Review
FAD-containing polyamine oxidases: a timely challenge for
researchers in biochemistry and physiology of plants
Marek S
&
ebela
a,*, Anna Radova´
a, Riccardo Angelini
b, Paraskevi Tavladoraki
b,
Ivo Fre´bort
a, Pavel Pecˇ
aaDepartment of Biochemistry,Faculty of Science,Palacky´ Uni6ersity,S&lechtitelu˚11,783 71Olomouc,Czech Republic bDepartment of Biology,Uni6ersity‘‘Roma Tre’’,Viale Guglielmo Marconi446,I-00146Rome,Italy
Received 13 July 2000; received in revised form 21 August 2000; accepted 4 September 2000
Abstract
Recent investigations on plant polyamine oxidase (PAO) are reviewed. The enzyme belongs to a new class of flavoenzymes with similar structural features including, among others, monoamine oxidase. Plant PAOs catalyse the oxidation of the polyamine
substrates spermidine and spermine. The reaction products are propane-1,3-diamine and 1-pyrroline or
1-(3-aminopropyl)pyrrolinium, respectively, along with hydrogen peroxide. Plant PAOs are predominantly localised in the cell wall. Purification procedures and molecular properties of several plant PAOs are compared. A special attention is being paid to the recently solved crystal structure of the maize enzyme and its implications for the substrate binding and catalytic mechanism.
Substrate specificity and inhibitors of plant PAOs are also described. The potential roles for PAO-generated H2O2 in lignin
biosynthesis and cell wall cross-linking reactions, which may regulate growth and contribute to cell defence, are discussed. © 2001 Elsevier Science Ireland Ltd. All rights reserved.
Keywords:Polyamine oxidase; Flavoprotein; FAD; Hydrogen peroxide; Spermine; Spermidine
www.elsevier.com/locate/plantsci
1. Introduction
The aliphatic diamines and polyamines show ubiquitous occurrence in prokaryotic and eukary-otic organisms [1]. Their implication in growth and key developmental processes has engaged the interest of an increasing number of researchers during the last three decades. Polyamines have been tentatively proposed as a new class of plant regulatory substances since they are involved in a variety of biological activities [2,3]. During recent years, interest has been growing in naturally oc-curring polyamines. The polyamines spermidine and spermine and the related diamine putrescine
appear to be essential for processes of growth, cell division and differentiation. A role for these com-pounds has been also postulated in macromolecu-lar synthesis, stress responses and senescence [4,5]. It is well known that polyamines bind DNA. However, there is evidence that some proteins possess a specific polyamine-binding site, possibly having a regulatory function [6]. Polyamine levels are finely controlled through a network regulatory system that includes pathways for polyamine biosynthesis and degradation as well as for their transport across the cell membrane [4,5].
In addition to a suggested role in the regulation of cellular polyamine levels, growing evidence arises indicating that polyamine catabolism in plants is associated with physiological events such as lignification, cell wall stiffening and cellular
defence [1,7]. The enzymes concerned with
* Corresponding author. Tel.: +420-68-5241198; fax: + 420-68-5221332.
E-mail address:[email protected] (M. S&ebela).
catabolism of these substances, which are widely distributed in living organisms, have been studied particularly in plants [1,2,8]. In comparison with large and detailed investigations focused on cop-per amine oxidases in last several years, only little
attention has been devoted to study of
flavoprotein polyamine oxidases (PAOs), which in addition to different substrate specificity also ex-hibit distinct pattern of substrate degradation [1,8]. The discovery that some polyamine ana-logues have strong anticancer activity indicates
that the enzymes involved in polyamine
metabolism may represent attractive targets for new antineoplastic drugs [9,10].
Recent investigations on plant PAOs are re-viewed in the present paper. New findings on the crystal structure, reaction mechanism and physio-logical role of the enzymes, which appeared within last few years, promise further and intense re-search work in future.
2. Reaction catalysed by flavin-containing polyamine oxidases
Polyamines (including diamines) are oxidatively deaminated by amine oxidases (AOs) that are
widespread in bacteria, fungi, higher plants and animals [1]. These enzymes are formally classified as copper-containing amine oxidases (CuAOs, E.C. 1.4.3.6) and flavin-containing polyamine oxi-dases (PAOs, E.C. 1.5.3.-). An alternative group-ing divides AOs into those that act on the primary amino group of di- and polyamines (diamine oxi-dases, DAOs) and those that act on the secondary amino group of polyamines (PAOs) [1,8,11]. As suggested by Morgan, the latter should be further subdivided according to whether propane-1,3-di-amine or 3-aminopropanal are the reaction prod-ucts [12].
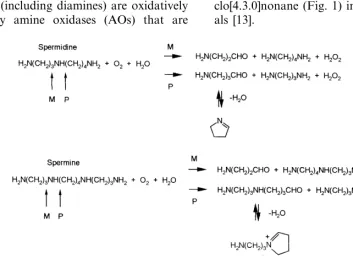
The exact nature of the reaction products de-pends on the enzyme source [1,8,11]. Mammalian PAOs transform spermidine and spermine into putrescine and spermidine, respectively, and 3-aminopropanal. On the contrary, plant and bacte-rial PAOs catalyse the conversion of spermidine
and spermine to 4-aminobutanal and
1-(3-aminopropyl)-4-aminobutanal, respectively, and propane-1,3-diamine (Fig. 1). The aminoaldehydes produced in the reaction spontaneously cyclise to 1-pyrroline and 1-(3-aminopropyl)pyrrolinium, re-spectively [1,12,13], the latter compound occurring mainly in the bicyclic form of 1,5-diazabicy-clo[4.3.0]nonane (Fig. 1) in leaves of various cere-als [13].
Fig. 2. Scheme of the oxidation of the substrate spermidine catalysed by maize PAO [15].
3. Purification and molecular features of plant polyamine oxidases
Flavin-containing PAOs have been isolated par-ticularly from plants belonging to the Gramineae.
The enzyme was found in oat (A6ena sati6a),
maize (Zea mays), barley (Hordeum 6ulgare),
wheat (Triticum aesti6um) and rye (Secale cereale)
[1]. PAOs from oat [21,22], maize [23,24] and barley [25] have been purified to homogeneity and
thoroughly studied. The enzyme from millet (Se
-taria italica) has been only partially purified [26].
Only PAOs from water hyacinth (Eichhornia cras
-sipes) [27] and lily (Lilium longiflorum) [28] have
been purified and characterised outside the
Gramineae in the monocotyledonous plants. In the dicotyledonous plants, putative PAO activity
has been detected in alfalfa (Medicago sati6a),
however, detailed properties of the enzyme remain unclear [29].
Purification procedures, which have been used for the isolation of plant PAO, included an extrac-tion of the enzyme from a crude plant or cell wall extract and its further purification using low pres-sure chromatography (LPC) techniques [21 – 28] or fast protein liquid chromatography (FPLC) [25]. For the extraction, acetone [22,23,26 – 28] or high ionic strength buffers [24,25] have been used. The LPC procedures utilised cation exchangers (CM-cellulose, CM-Sephadex, SP-Sepharose),
hydroxy-apatite and Sephadex gel matrices.
Affinity-interaction sorbents for efficient PAO purifications have been also reported, made on the basis of long-chain diamines (C6, C8) [27,28] or specific antibodies coupled to Sepharose 4B matrix [30].
Molecular properties of several plant PAOs are summarised in Table 1. All the enzymes are monomeric, some of them have been shown to be
glycoproteins. Chromatographic properties of
plant PAOs on cation exchangers indicate that their pI values fall into the acidic region. The enzymes are retained on CM-cellulose or SP-Sep-harose at pH 5.5 and then usually eluted by high
ionic strength buffers [22,24,25,27,28]. PAOs
purified to homogeneity show a typical absorption spectrum of oxidised flavoprotein with absorption maxima at 280, 380 and 450 to 460 nm [1]. The flavin cofactor confers a yellow colour to purified PAO preparations. It has been shown, that plant PAOs contain flavin adenine dinucleotide as the As typically observed in flavin-dependent
oxi-dases, the overall reaction catalysed by plant PAO can be divided into a reductive half reaction, in which the flavin is reduced upon polyamine oxida-tion, and an oxidative half reacoxida-tion, in which the reduced flavin is reoxidised by molecular oxygen with the release of hydrogen peroxide [14,15]. Polyamine oxidation is likely to result in the for-mation of an imino compound (azomethine), which is hydrolysed to produce the final products (Fig. 2). The stoichiometry of the reaction has been studied with barley PAO that produces 1 mol
of H2O2per mol of oxidised substrate spermine or
spermidine [16].
Contrary to CuAO reaction [17], the polyamine oxidation by PAO results in the production of
aminoaldehyde and hydrogen peroxide but
not in ammonia [1,11,12]. In higher plants, the
well-known CuAO of pea seedlings has
been shown to oxidise spermine only very slowly. By contrast, spermidine is rapidly converted to 1-(3-aminopropyl)-4-aminobutanal by the same
enzyme, which undergoes spontaneous
cyclisation to 1-(3-aminopropyl) pyrrolinium,
along with the formation of H2O2 and ammonia.
The relative rates of spermidine and spermine oxidation by the enzyme are 35 and 0.3%, respectively, when compared to that of putrescine [18]. CuAO of lentil seedlings utilises both spermidine and spermine as good substrates.
Sper-mine is oxidised at the terminal (primary)
cofactor in a ratio 1 mol of FAD per mol of the enzyme [23,25,28]. The addition in anaerobiosis of equimolar amounts of spermidine to oat or maize PAO results in the cofactor reduction as shown by the decrease in absorption at 380 and 450 nm. Reoxygenation then restores the spectrum [22,24]. The N-terminal amino acid sequences of maize [31,32], barley [25] and oat PAO (A. Radova´, M. S&ebela, S. Jacobsen, unpublished results) are pre-sented in Table 2, where homologous amino acid sequences of four other flavoenzymes: yeast acetylspermidine oxidase [33], bacterial tyramine oxidase [34] and tryptophan 2-monooxygenase [35] and human type B monoamine oxidase [36], are also given. The first complete amino acid sequence of a flavin-containing PAO has been reported for the maize enzyme [32]. The nucleotide sequence of maize PAO cDNA is 1737 bp in length. It shows a single open reading frame encoding a polypeptide (500 amino acids) starting with a 28 amino acid sequence showing the typical features of a secre-tion signal peptide [32]. This is in agreement with the apoplastic localisation of maize PAO as de-duced on the basis of available cytochemical and immunocytochemical evidence [37,38]. Maize PAO matches the signature motif of a new family of FAD-dependent oxidases, identified by sequence alignment. This family includes oxidoreductases catalysing different reactions such as phytoene
desaturase, protoporphyrinogen oxidase and
monoamine oxidase, all possessing similar ADP-ribityl binding sites [32,39]. A significant structural homology has been detected between maize PAO and vertebrate flavin-containing monoamine oxi-dases (MAO), even beyond the ADP-ribityl bind-ing motif [32,36]. PAO and MAO share the same overall folding topology and FAD-binding site, although this similarity does not extend, as ex-pected, to the substrate binding site [15]. Further-more, the Cys residue involved in the covalent
binding of FAD in vertebrate MAOs is replaced by a Thr residue in maize PAO [32,36] suggesting a non-covalent interaction of the FAD in plant PAOs [15,39].
Polyclonal antibodies raised against PAO from lily did not cross-react with PAOs from maize, oat and water hyacinth. This result suggests that the lily PAO does not share common epitopes with PAOs of the Gramineae [28]. Similarly, immuno-precipitation analysis performed with anti-maize PAO antiserum against oat and barley PAO re-vealed a high immunological compatibility be-tween the oat and maize enzyme, while the barley PAO shares few antigenic determinants with the latter [30].
4. Kinetic properties of plant polyamine oxidases
All plant PAOs show very restricted substrate specificity oxidising only spermine and spermidine [1]. This observation is in accordance with recently found ‘‘U-shaped’’ structure of the active site [15].
The respective Km values have been found in the
region between 10−5 M and 10−6 M. The pH
optima for the oxidation of the substrates vary among different species, being equal in the range 5.5 to 6.8 for most of the enzymes [22,24,26 – 28]. In the case of barley PAO, however, the pH optima 4.8 (spermine) and 8.0 (spermidine) have been reported [25,40]. Is it due to different contact and auxiliary amino acid side chains at the active site? The question has not yet been answered. Plant PAOs are usually equally active with sper-mine and spermidine [22,24,27]. The only excep-tion is barley PAO again, which oxidises spermine 14 times faster than spermidine at their respective pH optima [25,40]. Maize PAO also catalyses the
oxidation of N1-acetylspermidine, N1
-acetylsper-mine andN8-acetylspermidine at the same optimal
Table 1
Molecular properties of plant PAOs from oat [21,22], maize [15,24], barley [25], water hyacinth [27] and lily [28]
Maize Barley Water hyacinth
Property Oat Lily
53 000 63 000
Molecular weight (SDS-PAGE) 56 000 60 000 63 000
ND 53 000
80 000
Molecular weight (gel chromatography) 76 000 87 000
5.4 ND
Isoelectric point 4.7a ND ND
3 ND
Sugar content % (w/w) 2.5 0 5
FAD content (mol/mol enzyme) 1 1 1 1 1
pH 6.5 [41]. However, the enzyme is quickly inac-tivated during the reaction. Maize PAO cleaves
N1-acetylspermine and N8-acetylspermidine at the
same position as in spermine and spermidine reac-tion. These findings suggest that cell wall PAO from maize does not affect the interconversion pathway of acetylpolyamines [41]. The activity of oat PAO with spermine was stimulated by 1 M NaCl [42]. Similarly, the activity of lily PAO with spermine and spermidine was increased in the same way [28].
Calorimetric analysis on the maize enzyme re-vealed a single two-state transition at pH 6.0 with
Tm=49.8°C. At pH 5.0, the thermal stability is
increased by more than 14°C [31]. The pH opti-mum for the stability of the native enzyme is 5.0, similar to that of barley leaf PAO [31]. Maize and barley PAO do not reduce added electron carriers such as 2,6-dichlorophenolindophenol, phenazine methosulfate, or ferricyanide either in anaerobiosis or in the presence of oxygen [43,44]. However,
maize PAO is able to utilise p-benzoquinone as
electron acceptor in aerobiosis [43]. The coupled
reduction of cytochrome c or
2,6-dichloropheno-lindophenol could be effected by this system. For
barley, maize and oat PAOs, the respective Km
values for oxygen are relatively high [21,24]. The analysis of transient kinetic data revealed that the maize enzyme has a low rate constant for the reaction with oxygen, responsible for the high
value 0.2 mM ofKm(O2). Maize PAO is therefore
only partially oxidised even under fully aerobic condition and the oxygen concentration may be a
relevant rate-limiting factor in vivo [45]. On the other hand, the reaction with spermidine is much more efficient and both the turnover number (90
s−1) and the low Km value (27 mM) suggest that
the transformation proceeds rapidly under physio-logical conditions [45].
The activity of plant PAOs is measured either spectrophotometrically by means of a coupled re-action with horseradish peroxidase and guaiacol [21,25] or by measuring oxygen consumption using a Clark type electrode [22,24]. Specific activities of the final PAO preparations from cereals usually reach about 100 – 1500 nkat per mg with sper-midine or spermine substrates [22,24,27,28]. The preparations are stable without a significant loss of the activity for several weeks at 4°C [25,27] or
frozen at −15°C [21].
A loss of enzymatic activity during polyamine degradation has been observed for barley and oat PAO [16,25,46]. As it was shown for the maize enzyme, the inhibition is caused by the aminoalde-hydes produced during the reaction. Polyamine oxidation products, 4-aminobutanal and 1-(3-aminopropyl)-4-aminobutanal, behave as
competi-tive inhibitors of maize PAO (Ki10−4 M) [31].
The aminoaldehyde formed by the oxidation of
N1-acetylspermine also inhibits in a competitive
manner (Ki10−5 M) [41]. PAO from water
hy-acinth is competitively inhibited by
octamethylene-diamine (Ki10−6 M) [27]; similarly, the lily
enzyme is competitively inhibited by
hexa-methylenediamine (Ki10−6 M) [28]. Analogues
of spermidine and spermine, in which the length of
Table 2
N-terminal amino acid sequences of plant polyamine oxidases from barley, oat and maizea
Sequence
Enzyme Reference
[25]
01 GKGPRVIIVGAGMSGISAAKRLLDAGVVDL
Barley PAO
01 AAGPRVIIVGAGMSGISAGK
Oat PAO Radova´ et al. (unpublished results)
29 ATVGPRVIVVGAGMSGISAAKRLSEAGITDL
Maize PAOb [32]
[33] 02 TTVRIDAIVIGAGIAGVKASIELTKAGVSNI
ASO1b
[34]
TYOb 01 SNPHVVIVGAGFAGLVAARELQMAGVDVE
TR2Mb 35 GTPTPRVAIVGAGISGLVAATELLRAGVKDV [35]
MAO-Bb 01 MSNKCDVVVVG6GG6 ISG6MAA6AKLLHDSG6LNVV [36]
aThe N-terminal amino acid sequences of barley and oat PAOs were obtained by automated Edman degradation, for the
enzyme from maize seedlings the sequence was deduced from cloned cDNA. Translated sequences of homologous flavoenzymes:
yeast acetylspermidine oxidase (Candida boidinii, ASO1), bacterial tyramine oxidase (Micrococcus luteus, TYO), bacterial
tryptophan 2-monooxygenase (Pseudomonas syringae sa6astanoi, TR2M) and human type B monoamine oxidase (MAO-B), are
given for comparison. The identical residues of the three PAO sequences are in bold. Underlined amino acids in the MAO-B sequence are conserved in all seven sequences.
the tetramethylene chain was shortened or length-ened, caused competitive inhibition of polyamine
oxidation by barley and oat PAOs (Ki values
between 10−6and 10−4M) [40,42,44]. Oat PAO is
also inhibited by fungicide guazatine (Ki10−8
M) containing guanidine groups [21].
Methylgly-oxal-bis(guanylhydrazone), semicarbazide and b
-hydroxyethylhydrazine were shown to be
inhibitors of PAO from barley [16,25,44]. PAOs from cereals and water hyacinth are significantly
inhibited by acridine compounds, namely
quinacrine, at 1 mM concentration due to the presence of flavin cofactor [22 – 27].
5. Crystal structure of a plant polyamine oxidase
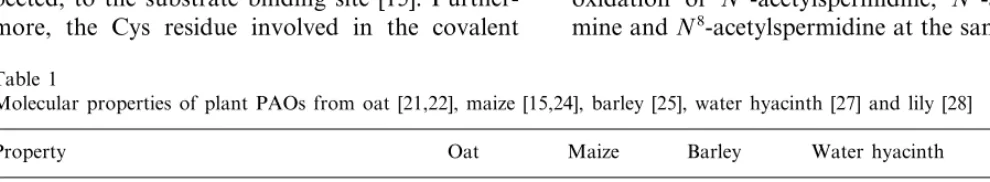
The only PAO that has been crystallised and whose three-dimensional structure has been solved up to date is the enzyme of maize seedlings [15,47]. Maize PAO is a monomeric enzyme [24] consisting
of 13ahelices and 19b strands, which fold to form
two well-defined domains (Fig. 3). The FAD-bind-ing domain comprises three fragments, whose
main structural elements are a central parallel b
sheet flanked by a b meander and three a helices.
The substrate-binding domain is composed of two fragments and is characterised by a six-stranded
mixed b sheet flanked by five a helices. The two
domains create a tunnel that defines the enzyme active site at their interface. The folding topology of maize PAO resembles that of several other flavoenzymes catalysing dehydrogenation
reac-tions such as glucose oxidase or D-amino acid
oxidase [15].
PAO from maize seedlings is a glycoprotein [24]. The glycosylation site has been identified to be Asn77 [15]. The FAD cofactor is non-covalently bound to the protein and is deeply buried within the structure. The isoalloxazine ring of FAD is located at the interface of the two domains [15]. With the exception for the flavin C5a, N5 and C4a atom that line the active site, all FAD atoms are solvent-inaccessible. The conformation of the oxi-dised flavin is nonplanar — the orientation might be important in precisely aligning the cofactor with respect to the polyamine substrate. The PAO active centre consists of remarkable ‘‘U-shaped’’ tunnel, which passes through the protein structure at the interface between the two above-mentioned domains [15]. The tunnel extends to a length of
about 30 A, . The U-shape brings its two openings
onto the same side of the protein surface. The turning point, around which the tunnel sharply bends and reverses its direction, represents the core of the catalytic centre, where the flavin ring is located (Fig. 3). There is a marked contrast in the chemical nature of the two arms of the U-shaped catalytic centre [15]. One arm is lined mainly by aromatic residues and it opens to the outside like a funnel with several acidic side-chains (Asp, Glu) on its rim (Fig. 4). In contrast, the other arm contains mostly oxygen atoms on its surface and displays a narrow entrance. In this respect, the ring of Asp and Glu residues seems to be suited to fulfil the role of guiding the polyamine substrate into the tunnel. Thus the substrate might be ad-mitted into its binding site preferentially through only one of the two tunnel openings [15].
6. Substrate binding and its implications for the catalytic mechanism
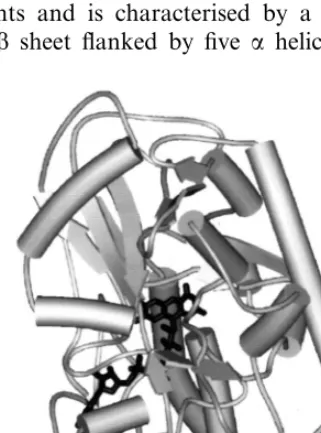
The precise mechanism by which plant PAOs catalyse the oxidation of the polyamine substrates is still unknown. Overall, the catalytic cycle con-sists of two steps. During the first step (reductive), FAD is reduced upon polyamine oxidation. The second step (oxidative) represents re-oxidation of Fig. 3. The overall three-dimensional structure of maize PAO.
The crystal structure has been determined to the resolution of
1.9 A,. The isoalloxazine ring of the cofactor FAD can be seen
Fig. 4. Schematic representation of the catalytic tunnel in the active site of maize PAO during binding of the inhibitor
N,N%-bis(2,3-butadienyl)-1,4-butanediamine. Hydrogen bonds
are indicated by dashed lines. The 3.8 A, contact between the
inhibitor C8 and the flavin N5 is outlined. The distances shown are in angstro¨ms [15].
well positioned to perform the hydrolytic attack on the imino compound resulting from polyamine oxidation to produce the final aldehyde product (Fig. 2). The three-dimensional structure shows that MDL72527 is a non-covalent inhibitor of maize PAO [15]. In this respect the plant PAO differs from the mammalian enzymes. It may be related to the different substrate cleavage dis-played by mammalian and plant PAOs. The in-hibitor differs from the spermine substrate only in the absence of the primary amino groups. Thus, the substrate primary amino groups are strictly required by the enzyme in order to catalyse sub-strate oxidation. Primary nitrogen atoms of the substrate spermine possess a positive charge,
which may affect pKa values of the surrounding
catalytic residues and those of the secondary sub-strate amino groups. The primary amino groups are likely to affect the binding mode of the sub-strate in particular [15].
7. Physiological aspects of plant polyamine oxidases
Earlier studies on PAO from barley shoots showed that the enzyme was associated with a particle fraction [16]. Barley and oat PAO could be solubilised by washing the pellet with 0.5 M NaCl or KCl, whereas sonication, Triton X-100 or acetone treatments were ineffective. Moreover, the enzyme could not be eluted by the effect of nucle-ases, thus making a nuclear localisation of the enzyme unlikely [46]. PAO activity was absent in oat leaf protoplasts isolated after the digestion of the leaves with cellulase in hypertonic osmoticum. When the protoplasts were cultured in a medium suitable for regeneration of cell wall, the PAO activity was detected as the cell wall developed. Therefore it has been suggested that the enzyme is localised in cell wall [49].
Histochemical studies revealed the association of the enzyme with the vascular system and stom-ata [49]. Specific PAO activity is noticeably higher in extracellular fluids obtained from maize meso-cotyls, exceeding by 100 times that found in crude homogenate of the whole organ [50]. Similarly, a highly active enzyme could be solubilised from oat primary leaves using the vacuum infiltration tech-nique that selectively extracts soluble and ionically bound apoplastic proteins [51]. The method can be the reduced flavin cofactor by molecular oxygen
with the release of H2O2 (Fig. 2). In this respect,
the three-dimensional structure of the maize en-zyme in complex with substrate analogues reveals several relevant features [15]. The substrate
ana-logue N,N%-bis(2,3-butadienyl)-1,4-butanediamine
(MDL72527) [48], which competitively inhibits
maize PAO (Ki10−7 M) (R. Federico,
unpub-lished results) was used for substrate-binding stud-ies. MDL72527 binds in the central part of the catalytic tunnel, adopting a conformation that closely matches the shape of the tunnel (Fig. 4). The ligand binding does not cause any significant conformational change of the enzyme. The bound inhibitor molecule is completely solvent-inaccessi-ble and it is involved in extensive H-bond and van der Waals contacts with the surrounding protein atoms. Moreover, it interacts with the flavin ring. The flavin C4a-N5-C5 locus becomes shielded from the solvent after the inhibitor binding. Six of the twelve inhibitor carbons are located in close proximity to protein oxygens. These PAO-in-hibitor interactions appear to have the
stereo-chemical characteristics of the so-called CHO
used for rapid purification of the enzyme. Unlike the oat and maize enzyme, PAO is apparently symplastic in barley primary leaves [52]. The apo-plastic nature of maize PAO has been recently confirmed by an electron-microscopic
cytochemi-cal method based on the precipitation of H2O2
generated by the in situ polyamine oxidation. PAO activity staining was most intense in the middle lamellar region of the wall and in cells exhibiting highly lignified walls [37]. PAO activity is very low in resting seeds and can be detected as soon as germination begins. During the first phases of growth, a continuous increase of enzyme activity is observable in the shoot [53].
Sub-cellular and tissue distribution of PAO ac-tivity has been investigated in maize seedlings. It was observed that from 30 to 90% of maize PAO activity is tightly bound to cell walls of maize seedlings (in the shoot and root, respectively), while the remainder can be eluted with high ionic strength buffers [54]. Despite some apparent dif-ferences, the ionically bound and the tightly wall-bound PAOs are probably the same protein [55]. Tissue and organ distribution of PAO in the maize seedling, studied by a histochemical approach, shows an apparent PAO localisation mainly in the cell walls of xylem (coleoptile), xylem and xylem parenchyma (mesocotyl, root), rhizodermis, hypo-dermis and endohypo-dermis (root) and epihypo-dermis (coleoptile, mesocotyl) [54]. Apparent histochemi-cal peroxidase activity shows the same tissue and organ distribution indicating the preferential asso-ciation of the two enzymes with the cell walls of tissues where lignification, suberisation, and wall stiffening occur [54].
Moreover, ultrastructural localisation of PAO has been done by means of immuno-gold experi-ments with antibodies specific for the polypeptide moiety in different organs of the maize seedling [38]. The antibodies have been raised in order to develop immunocytochemical method clearly dis-tinguishing between CuAO and PAO coexisting in plant tissues. The occurrence of both enzymes has been recently demonstrated in barley [56] and maize seedlings [57]. In the endodermal cells of the primary and lateral roots only the cells with sec-ondary walls were labelled. No labelling was ob-served in the intercellular spaces, the suberin layer or in the primary wall. All the secondary walls of the xylem cells were labelled. In the etiolated mesocotyl, labelling was limited to the secondary
walls of large vessel and vascular parenchyma, while the parenchymal cells were unreactive. The gold particles were limited to the secondary thick-enings, in particular in the differentiating cells that were involved in secondary wall deposition. No labelling was found over the primary wall. PAO was detected only rarely over the epidermal cell walls. All these findings indicate that PAO may play a crucial role in cell wall differentiation [38]. The available evidence suggests a considerable similarity for the physiological role of CuAO and PAO in the apoplast with regard to the functional correlation with peroxidase [58]. Indeed, it has been reported that peroxidase-mediated oxidative cross-linking reactions between phenolic residues of matrix polysaccharides, as well as those of extensins may partially account for wall stiffening, and have remarkable consequences for the wall expansion during growth and differentiation [59].
It could indicate that wall stiffening in 6i6o is
controlled by the synthesis of hydrogen peroxide in the cell wall. Unravelling the biochemical
sys-tems providing H2O2 to peroxidase in the
above-mentioned reactions, as well as establishing their functional correlation with light-mediated growth, represents a major unsolved problem. Several lines of evidence suggest that PAO may be functionally correlated with peroxidase and may have a key role in the production of reactive oxygen interme-diates in the apoplast [38,58].
pre-treatment of mesocotyl and coleoptile segments with 1 mM spermidine, but not propane-1,3-di-amine, inhibited elongation growth induced by indoleacetic acid; this phenomenon being reverted
by catalase. Similarly, spermidine-grown Ara
-bidopsis thaliana plants showed a different mor-phology (shorter stalks and darker green-leaves) when compared with control ones. In most plant organs, the uptaken spermidine was converted to putrescine. The successive increase in putrescine
level suggests the presence of an
intercon-version pathway mediated by a putative
polyamine oxidase [60]. These results indicate that
PAO activity is important in producing H2O2 in
vivo for wall stiffening reactions and may be in-volved in the modulation of growth and cell wall differentiation [7]. In this context, it was previ-ously observed that spermidine incubation of in-tact maize roots resulted in an increase of PAO and peroxidase activity, associated to an earlier differentiation of xylem tissues [61]. Among oth-ers, these aspects of polyamine catabolism may shed new light on the modulation of cell wall redox status in the context of programmed cell death, host – pathogen interactions and resistance to abiotic stress.
8. Conclusions and future perspectives
Plant PAOs play an important role in several
physiological processes including polyamine
homeostasis and cell death [1]. The enzymes ex-hibit many similar properties at the molecular level. However, the situation in higher plants is quite puzzling because the enzymes occur at a high concentration only in some species. Two explana-tions are possible: some species have alternative mechanisms of polyamine catabolism, or the ap-parent lack of PAO activity is due to a very low
enzyme concentration in plant tissues and/or to its
inhibition by still unknown factors [1]. The poten-tial roles for amine oxidase-generated hydrogen peroxide in lignin biosynthesis and cell wall cross-linking reactions that may regulate growth as well as defence and cell death responses have been discussed. In addition to polyamine catabolism, the enzymes and their reaction products may have other important functions in some species. In this light, the study of polyamine catabolism merits a renewed interest and further experimental effort.
Several ways for pursuing the research work on plant PAOs may be outlined. The cloning of the maize enzyme opened new horizons for studying PAO gene expression. There are many chemical and physiological factors that can influence such a delicate process. The expression also depends on the stage of plant development, it could be influ-enced by growth conditions and by stress during pathogen infections. Open topics for further re-search on plant PAOs also include studies on distribution of the enzyme protein and the activity on conditions of growth, pathogen infections, in-juries and wound healing. Moreover, site-directed mutagenesis experiments using overexpressed PAO will contribute to a deeper insight into structure – function relationships and biotechnological appli-cations of this class of enzymes. Appliappli-cations of PAOs in food industry and agriculture are under development. Just recently, a polyamine oxidase-based biosensor appeared [62]. The aminoaldehyde products of the enzyme reaction show antiproto-zoal effects [63] suggesting possible application of PAOs in medicine. Regarding to enzymologic studies, a big attention is being paid to search for new substrates and sensitive and potent inhibitors. Chemical synthesis of highly reactive mechanism-based inhibitors (made on the basis of a natural substrate) has the top priority. Kinetic studies with substrates and inhibitors could bring informations about intermediates of the catalytic cycle of plant PAOs, which still remain unclear. Many questions could be answered by comparative experiments on expression, activity modulation and physiological functions of plant FAD-containing PAOs and copper-containing DAOs. Both types of amine oxidases have been found in barley, which seems to be very suitable subject for such investigations.
Acknowledgements
The authors thank Dr Susanne Jacobsen, De-partment of Biochemistry and Nutrition, Danish Technical University, Kongens Lyngby for collab-oration on barley and oat PAO sequencing. This
work was supported by the grants 203/97/0097
from the Grant Agency, Czech Republic, No. 153100010 from the Ministry of Education, Czech
Republic, IG 31903008/99 from the Faculty of
Science, Palacky´ University, Olomouc and the
The National Research Council of Italy (CNR Target Project on Biotechnology) is also gratefully acknowledged.
References
[1] R. Federico, R. Angelini, Polyamine catabolism in plants, in: R.D. Slocum, H.E. Flores (Eds.), Biochem-istry and Physiology of Polyamines in Plants, CRC Press, Boca Raton, 1991, pp. 41 – 56.
[2] T.A. Smith, Polyamines, Annu. Rev. Plant Physiol. 36 (1985) 117 – 123.
[3] S.S. Cohen, A Guide to Polyamines, Oxford University Press, New York, 1998, pp. 396 – 442.
[4] A.F. Tiburcio, T. Altabella, A. Borrell, C. Masgrau, Polyamine metabolism and its regulation, Physiol. Plant. 100 (1997) 664 – 674.
[5] A. Bouchereau, A. Aziz, F. Larher, J. Martin-Tanguy, Polyamines and environmental challenges: recent devel-opment, Plant Sci. 140 (1999) 103 – 125.
[6] D. Leroy, J.K. Heriche, O. Filhol, E.M. Chambaz, C. Cochet, Binding of polyamines to an autonomous do-main of the regulatory subunit of protein kinase CK2 induces a conformational change in the holoenzyme, J. Biol. Chem. 272 (1997) 20 820 – 20 827.
[7] M. Laurenzi, G. Rea, R. Federico, P. Tavladoraki, R. Angelini, De-etiolation causes a phytochrome-mediated increase of polyamine oxidase expression in outer tissues of the maize mesocotyl: a role in the photomodulation of growth and cell wall differentiation, Planta 208 (1999) 146 – 154.
[8] S.S. Cohen, A Guide to Polyamines, Oxford University Press, New York, 1998, pp. 69 – 93.
[9] A.E. Pegg, R.H. Hu, Effect of polyamine analogues and
inhibition of polyamine oxidase on spermidine/spermine
N1-acetytransferase activity and cell proliferation, Can-cer Lett. 95 (1995) 247 – 252.
[10] L.J. Marton, A.E. Pegg, Polyamines as targets for thera-peutic intervention, Annu. Rev. Pharmacol. Toxicol. 35 (1995) 55 – 91.
[11] T.A. Smith, The di- and polyamine oxidases of higher plants, Biochem. Soc. Trans. 13 (1985) 319 – 322. [12] D.L. Morgan, Polyamine oxidases, Biochem. Soc. Trans.
13 (1985) 322 – 326.
[13] T.A. Smith, S.J. Croker, R.S.T. Loeffler, Occurence in higher plants of 1-(3-aminopropyl)pyrrolinium and pyrroline: products of polyamine oxidation, Phytochem-istry 25 (1986) 683 – 689.
[14] N. Seiler, Polyamine oxidase, properties and functions, Prog. Brain. Res. 106 (1995) 333 – 344.
[15] C. Binda, A. Coda, R. Angelini, R. Federico, P. Ascenzi,
P.A. Mattevi, A 30 A, long U-shaped catalytic tunnel in
the crystal structure of polyamine oxidase, Structure Fold. Des. 7 (1999) 265 – 276.
[16] T.A. Smith, Purification and properties of the polyamine oxidase of barley plants, Phytochemistry 11 (1972) 899 – 910.
[17] R. Medda, A. Padiglia, G. Floris, Plant copper-amine oxidases, Phytochemistry 39 (1995) 1 – 9.
[18] J.M. Hill, P.J.G. Mann, Further properties of the di-amine oxidase of pea seedlings, Biochem. J. 91 (1964) 171 – 182.
[19] A. Cogoni, A. Padiglia, R. Medda, P. Segni, G. Floris, Oxidation of spermine by an amine oxidase from lentil seedlings, Plant Physiol. 95 (1991) 477 – 479.
[20] A. Padiglia, R. Medda, M. Paci, M. Sette, A. Lorrai, G. Floris, Characterization of a cyclic compound formed after spermine oxidation by lentil amine oxidase, Biochem. Molec. Biol. Int. 41 (1997) 407 – 413.
[21] T.A. Smith, Polyamine oxidase (oat seedlings), in: H. Tabor, C.W. Tabor (Eds.), Methods in Enzymology, vol. 94, Academic Press, New York, 1983, pp. 311 – 314. [22] R. Federico, C. Alisi, F. Forlani, R. Angelini, Purifica-tion and characterizaPurifica-tion of oat polyamine oxidase, Phy-tochemistry 28 (1989) 2045 – 2046.
[23] Y. Suzuki, H. Yanagisawa, Purification and properties of maize polyamine oxidase: a flavoprotein, Plant Cell Physiol. 21 (1980) 1085 – 1094.
[24] R. Federico, C. Alisi, F. Forlani, Properties of polyamine oxidase from the cell wall of maize seedlings, Phytochemistry 28 (1989) 45 – 46.
[25] A. Radova´, M. S&ebela, P. Galuszka, I. Fre´bort, S.
Jacobsen, H.G. Faulhammer, P. Pec, Barley polyamine oxidase: characterisation and analysis of the cofactor and the N-terminal amino acid sequence, Phytochem Anal, in press.
[26] E. Hirasawa, H. Watanabe, Y. Suzuki, Polyamine ox-idase of millet shoots, Phytochemistry 25 (1986) 1739 – 1740.
[27] H. Yanagisawa, A. Kato, S. Hoshiai, A. Kamiya, N. Torii, Polyamine oxidase from water hyacinth, Plant Physiol. 85 (1987) 906 – 909.
[28] H. Yanagisawa, N. Hamashima, T. Kato, Polyamine
oxidase from leaves of Lilium longiflorum: purification
and properties, J. Plant Physiol. 149 (1996) 657 – 662. [29] S. Bagga, A. Dharma, G.C. Phillips, G.D. Kuehn,
Evi-dence of the occurence of polyamine oxidase in the
dicotyledonous plant Medicago sati6aL. (alfalfa), Plant
Cell Reports 10 (1991) 550 – 554.
[30] R. Federico, C. Alisi, A. Cona, R. Angelini, Purification of polyamine oxidase from maize seedlings by im-munoadsorbent column, in: V. Zappia, A.E. Pegg (Eds.), Progress in Polyamine Research, Plenum Press, New York, 1989, pp. 617 – 623.
[31] R. Federico, A. Cona, R. Angelini, M.E. Schinina`, A. Giartosio, Characterization of maize polyamine oxidase, Phytochemistry 29 (1990) 2411 – 2414.
[32] P. Tavladoraki, M.E. Schinina`, F. Cecconi, S. di Agostino, F. Manera, G. Rea, P. Mariottini, R. Fed-erico, R. Angelini, Maize polyamine oxidase: primary structure from protein and cDNA sequencing, FEBS Lett. 426 (1998) 62 – 66.
[33] M. Nishikawa, T. Hagishita, H. Yurimoto, N. Kato, Y. Sakai, T. Hatanaka, Primary structure and expression of peroxisomal acetylspermidine oxidase in the
methy-lotrophic yeastCandida boidinii, FEBS Lett. 476 (2000)
[34] J.H. Roh, J. Wouters, E. Depiereux, H. Yukawa, M. Inui, H. Minami, H. Suzuki, H. Kumagai, Purification,
cloning and three-dimensional structure of Micrococus
luteusFAD-containing tyramine oxidase, Biochem. Bio-phys. Res. Commun. 268 (2000) 293 – 297.
[35] T. Yamada, C.J. Palm, B. Brooks, T. Kosuge,
Nucle-otide sequences of the Pseudomonas sa6astanoi
in-doleacetic acid genes show homology with
Agrobacterium tumefaciens T-DNA, Proc. Natl. Acad. Sci. USA 82 (1985) 6522 – 6526.
[36] A.W.J. Bach, N.C. Lan, D.L. Johnson, C.W. Abell, M.E. Bembenek, S.W. Kwan, P.H. Seeburg, J.C. Shih, cDNA cloning of human liver monoamine oxidase A and B: molecular basis of differences in enzymatic prop-erties, Proc. Natl Acad. Sci. USA 85 (1988) 4934 – 4938. [37] R.D. Slocum, M.J. Furey, Electron-microscopic cyto-chemical localization of diamine and polyamine oxidases in pea and maize tissues, Planta 183 (1991) 443 – 450. [38] R. Angelini, R. Federico, P. Bonfante, Maize polyamine
oxidase: antibody production and ultrastructural local-ization, J. Plant Physiol. 145 (1995) 686 – 692.
[39] M.W. Fraaije, A. Mattevi, Flavoenzymes: diverse cata-lysts with recurrent features, Tibs 25 (2000) 126 – 132. [40] T.A. Smith, Polyamine oxidation by enzymes from
Hordeum 6ulgare and Pisum sati6um seedlings, Phyto-chemistry 13 (1974) 1075 – 1081.
[41] R. Federico, L. Ercolini, M. Laurenzi, R. Angelini, Oxidation of acetylpolyamines by maize polyamine ox-idase, Phytochemistry 43 (1996) 339 – 341.
[42] T.A. Smith, Further properties of the polyamine oxidase from oat seedlings, Phytochemistry 16 (1977) 1647 – 1649.
[43] E. Hirasawa, Y. Suzuki, Purification and properties of a
polyamine oxidase from Zea mays, Phytochemistry 14
(1975) 99 – 101.
[44] T.A. Smith, D.A. Bickley, Further properties of the polyamine oxidase of barley leaves, Phytochemistry 13 (1974) 2437 – 2443.
[45] A. Bellelli, R. Angelini, M. Laurenzi, R. Federico,
Tran-sient kinetics of polyamine oxidase from Zea mays L,
Arch. Biochem. Biophys. 343 (1997) 146 – 148.
[46] T.A. Smith, Polyamine oxidase from barley and oats, Phytochemistry 15 (1976) 633 – 636.
[47] C. Binda, A. Coda, R. Angelini, R. Federico, P. Ascenzi, A. Mattevi, Crystallization and preliminary X-ray
analy-sis of polyamine oxidase fromZea maysL, Acta
Crystal-logr. D Biol. CrystalCrystal-logr. 54 (1998) 1429 – 1431. [48] F.N. Bolkenius, P. Bey, N. Seiler, Specific inhibition of
polyamine oxidase in vivo is a method for the elucida-tion of its physiological role, Biochim. Biophys. Acta 838 (1985) 69 – 76.
[49] R. Kaur-Sawhney, H.E. Flores, A.W. Galston,
Polyamine oxidase in oat leaves: a cell wall-localized enzyme, Plant Physiol. 68 (1981) 494 – 498.
[50] R. Federico, C. Alisi, R. Angelini, On the occurence of oxidoreductases in the apoplast of Leguminosae and Gramineae and their significance in the study of plasma membrane-bound redox activities, in: F.L. Crane, D.J. Morre, H. Low (Eds.), Plasma Membrane Oxidoreduc-tases in Control of Animal and Plant Growth, Plenum Press, New York, 1988, pp. 333 – 337.
[51] Z.C. Li, Rapid purification of polyamine oxidase from oat primary leaves, Phytochemistry 34 (1993) 611 – 612. [52] Z.C. Li, J.W. McClure, Polyamine oxidase of primary
leaves is apoplastic in oats but symplastic in barley, Phytochemistry 28 (1989) 2255 – 2559.
[53] P. Torrigiani, V. Scoccianti, N. Bagni, Polyamine ox-idase activity and polyamine content in maize during seed germination, Physiol. Plant. 74 (1988) 427 – 432. [54] M. Augeri, R. Angelini, R. Federico, Sub-cellular
local-ization and tissue distribution of polyamine oxidase in
maize (Zea mays L.) seedlings, J. Plant Physiol. 136
(1990) 690 – 695.
[55] R. Federico, R. Angelini, A. Cona, A. Niglio, Polyamine
oxidase bound to cell walls from Zea mays seedlings,
Phytochemistry 31 (1992) 2955 – 2957.
[56] A. Cogoni, C. Piras, R. Farci, A. Melis, G. G. Floris,
Hordeum 6ulgare seedlings amine oxidase. Purification
and properties, Plant Physiol. 93 (1990) 818 – 821. [57] Y. Suzuki, M. Hagiwara, Purification and
characteriza-tion of diamine oxidase from Zea mays shoots,
Phyto-chemistry 33 (1993) 995 – 998.
[58] R. Angelini, R. Federico, Histochemical evidence of polyamine oxidation and hydrogen peroxide production in the cell wall, J. Plant Physiol. 135 (1989) 212 – 217. [59] M. Hohl, H. Greiner, P. Schopfer, The cryptic growth
response of maize coleoptiles and its relationship to
H2O2-dependent cell-wall stiffening, Physiol. Plant. 94
(1995) 491 – 498.
[60] A. Tassoni, M. van Buuren, M. Franceschetti, S. For-nale, N. Bagni, Polyamine content and metabolism in
Arabidopsis thailiana and effect of spermidine on plant development, Plant Physiol. Biochem. 38 (2000) 383 – 393.
[61] M. de Agazio, R. Federico, R. Angelini, Spermidine pretreatment or root tip removal in maize seedlings:
effects on K+uptake and tissue modifications, J. Plant
Physiol. 140 (1992) 741 – 746.
[62] M. Esti, G. Volpe, L. Massignan, D. Compagnone, E. La Notte, G. Paleschi, Determination of amines in fresh and modified atmosphere packaged fruits using electro-chemical biosensors, J. Agric. Food. Chem. 46 (1998) 4233 – 4237.
[63] A. Cona, R. Federico, M. Gramiccia, S. Orsini, L. Gradoni, The aminoaldehydes produced by spermine and spermidine oxidation with maize polyamine oxidase have anti-leishmanial effect, Biotech. Appl. Biochem. 14 (1991) 54 – 59.
![Fig. 2. Scheme of the oxidation of the substrate spermidinecatalysed by maize PAO [15].](https://thumb-ap.123doks.com/thumbv2/123dok/1031775.928217/3.612.28.266.46.157/fig-scheme-oxidation-substrate-spermidinecatalysed-maize-pao.webp)