20].
The properties of some enzymes are distinct between monocotyledonous and dicotyledonous plants; for example, acetyl-CoA carboxylase is particularly distinct in gramineae such as rice [21]. Although there have been a considerable number of genetic studies on rice [14,17,19,22], little is known of the purification and properties of the rice 26S proteasome. The relationship between the properties of rice and spinach 26S proteasomes has never been clarified. In this paper, we report the purification and some properties of the 26S proteasome from cultured rice cells, compare the properties of rice and spinach 26S proteasomes, and discuss the functional differences between higher plant and mammalian 26S proteasomes.
2. Materials and methods
2.1. Materials
The materials used were as follows:
succinyl-Leu-Leu-Val-Tyr-4-methyl-coumaryl-7 amide
(Suc-LLVY-MCA) (Peptide Institute, Minoh,
Japan); t
-butyloxycarbonyl-Phe-Ser-Arg-4-methyl-coumaryl-7 amide (Boc-FSR-MCA) (Peptide
Insti-tute);
carbobenzoxy-Leu-Leu-Glu-4-methyl-cou-maryl-7 amide (Z-LLE-MCA) (Peptide Institute); Biogel A-1.5m (Bio-Rad Laboratories, Richmond, CA, USA); carbobenzoxy-Ile-Glu(OBut)-Ala-Leu-H (aldehyde) (PSI) (Peptide Institute),
carboben-zoxy-Leu-Leu-Leu-H (aldehyde) (MG132)
(Peptide Institute); carbobenzoxy-Leu-Leu-Nva-H (MG115) (Peptide Institute); lactacystin (Kyowa medix, Tokyo, Japan); leupeptin (Peptide Insti-tute); ATP (Oriental Yeast, Osaka, Japan);
ubiqui-tin (Ub) (Sigma); and Na125I (New England
Nuclear).
2.2. Plant materials
Suspension cultures of rice cells (Oryza sati6aL.
cv. Nipponbare) were carried out according to Muller and Grafe [23]. The growth of rice cells was initiated by cultivation of seed embryos [24]. After transfer to fresh media, the cells were sub-cultured with shaking at 100 rpm. Protein concen-tration was determined by the method of Bradford [25] with bovine serum albumin as standard.
2.3. Assay of peptidase acti6ity
Fluorogenic substrates, such as Suc-LLVY-MCA, Z-LLE-Suc-LLVY-MCA, or Boc-FSR-MCA were in-cubated with a test preparation for 10 min at 37°C in the presence or absence of 0.02% sodium dode-cyl sulfate (SDS) in 100 mM Tris – HCl (pH 8.0), as described previously [26]. The reaction was
stopped by the addition of 100 ml 10% SDS and 2
ml 100 mM Tris – HCl (pH 9.0), and the fluores-cence of the reaction products was measured. Protease inhibitors, such as PSI, MG132, MG115, or lactacystin were dissolved in dimethyl sulfoxide
and added at a final concentration of 10 mM.
2.4. Assay of protease acti6ity
About 18,000 cpm of poly-Ub-125I-lysozyme
conjugates were incubated at 37°C for 0 – 60 min
in a total volume of 100 ml reaction mixture
con-sisting of 50 mM Tris – HCl (pH 7.8), 10 mM
MgCl2, 2 mM ATP, 1 mM dithiothreitol, and a
suitable amount of test preparation. The reaction was then terminated by adding the SDS sample buffer. For exact measurement of the activity
without Mg2+, 20 mM EDTA was added to the
assay mixture. The method for preparation of radiolabeled Ub-lysozyme conjugates was as previ-ously described [27].
2.5. Purification of 26S proteasomes from spinach and rat
The 26S proteasomes from spinach leaves [10] and rat liver [28] were purified as described previously.
2.6. Assay of ornithine decarboxylase degrading acti6ity
Degradation of ornithine decarboxylase (ODC) in vitro was determined in the presence of recom-binant antizyme and ATP, as described previously
[29].35S-ODC was prepared by in vitro translation
using rabbit reticulocyte lysate (Wako, Osaka,
Japan), rat ODC mRNA and 35S-labeled
methion-ine (DuPont NEN), and purified by
im-munoaffinity chromatography as described
2.7. Electrophoretic analysis
Polyacrylamide gel electrophoresis (PAGE) was carried out in 2.5% polyacrylamide gel containing 0.5% agarose under nondenaturing conditions. SDS-PAGE was carried out by the method of Laemmli [31] in 12.5% slab gel. Protein was de-tected by staining with Coomassie Brilliant Blue G-250. Kaleidoscope prestained standards (Bio-Rad Laboratories) were used for SDS-PAGE.
2.8. Immunological analysis
Polyclonal antibody against the spinach 26S proteasome has been described previously [32]. An immunoelectrophoretic blot analysis was carried out according to the method of Towbin et al. [33]. Samples were separated by PAGE and transferred electrophoretically to Immobilon polyvinylidene difluoride membranes (Millipore, Bedford, USA). Anti-rabbit immunoglobulin G conjugated with alkaline phosphatase (Promega, Madison, USA) was used as a secondary antibody. Nitroblue tetra-zolium and 5-bromo-4-chloro-3-indolyl phosphate were used as substrates of alkaline phosphatase.
3. Results
3.1. Purification of the 26S proteasome from rice cells
To purify the 26S proteasome on a large-scale, we used rice suspension-cultured cells collected in the logarithmic phase of growth. All purification procedures were performed at 4°C, using standard buffer consisting of 50 mM Tris – HCl (pH 7.5), 10 mM 2-mercaptoethanol, 2 mM ATP, 5 mM
MgCl2, 20% glycerol, and 10mM leupeptin, unless
otherwise specified.
Fresh rice cells (40 g) were homogenized with 100 ml of standard buffer in a Teflon homoge-nizer. The homogenate was centrifuged for 1 h at 30,000 rpm in a Hitachi RP50A rotor (average,
70,000×g), and the resulting supernatant was
fur-ther centrifuged for 5 h at 33,000 rpm in a Hitachi
RP50A rotor (average, 85,000×g). The crude
ex-tract was prepared by dissolving the precipitate in a suitable volume (about 1 – 2 ml) of standard buffer. This crude extract (approximately 217 mg of protein) was applied to a Biogel A-1.5m column
(2.5×100 cm) equilibrated with standard buffer.
Three-milliliter fractions of the eluate were
col-lected at a flow rate of 20 ml/h. A single peak of
material showing Suc-LLVY-MCA-degrading ac-tivity was obtained. The active fraction from the Biogel A-1.5m gel filtration column was dialyzed against standard buffer without glycerol and leu-peptin, and then concentrated in a Centriprep-50 concentrator (Amicon, Beverly, USA). The con-centrated sample was loaded onto a linear gradient
of 10 – 40% (v/v) glycerol in standard buffer
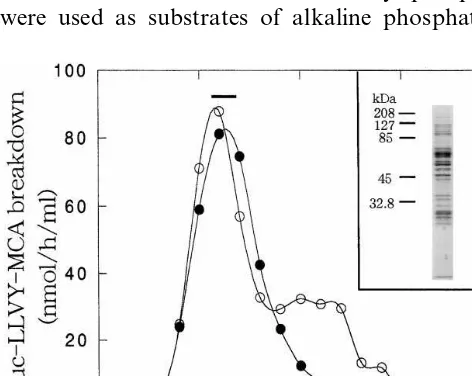
with-out glycerol and leupeptin. After centrifugation at 25,000 rpm for 22 h in a Hitachi SRP28SA rotor (Hitachi, Tokyo, Japan), the gradient was sepa-rated into 30 fractions of 1 ml each, and peptidase activity was assayed. As shown in Fig. 1, a single major peak of peptidase activity in the absence of SDS was eluted in about fraction 11, but a small shoulder of Suc-LLVY-MCA degrading activity in the presence of 0.02% SDS was observed in a lighter fraction. By SDS-PAGE analysis, the rice 26S proteasome gave multiple bands with molecu-lar masses of 25 – 120 kDa (Fig. 1, inset). Those with molecular masses of 25 – 35 kDa were as-sumed to be components of the 20S proteasomal portion, and those of 42 – 120 kDa were assumed to be components of the regulatory part, called
Fig. 1. Fractionation of the 26S proteasome from cultured rice (Oryza sati6a) cells by glycerol density-gradient
Fig. 2. Electrophoretic analysis of degradation of
poly-Ub-125I-lysozyme conjugates by the purified 26S proteasome. (A)
The purified 26S proteasome (7.5mg) was incubated with 0.2
mg of poly-Ub-125I-lysozyme conjugates for the indicated
times in the presence of 10 mM Mg2+ and 2 mM ATP. The
resulting products were then separated by SDS-PAGE and detected by a Fuji Bio-imaging analyzer BAS 2000. (B) Anal-ysis was performed as for (A), except that 20 mM EDTA was added to the reaction mixture instead of Mg2+and ATP. The
arrow indicates the position of125I-lysozyme.
of the 26S proteasome.
The purification method described usually led to a 14.7-fold increase in specific Suc-LLVY-MCA-degrading activity in the absence of SDS (i.e. that of the 26S proteasome) from the crude extract, and the overall yield was approximately 4.0%.
3.2. Degradation of ubiquitinated lysozyme by the 26S proteasome
To clarify whether the 26S proteasome purified from rice is capable of degrading ubiqitinated proteins, we electrophoretically monitored changes
in poly-Ub-125I-lysozyme conjugates during
incu-bation with the 26S proteasome. Incuincu-bation of
poly-Ub-125I-lysozyme conjugates with the 26S
proteasome in the presence of Mg2+ and ATP
resulted in a marked loss of material in main bands (approximately 200 kDa) of conjugates (Fig. 2A), while little significant degradation was
observed without Mg2+ (Fig. 2B). Moreover,
un-modified 125I-lysozyme was not degraded
apprecia-bly when incubated with the 26S proteasome in
the presence of Mg2+ and ATP (data not shown).
These results strongly suggest the idea that the purified 26S proteasome complex hydrolyzes Ub-ligated proteins ATP dependently.
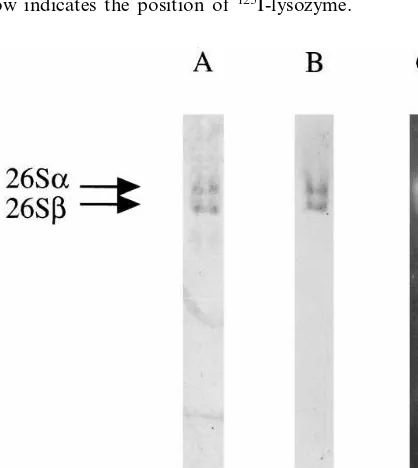
3.3. Identification of two isoforms of the 26S proteasome complex
As reported for the enzymes from rat liver [28], rabbit reticulocytes [34], spinach leaves [10], and yeast [35], the purified rice 26S proteasome gave two protein bands on nondenaturing PAGE (Fig. 3A). As shown in Fig. 3B, the anti-26S protea-some antibodies from spinach reacted with both bands, indicating that the protein is present as two isoforms, because preimmune serum reacted with neither band (data not shown). We named these
isoforms rice 26Sa and 26Sb proteasomes,
accord-ing to our nomenclature for the rat liver enzyme [28]. To determine whether these two isoforms have proteolytic activity, after their separation by electrophoresis, the polyacrylamide gel was over-loaded with a solution of a fluorogenic substrate (Suc-LLVY-MCA) and incubated for about 15 min at room temperature, and peptide degrading activity was detected under ultraviolet light. Suc-LLVY-MCA degrading activity was detected in both bands (Fig. 3C). Thus, rice cells contain two
Fig. 3. Electrophoretic and immunological analyses of the 26S proteasome. The purified 26S proteasome (2 mg) was elec-trophoresced in 2.5% polyacrylamide gel – 0.5% agarose gel. (A) The gel was stained for protein with Coomassie Brilliant Blue; (B) immunoblot analysis was performed with anti-26S proteasomal polyclonal antibodies from spinach; (C) Suc-LLVY-MCA degrading activity was detected in the gel as described in Section 2. Arrows show the position of the isoforms of the 26S proteasome (26Sa and 26Sb).
PA700. Pooled active fractions (approximately 600
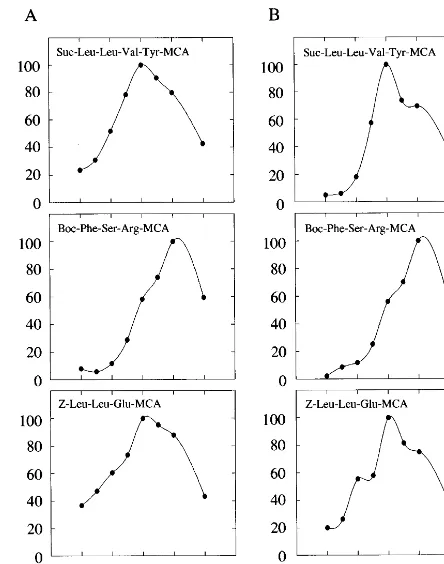
Fig. 4. Effect of pH on three peptidase activities. The degra-dation activities by rice (A) and spinach (B) 26S proteasomes for the indicated substrates were measured at various pH values in the absence of SDS. Values are shown as percent-ages of the maximum activity.
was 9.0. Comparison of the effects of pH on the rice 26S proteasome with those on the spinach enzyme (Fig. 4B) revealed that the effects of pH are similar in monocotyledonous and dicotyle-donous plants, although the rice 26S proteasome was slightly more active at acidic pH values than the spinach one.
3.5. Inhibitor sensiti6ity of the purified 26S
proteasome
Chymotrypsin-like and trypsin-like activities of rice and spinach 26S proteasomes were strongly inhibited by PSI, MG132, and MG115 (Table 1). Peptidylglutamyl-peptide hydrolase activities of rice and spinach 26S proteasomes were inhibited approximately 10 and 50% by these inhibitors, respectively. Chymotrypsin-like, trypsin-like and peptidylglutamyl-peptide hydrolase activities of these 26S proteasomes were also partially inhibited
by 10 mM lactacystin.
3.6. ODC degradation by 26S proteasomes from plants
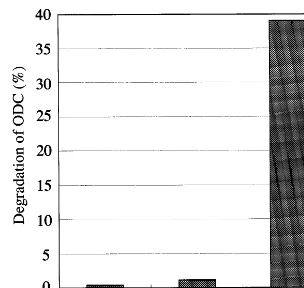
Previously, ODC was shown to be degraded by the 26S proteasome purified from rat liver without ubiquitination, but requiring antizyme and ODC inhibitory protein instead of ubiquitin [29]. We examined whether plant 26S proteasomes degrade
35S-labeled ODC in vitro. As shown in Fig. 5,
ODC was degraded rapidly by purified rat liver 26S proteasome in an antizyme- and ATP-depen-dent fashion, as previously reported [29]. How-ever, unexpectedly, no appreciable degradation of ODC by 26S proteasomes from rice and spinach was observed, even in the presence of antizyme and ATP, indicating strongly that 26S protea-somes from plants are functionally different from
active isoforms of the 26S proteasome, 26Sa and
26Sb.
3.4. Effect of pH on peptidase acti6ities
Fig. 4 shows the influence of pH on the activi-ties of the enzyme with three substrates. In rice 26S proteasome, as shown in Fig. 4A, the opti-mum pH value for chymotrypsin-like and pep-tidylglutamyl-peptide hydrolase activities was 8.0. The optimum pH value for trypsin-like activity
Table 1
Effect of inhibitors of rice and spinach 26S proteasomesa
Compound Concentration Inhibition (%)
Chymotrypsin-like activity Trypsin-like activity PGPH activity
Rice Spinach Rice Spinach Rice Spinach
97.191.85 98.291.09 82.291.27 79.299.52 10.291.16
PSI 10 43.794.27
53.793.26 7.9093.52
81.790.99 78.392.83
MG132 10 81.390.96 96.891.79
10
MG115 95.992.04 96.890.73 85.192.97 94.792.20 25.396.71 55.292.81
67.390.35 23.793.32 30.093.80 2.6092.54 16.191.46
10 71.691.85
Lactacystin
Fig. 5. Degradation of rat ODC by purified 26S proteasomes from rice, spinach and rat. The ATP- and antizyme-depen-dent degradation of 35S-labeled ODC with purified 26S
proteasomes (1mg) was assayed by measuring the conversion into TCA-soluble fraction as described in Section 2.
26S proteasome from rice cells, however, could
only be isolated with buffers containing 10 mM
leupeptin, as well as 2 mM ATP, since dissociation of the complex into smaller particles occurred very rapidly in the absence of leupeptin. This dissocia-tion was also suppressed by casein, but not by pepstatin A or ATP alone (data not shown). Therefore, we suggest that some proteases should be taken into consideration during the purification of 26S proteasomes from plants.
In this work, we have shown that the purified rice 26S proteasome can be separated into two forms by slab electrophoresis on a nondenaturing polyacrylamide gel (Fig. 3). These isoforms
proba-bly include double (26Sa) and single (26Sb)
regu-latory particle(s), as the spinach 26S proteasome [10]. Using SDS-PAGE analysis, we found that the enzyme is a multisubunit complex consisting of subunits with molecular masses of 25 – 120 kDa. The heterogeneity of subunit composition ana-lyzed by SDS-PAGE is highly conserved among 26S proteasomal complexes isolated from other sources [11 – 13,35].
In addition, substrate specificity and pH depen-dence of the 26S proteasome indicate that the enzyme has at least three distinct activities, namely chymotrypsin-like, trypsin-like, and peptidylglu-tamyl-peptide hydrolase activities. Each of the en-zyme activities exhibits a maximum at neutral and weakly alkaline pH values, similar to the 20S proteasome from spinach leaves. However, it is noticeable that three peptidase activities of spinach 20S proteasome exhibit a maximum at higher pH values than those of rice and spinach 26S protea-somes [7]. Chymotrypsin-like, trypsin-like, and peptidylglutamyl-peptide hydrolase activities of 26S proteasomes from rice and spinach were sensi-tive to PSI, MG132, MG115 and lactacystin; how-ever, peptidylglutamyl-peptide hydrolase activity exhibited only weak sensitivity to them. The sensi-tivities of plant 26S proteasomes are similar to that of mammalian 26S proteasome.
The purified 26S proteasome complex rapidly
degraded poly-Ub-125I-lysozyme conjugates in an
ATP-dependent fashion (Fig. 2), suggesting that
covalent modification of substrate proteins
through ubiquitination is essential for their prote-olysis by the 26S proteasome. Recently, we also found a multiubiquitin-binding subunit of the proteasome, a homolog to S5a, from rice [19]. Thus, the rice 26S proteasome may have the abil-those from mammals. Nonetheless, they can
hy-drolyze Ub-ligated proteins and their subunit mul-tiplicity is common in the size range of 22 – 120 kDa among all eukaryotic 26S proteasomes from yeast, plants and mammals [10 – 12,35] (this study).
4. Discussion
So far 20S proteasomes have been purified from various higher plants, such as spinach leaves [7], pea seeds [8], and wheat leaves [9], the biochemical characteristics of proteasomes isolated from these three plant sources being remarkably similar to those of 20S proteasomes found in other eukary-otes, such as yeast and mammals. Despite the fact that the 26S proteasome plays a vital role in cellular functions, with respect to higher plants, only our group has reported the purification of the 26S proteasome from spinach leaves [10]. The 26S proteasomes from other organisms are currently receiving much attention (for reviews, see [1 – 4,36]), and the similarity among all eukaryotic proteasomes implies that much of the information derived will be broadly applicable to all plants. However, it is also apparent that each system has its own unique aspects.
ity to recognize multiubiquitin chains bound to proteins for their specific breakdown.
Previously, it has been reported that mam-malian ODC was degraded in an ATP-dependent manner by the 26S proteasome in the presence of antizyme, an ODC inhibitory protein induced by polyamines [29]. Surprisingly, in this work, we have demonstrated that rat ODC was scarcely degraded by purified 26S proteasomes from rice and spinach, even in the presence of antizyme and ATP, implying that plant 26S proteasomes could not recognize the rat AZ-ODC complex as a pro-teolytic target. However, this is not curious, be-cause it was reported that mouse ODC was not degraded rapidly when ODC together with
an-tizyme had been expressed in Trypanosome bruce,
suggesting that trypanosomes do not have the proteolytic machinery present in mammalian cells, which are responsible for the rapid degradation of ODC [37]. In addition, it was found that the 26S proteasome degrades mouse and yeast ODCs simi-larly in yeast cells, and both ODCs were strongly stabilized in the cells of carrying the mutant 26S proteasome gene [38]. However, yeast cells lack the antizyme gene, because polyamines are proba-bly necessary for the proliferation of these cells, indicating clearly that the yeast 26S proteasome efficiently degrades mouse ODC in the absence of antizyme. These observations, together with the present results, indicate clearly that 26S protea-somes from trypanoprotea-somes, yeast, and plants are functionally different from those from mammals with respect to the degradation mechanism of ODC. Rice crude extract, however, could degrade rat ODC at a fairly high speed, even in the ab-sence of antizyme (data not shown). Additional factor(s), e.g. an antizyme-like protein, would be necessary for ODC degradation by the rice 26S proteasome. Alternatively, it is possible that the ubiquitination pathway contributes to the degra-dation of ODC in rice. There is also the possibility that other unknown protease(s) can degrade rice ODC. Thus, we suggest that there may be distinct ODC recognition or degradation systems that dif-fer between plants and mammals. The investiga-tion of this possibility must be the subject of future research.
Accumulated evidence indicates that the ubiqui-tin proteasome pathway has been conserved among all eukaryotic organisms studied so far; thus, it is plausible that this regulatory system
fulfills similar functions in all eukaryotes. As demonstrated for yeast and mammalian cells, this pathway seems to be involved in cell cycle progres-sion [4,5,14,16,19,39,40] and stress response in-cluding resistance to pathogens in plants [41 – 43], as well as in the degradation of short-lived regula-tory proteins. Also, several reports indicate that the ubiquitin proteasome pathway may participate in germination [16] and senescence [17,42,43], which are unique aspects of plants. The occurrence of the 26S proteasome in rice cells reported here could contribute to a better understanding of the proteolytic pathway in plants.
Acknowledgements
We thank Dr. Atsushi Komamine for providing the suspension culture of rice cells. This work was supported in part by a project grant from the ‘Research for the Future’ program (JSPS-RFTF 96L00604) of the Japan Society for the Promotion of Science, and a grant from the ‘Hamaguchi Biochemical Foundation’ (to H.N.).
References
[1] O. Coux, K. Tanaka, A.L. Goldberg, Structure and functions of the 20S and 26S proteasomes, Annu. Rev. Biochem. 65 (1996) 801 – 847.
[2] W. Baumeister, J. Walz, F. Zu¨hl, E. Seemu¨ller, The proteasome: paradigm of a self-compartalizing protease, Cell 92 (1998) 367 – 380.
[3] M. Rechsteiner, The 26S proteasome, in: J.-M. Peters, J.R. Harris, D. Finley (Eds.), Ubiquitin and the Biology of the Cell, Plenum Press, New York, 1998, pp. 147 – 189. [4] K. Tanaka, Proteasome: structure and biology, J.
Biochem. 123 (1998) 195 – 204.
[5] A. Hershko, A. Ciechanover, The ubiquitin system, Annu. Rev. Biochem. 67 (1998) 425 – 479.
[6] M. Schliephacke, A. Kremp, H.P. Schmid, K. Kohler, U. Kull, Prosomes (proteasome) of higher plants, Eur. J. Cell Biol. 55 (1991) 114 – 121.
[7] M. Ozaki, K. Fujinami, K. Tanaka, Y. Amemiya, T. Sato, N. Ogura, H. Nakagawa, Proteasome and initial characterization of the proteasome from the higher plant Spinacia oleracia, J. Biol. Chem. 267 (1992) 21678 – 21684.
[8] B. Skoda, L. Malek, Dry pea seed proteasome: purifica-tion and enzymatic activities, Plant Physiol. 99 (1992) 1515 – 1519.
[10] K. Fujinami, N. Tanahashi, K. Tanaka, A. Ichihara, Z. Cejka, W. Baumeister, M. Miyawaki, T. Sato, H. Naka-gawa, Purification and characterization of the 26S proteasome from spinach leaves, J. Biol. Chem. 269 (1994) 25905 – 25910.
[11] J.-M. Peters, Z. Cejka, J.R. Harris, J.A. Kleinschmidt, W. Baumeister, Structural features of the 26S protea-some complex, J. Mol. Biol. 234 (1993) 932 – 937. [12] T. Yoshimura, K. Kameyama, T. Takagi, A. Ikai, F.
Tokunaga, T. Koide, N. Tanahashi, T. Tamura, Z. Ce-jka, W. Baumeister, K. Tanaka, A. Ichihara, Molecular characterization of the ‘‘26S’’ proteasome complex from rat liver, J. Struct. Biol. 111 (1993) 200 – 211.
[13] M.H. Glickman, D.M. Rubin, O. Coux, I. Wefes, G. Pfeife, Z. Cjeka, W. Baumeister, V.A. Fried, D. Finley, A subcomplex of the proteasome regulatory particle required for ubiquitin-conjugate degradation and related to the COP9-signalosome and eIF3, Cell 94 (1998) 615 – 623.
[14] I. Suzuka, Y. Koga-Ban, Y. Minobe, J. Hashimoto, Identification of cDNA clones for rice homologs of the human immunodeficiency virus-1 Tat binding protein and subunit 4 of human 26S protease (proteasome), Plant Sci. 103 (1994) 33 – 40.
[15] Y. Parmentier, D. Bouchez, J. Fleck, P. Genschik, The 20S proteasome gene family in Arabidopsis thaliana, FEBS Lett. 416 (1997) 281 – 285.
[16] M. Umeda, N. Fujii, Y. Manabe, H. Uchimiya, Molecu-lar and biochemical characterization of a proteasome subunit from rice and carrot cells, Mol. Gen. Genet. 255 (1997) 19 – 27.
[17] N. Ito, K. Tomizawa, K. Tanaka, M. Matsui, R.E. Kendrick, T. Sato, H. Nakagawa, Characterization of 26S proteasomea- andb-type and ATPase subunits from spinach and their expression during early stages of seedling development, Plant Mol. Biol. 34 (1997) 307 – 316.
[18] H. Fu, J.H. Doelling, C.S. Arendt, M. Hochstrasser, R.D. Vierstra, Molecular organization of the 20S protea-some gene family from Arabidopsis thaliana, Genetics 149 (1998) 677 – 692.
[19] Y. Yanagawa, T. Ueda, K. Yamamoto, T. Sasaki, K. Tanaka, J. Hashimoto, T. Sato, H. Nakagawa, Cloning and sequencing of cDNA from Oryza sati6aencoding a
homolog to non-ATPase subunit, MBP1, of 26S protea-some inArabidopsis thaliana, Plant Biotechnol. 15 (1998) 147 – 150.
[20] S.F. Kwok, J.M. Staub, X.W. Deng, Characterization of two subunits of Arabidopsis 19S proteasome regulatory complex and its possible interaction with the COP9 complex, J. Mol. Biol. 285 (1999) 85 – 95.
[21] T. Konishi, Y. Sasaki, Compartmentalization of two forms of acetyl-CoA carboxylase in plants and the origin of their tolerance toward herbicides, Proc. Natl. Acad. Sci. U.S.A. 91 (1994) 3598 – 3601.
[22] I. Suzuka, Y. Yanagawa, K. Yamazaki, T. Ueda, H. Nakagawa, J. Hashimoto, Biochemical and immunologi-cal characterization of rice homologues of the human immunodeficiency virus-1 Tat binding protein and sub-unit 4 of human 26S proteasome subsub-units, Plant Mol. Biol. 37 (1998) 495 – 504.
[23] A.J. Muller, R. Grafe, Isolation and characterization of cell lines ofNicotiana tobacumlacking nitrate reductase, Mol. Gen. Genet. 161 (1978) 67 – 76.
[24] S. Nakasone, E. Minami, T. Imai, F. Akiyama, Y. Ohashi, Synchronous cell division in rice suspension cultures and cell cycle specific expression of histone H3 and PCNA genes, Bull. Natl Inst. Agrobiol. Resources Jpn. 8 (1993) 1 – 10.
[25] M.M. Bradford, A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding, Anal. Biochem. 72 (1976) 248 – 254.
[26] K. Tanaka, T. Yoshimura, A. Kumatori, A. Ichihara, A. Ikai, A. Nishigai, M. Kameyama, T. Takagi, Proteasome (multi-protease complexes) as 20S ring-shaped particles in a variety of eukaryotic cells, J. Biol. Chem. 263 (1988) 16209 – 16217.
[27] M. Fujimuro, H. Sawada, H. Yokosawa, Production and characterization of monoclonal antibodies specific to multi-ubiquitin chains of polyubiquitinated proteins, FEBS Lett. 349 (1994) 173 – 180.
[28] S. Ugai, T. Tamura, N. Tanahashi, S. Takai, N. Komi, C.-H. Chung, K. Tanaka, A. Ichihara, Purification and characterization of the 26S proteasome complex catalyz-ing ATP-dependent breakdown of ubiquitin-ligated proteins from rat liver, J. Biochem. 113 (1993) 754 – 768. [29] Y. Murakami, S. Matsufuji, T. Kameji, S. Hayashi, K. Igarashi, T. Tamura, K. Tanaka, A. Ichihara, Ornithine decarboxylase is degraded by the 26S proteasome with-out ubiquitinataion, Nature 360 (1992) 597 – 599. [30] M. Nishiyama, S. Matsufuji, R. Kanamoto, M. Takano,
Y. Murakami, S. Hayashi, Two-step purification of mouse kidney ornithine decarboxylase, Prep. Biochem. 18 (1988) 227 – 238.
[31] U.K. Laemmli, Cleavage of structural proteins during the assembly of the fead of bacteriophage T4, Nature 227 (1970) 680 – 685.
[32] M. Miyawaki, M. Aito, N. Ito, Y. Yanagawa, R.E. Kendrick, T. Keiji, T. Sato, H. Nakagawa, Change in proteasome levels in spinach (Spinacia oleracea) seeds during imbibition and germination, Biosci. Biotech. Biochem. 61 (1997) 998 – 1001.
[33] H.S. Towbin, J. Staehelin, J. Gordon, Electrophoretic transfer of proteins from polyacrylamide gels to nitrocel-lulose sheets: procedure and some applications, Proc. Natl. Acad. Sci. U.S.A. 76 (1979) 4350 – 4354.
[34] L. Hoffman, G. Pratt, M. Rechsteiner, Multiple forms of the 20S multicatalytic and the 26S ubiquitin/ ATP-depen-dent proteases from rabbit reticulocyte lysate, J. Biol. Chem. 267 (1992) 22362 – 22368.
[35] M.H. Glickman, D.M. Rubin, V.A. Fried, D. Finley, The regulatory particle of the Saccharomyces cere6isiae
proteasome, Mol Cell. Biol. 18 (1998) 3149 – 3162. [36] R.D. Vierstra, Proteolysis in plants: mechanisms and
functions, Plant Mol. Biol. 32 (1996) 275 – 302.
[37] K.E. Bass, J.M. Sommer, Q.-L. Cheng, C.C. Wang, Mouse ornithine decarboxylase is stable inTrypanosoma brucei, J. Biol. Chem. 267 (1992) 11034 – 11037.
[39] P. Genschik, E. Jamet, G. Philipps, Y. Parmentier, C. Gigot, J. Fleck, Molecular characterization of b b-type proteasome subunit from Arabidopsis thaliana co-ex-pressed at a high level with ana-type proteasome subunit early in the cell cycle, Plant J. 6 (1994) 537 – 546. [40] P. Genschik, M.C. Criqui, Y. Parmentier, A. Derevier, J.
Fleck, Cell cycle-dependent proteolysis in plants. Identifi-cation of the destruction box pathway and metaphase arrest produced by the proteasome inhibitor MG132, Plant Cell 10 (1998) 2063 – 2076.
[41] F. Becker, E. Buschfeld, J. Schell, A. Bachmair, Altered response to viral infection by tobacco plants perturbed in ubiquitin system, Plant J. 3 (1993) 875 – 881.
[42] S. Homma, A. Horsch, M.N. Pouch, F. Petit, Y. Briand, H.P. Schmid, Proteasomes (prosomes) inhibit the trans-lation of tobacco mosaic virus RNA by preventing the formation of initiation complexes, Mol. Biol. Rep. 20 (1994) 57 – 61.
[43] W.P. Belknap, J.E. Garbarino, The role of ubiquitin in plant senescence and stress responses, Trends Plant Sci. 1 (1996) 331 – 335.