The promoter of the
Vicia faba
L. gene VfEnod12 encoding an
early nodulin is active in cortical cells and nodule primordia of
transgenic hairy roots of
Vicia hirsuta
as well as in the prefixing
zone II of mature transgenic
V
.
hirsuta
root nodules
Martin Fru¨hling, Gerald Schro¨der, Natalija Hohnjec, Alfred Pu¨hler,
Andreas M. Perlick, Helge Ku¨ster *
Biologie VI(Genetik),Uni6ersita¨t Bielefeld,Fakulta¨t fu¨r Biologie,Postfach100131,D-33501Bielefeld, Germany
Received 6 April 2000; received in revised form 7 July 2000; accepted 9 August 2000
Abstract
A full-length cDNA encoding theVicia fabaL. early nodulin VfEnod12 was isolated. The deduced protein sequence specified a 90 amino acid protein with a MW of 10 206 and contained a putative signal peptide sequence followed by PPX3 repeats
characteristic of Enod12 proteins. The VfEnod12 gene was found to be expressed specifically in root nodules as early as 3 days post inoculation withRhizobium leguminosarumbv.6iciae. In mature nodules, VfEnod12 transcripts were confined to the prefixing
zone II. A 3.3 kb genomic fragment carrying the complete VfEnod12 coding region was isolated. No intervening sequences were identified in the coding region. A promoter fragment carrying the -692/-41 region mediated reporter gene expression in root cortical cells, nodule primordia and the prefixing zone II of transgenic Vicia hirsuta root nodules. This fragment contained a putative binding site for the transcription factor ENBP1. In contrast to the highly conserved terminal AATAA motif of the ENBP1 binding site of known Enod12 promoters, the VfEnod12 promoter was characterized by an altered terminal AATAT sequence. This alteration did not interfere with VfEnod12 promoter activity in transgenic roots and nodules ofV.hirsuta. © 2000 Elsevier Science Ireland Ltd. All rights reserved.
Keywords:Early nodulin; ENBP1 binding site; Hairy roots; Promoter activity;Vicia faba;Vicia hirsuta
www.elsevier.com/locate/plantsci
1. Introduction
During the symbiotic interaction of rhizobia with legume plants the bacterial microsymbionts induce a developmental programme that leads to the formation of a completely new symbiotic or-gan, the root nodule. The root nodule is infected and the central tissue is subsequently colonized by the microsymbionts [4,9]. In the nodule tissue, the plant provides the environment necessary for the microsymbiont to convert atmospheric dinitrogen to ammonia. During all stages of recognition,
organogenesis and function, a complex network of molecular communication accompanied by the specific activation of genes in both partners is involved [8,22,12]. The crucial signalling factors
that are synthesized by Rhizobium and that are
perceived by the plants are substituted
lipochi-tooligosaccharides, the so-called nodulation
(NOD) factors. These bacterial signal molecules are responsible for the specificity of the legume-Rhizobium interaction [15,34]. Up to now, a num-ber of plant genes expressed exclusively in root nodules in response to rhizobial infection were identified [7,40,23]. The encoded gene products were designated nodulins and were divided into early and late nodulins according to their timing of synthesis [39]. In contrast to late nodulins
com-* Corresponding author. Tel.: +49-521-1065620; fax: + 49-521-1065626.
E-mail address:[email protected] (H. Ku¨ster).
prising leghemoglobins and enzymes of nodule carbon and nitrogen metabolism, early nodulins are mainly structural proteins involved in infection or nodule organogenesis [32]. One of the first early nodulin genes specifically activated after infection of legume roots by rhizobia are the Enod12 genes. Up to now, Enod12 sequences were isolated from Pisum sati6um [13,31], Medicago sati6a [1],
Medicago truncatula [26] and Vicia sati6a [41].
Enod12 genes code for proline-rich proteins
char-acterized by different numbers of PPX3
pentapep-tide repeats preceded by a signal peppentapep-tide. The two
similar Enod12 genes encoded in P. sati6um
(PsEnod12a and-b) and M. sati6a (MsEnod12a
and-b) differ in the number of PPX3 repeats. The
occurence of PPX3 pentapeptide repeats defines a
category of structural cell-wall proteins designated hydroxyproline rich glycoprotein (HRGPs, [35]). Hence, Enod12 proteins are assumed to be struc-tural components of plant cell walls in root nod-ules involved in the reaction of the plant to an infection by rhizobia [31]. In addition to the
Enod12 proteins, several proline-rich early
nodulins were identified [32], e.g. the well-known Enod5 and Enod2 nodulins as well as the Enod10 [21] and MtPRP4 proteins [44]. Csanadi et al. [6] identified an alfalfa line that does not contain any Enod12 gene. In such plants nodule formation was not impaired, nor was nitrogen fixation reduced.
Obviously, at least in M. sati6a root nodule
organogenesis and function is not dependent on Enod12 proteins. This might be explained by the existence of similar PRPs that substitute for Enod12 proteins.
In infected roots, PsEnod12 transcripts were localized in cells containing an infection thread and cells placed in front of the growing infection thread leading to the suggestion that Enod12 might be involved in the infection process [31]. Later it was demonstrated that the Enod12 gene from M. truncatula is activated in root hairs as early as 3 h after infection [26]. In mature root nodules, Enod12 transcripts were localized in the prefixing zone II [2,31,41]. In contrast to the PsEnod12a and PsEnod12b genes, which are ex-pressed comparably, for the two Enod12 genes
fromM.sati6amarkedly different expression
char-acteristics were found. MsEnod12a was activated exclusively in the proximal part of the prefixing zone II of root nodules dependent on the presence of an active meristem, whereas MsEnod12b was
detected in root hairs within few hours after appli-cation of NOD factors [2] as it was already demonstrated for PsEnod12a [17] and MtEnod12 [26]. Enod12 genes are also activated in spontanu-ous nodules [27] or by phytohormones [3] indicat-ing that Enod12 gene expression is part of the preexisting plant programme underlying nodule formation.
Using fusions of Enod12 promoters from M.
sati6a and M. truncatula to the gusA reporter
gene, regions mediating activity were identified [3,26]. In case of the PsEnod12 promoters, essen-tial regions were localized within 200 bp upstream of the transcription start sites [42]. Recently, a promoter element was identified in the PsEnod12b promoter that was able to specifically interact with
the transcription factor ENBP1 from Vicia sati6a
[5]. Interestingly, mutations in this element com-pletely abolished PsEnod12b promoter activity in
transgenic root nodules of Vicia hirsuta [14].
The characteristic expression properties of
Enod12 genes made them valuable tools to analyse
early aspects of legume-Rhizobiuminteractions. To
investigate organ-specific gene expression in broad
bean (Vicia faba L.) nodules, we constructed a
nodule-specific cDNA library by differential hy-bridization [24]. Sequence analysis of a cDNA
from clone group VfNDS-X7 (V6 icia f6aba n6odule
d6ifferential s6creening, group X7) of this library
revealed that this incomplete cDNA encoded a broad bean Enod12 protein. We here report on
the expression properties of a Vicia faba Enod12
gene and analyze the promoter in transgenic hairy
roots and nodules of Vicia hirsuta. We show that
the VfEnod12 promoter fragment isolated is active although it contains a binding site for the tran-scription factor ENBP1 that is altered in a subele-ment that is exactly conserved in all other Enod12 genes identified so far.
2. Methods
2.1. Plant material
Vicia faba L. cv. Kleine Thu¨ringer plants were grown in the greenhouse in sand or Seramis (clay granules). Two days after sowing, the seedlings
were inoculated withRhizobium leguminosarumbv.
6iciae VF39 [28] to obtain infected plants. To
sterile soil as described [24]. Vicia hirsuta (hairy tare) seeds were obtained from John Chambers Wild Flower Seeds, Kettering, UK.
2.2. cDNA libraries, genomic libraries and cloning of reporter gene fusions
A nodule cDNA library and a nodule-specific
cDNA library were constructed in lgt11 [18]
from polyA+ mRNA isolated from root nodules
of V. faba L. cv. Kleine Thu¨ringer [24]. A
ge-nomic library of V. faba L. cv. Kleine Thu¨ringer
was prepared in lEMBL3 [10] according to
Sambrook et al. [30]. The complete -1955/-41
VfEnod12 promoter region was PCR-amplified using the M13 reverse primer in conjunction
with an oligonucleotide spanning the -36/-60
re-gion of the VfEnod12 promoter where positions
-36/-41 were modified to form an EcoRI site. As
a template, plasmid pUC18:16-69 was used that contained the 3.3 kb genomic VfEnod12
frag-ment cloned in the EcoRI site of plasmid
pUC18 [45]. The -692/-41 region was
subse-quently released as EcoRV/EcoRI fragment and
was cloned into the SmaI/EcoRI sites in front of
the gusAint gene in plasmid pGUS-INT [19].
From the resulting plasmid, the pVfEnod12-gu
-sAint fusion was subcloned as SphI/SalI
frag-ment into the TL DNA integration vector pIV2 [19] and the resulting plasmid was transferred to A. rhizogenes ARqua1 using E. coli S17-1 medi-ated conjugation [36]. Correct integration in the TL DNA was verified by Southern hybridiza-tions.
2.3. DNA sequencing and sequence analysis
Sequencing reactions were carried out accord-ing to Zimmermann et al. [46] usaccord-ing the ‘Au-toRead Sequencing Kit’ (Pharmacia). Sequencing gels were run on the ‘A.L.F. DNA Sequencer’ (Pharmacia) using sequencing gel mixes of stan-dard composition. All sequences reported here were determined from both strands. Nucleic acid sequences were read using the ‘A.L.F. MAN-AGER V3.0’ software (Pharmacia). Deduced amino acid sequences were analysed according to von Heijne [43] and Straden [37] and by using
the PC/Gene software package (Intelligenetics,
release 6.80).
2.4. Isolation of RNA, northern, cDNA-cDNA and tissue-print hybridizations
Total RNA was isolated from nodules (32 days after sowing), uninfected roots (32 days af-ter sowing), leaves (32 days afaf-ter sowing), seeds (90 days after sowing), epicotyls (8 days after sowing), stems (12 days after sowing) and flow-ers (60 days after sowing) of broad bean accord-ing to Perlick and Pu¨hler [24]. For time-course hybridizations, total RNA was prepared accord-ingly from infected roots harvested in 2 day
in-tervals from day 3 after inoculation with R.
leguminosarum bv. 6iciae VF39. For Northern
blotting, the amount of 30 mg of total RNA
from different tissues was separated
elec-trophoretically and blotted onto Hybond – N
ny-lon membranes (Amersham) according to
standard protocols [30]. Fifty nanograms of
VfEnod12 probe DNA were labelled with 50 mCi
of [a32P]dATP and hybridizations were carried
out as described [24]. Stringent washes were
car-ried out at room temperature using 2×SSC,
0.1% (w/v) SDS (5 min) and at 68°C using
0.2×SSC, 0.1% (w/v) SDS (twice for 30 min).
cDNA – cDNA hybridizations were carried out according to Fru¨hling et al. [11]. Tissue print hybridizations were carried out as described by Schro¨der et al. [33]. To relate hybridizing regions of the print to distinct nodule zones, nodule sec-tions corresponding to the print were stained for
starch in a solution containing 1% (w/v) KI and
1% (w/v) I2 in distilled water. Subsequently,
stained sections were rinsed in distilled water and photographed at the same magnification as the tissue print filter.
2.5. Induction and analysis of transgenic hairy roots and root nodules of V. hirsuta
V. hirsuta seeds were surface-sterilized and plants were grown in petri dishes in a growth chamber as described [29]. Transgenic hairy
roots of V. hirsuta were generated using A. rhi
3. Results and discussion
3.1. Isolation of a full-length cDNA encoding an Enod12 protein from V. faba
During a differential screening for nodulin genes
of V. faba, the cDNA 66-1 from the
cross-hy-bridizing clone group X7 was isolated that con-tained an incomplete Enod12 transcript sequence [24]. A full-length VfEnod12 cDNA was isolated by rescreening the nodule cDNA library with the 66-1 probe. Clone 114 – 96 was sequenced and contained a 533 bp cDNA covering the complete
Enod12 coding region (EMBL accession
AJ277289). The deduced protein sequence con-sisted of 90 amino acids and had a MW of 10 206. According to von Heine [43], the VfEnod12 protein was predicted to contain an N-terminal secretory signal peptide with a cleavage site be-tween amino acids 24 and 25 (Fig. 1). The C-ter-minal region was composed of the proline-rich
PPX3 pentapeptide repeats typical of Enod12
proteins [31]. Comparing VfEnod12 with other Enod12 sequences identified so far, 10 such repeats were identified. In four cases one of the proline residues (NPAYE, TPVHK, PSYGK, HPTES) and in one case both prolines were changed (SHLHV, Fig. 1). In the homologous region, VfEnod12 was almost identical to PsEnod12a [31]. Within 90 amino acids only four changes occured, three in the signal peptide region and one in the
first PPX3 repeat. Nevertheless, four PPX3 repeats
corresponding to PsEnod12a repeats were not present in the broad bean sequence (Fig. 1).
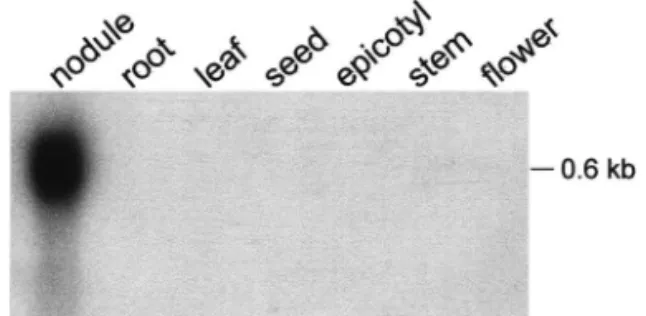
Inter-Fig. 2. Expression of the VfEnod12 gene in broad bean tissues. A Northern blot containing total RNA isolated from root nodules, uninfected roots, leaves, seeds, epicotyls, stems, and flowers was probed with the full-length VfEnod12 cDNA. The size of the hybridizing transcript was determined using molecular weight standards. Abbreviation: kb, kilo bases.
estingly, these four PPX3repeats were also missing
in the PsEnod12b sequence which on the other hand showed a reduced sequence homology with
VfEnod12. Hence, VfEnod12 resembled
PsEnod12a on the sequence level but PsEnod12b on the structural level. Comparable differences
existed for the twoM. sati6a Enod12 nodulins [1].
Here, MsEnod12a is a 93 amino acid protein whereas MsEnod12b is a 113 amino acid protein
containing four additional PPX3 repeat as does
PsEnod12a. Due to the presence of PPX3 repeats,
the structure of VfEnod12 and other Enod12 proteins is very similar to proline-rich proteins (PRPs) found in a variety of plants independently of the presence of any symbiotic interaction. The highest homologies existed with SbPRP3, a 90
amino acid protein with 11 PPX3 repeats present
in the aerial parts of soybean plants ([16], data not
shown). Due to the presence of PPX3 repeats
characteristic of plant cell wall proteins of the hydroxyproline rich glycoprotein (HGRP) type [35], Enod12 proteins are thought to be integral cell wall proteins [31]. Considering this and the homologies identified, we propose that VfEnod12 constitutes a cell wall protein from broad bean root nodules.
3.2. VfEnod12 transcripts accumulate early during root nodule organogenesis
The cDNA 114 – 96 was used as a probe to analyse VfEnod12 gene expression in different tis-sues. An appr. 600 nt transcript corresponding in size to the full-length VfEnod12 cDNA was present exclusively in nodules, whereas no tran-scripts were detected in uninfected roots, seeds,
Fig. 1. Comparison of Enod12 protein sequences from differ-ent legumes. Sequences of Enod12 proteins fromVicia sati6a
(VsEnod12; [41]), Medicago sati6a (MsEnod12a and b; [1]), Pisum sati6um (PsEnod12a and b; [13,31]) and Medicago truncatula (MtEnod12, [26]) were compared with VfEnod12. The potential cleavage site between the signal peptides and the mature proteins containing characteristic PPX3repeats is
epicotyls, shoots, and flowers (Fig. 2). This corre-sponds to the expression pattern of MsEnod12a [2] and MtEnod12 [26], but is in contrast to the PsEnod12a, PsEnod12b and MsEnod12b gene pression [2,13,31]. The latter genes are also ex-pressed at lower levels in other tissues, in particular in flowers. In a time-course experiment, VfEnod12 transcripts accumulated earlier after
in-fection by R. leguminosarum bv. 6iciae than all
other 19 broad bean nodulin genes identified so far. In detail, VfEnod12 transcripts were present three post inoculation, whereas transcripts from the early nodulin gene VfEnod2 were detected 2 days later and most other nodulin genes including the leghemoglobin genes were detected 6 days later [20,25]. To localize the VfEnod12 site of expres-sion, tissue-print hybridizations were carried out. Fig. 3 shows that VfEnod12 transcripts were de-tected in the prefixing zone II of mature root nodules with the highest amount in the distal part of this region. Apparently, no VfEnod12 tran-scripts were present in the nodule meristem. This type of expression is more similar to the expres-sion pattern observed for the Enod12 gene from
V.sati6a[41] than to the expression patterns of the
PsEnod12 genes that are expressed in the most distal parts of the prefixing zone II [13,31]. Hence, VfEnod12 constitutes a proline-rich early nodulin that might function as a cell wall protein in the prefixing zone II of broad bean root nodules,
possibly by specifically modifying plant cell walls.
3.3. The VfEnod12 gene contains no introns and is preceded by a putati6e ENBP1 binding site
In contrast to the two different P. sati6um
Enod12 genes that are expressed similarly [13,31], the expression properties of the two alfalfa Enod12 genes are different [2]. To be able to
investigate the expression properties of the
VfEnod12 gene using fusions of its promoter to reporter genes, we isolated a broad bean genomic clone containing VfEnod12 sequences. A 3332 bp
EcoRI fragment of this clone contained not only
the complete coding region without any
interven-ing sequences, but also 1955 bp of the 5%and 1104
bp of the 3% region (EMBL accession AJ277288).
The fact that the VfEnod12 gene contains no introns is a common feature of Enod12 genes [1,13,26,41]. Sequence comparisons between the VfEnod12 cDNA 114-96 and the VfEnod12 ge-nomic sequence revealed two nucleotide changes in the coding region and 3 nucleotide changes in
the 3% UTR, whereas insertions or deletions were
not detected (data not shown). Considering the limited allelic variation that exists in our broad bean cultivar [20], the genomic fragment isolated corresponds to the full-length VfEnod12 cDNA.
Further upstream in the 5% region, homologies to
gag proteases were identifed from position -856 onwards indicating the presence of a
retrotranspo-son in the -856/-1955 upstream region of
VfEnod12. The putative gag coding region is ori-ented in the opposite direction of VfEnod12 tran-scription (data not shown).
At position -47/-52 relative to the start codon a
putative TATA-box was localized, whose position was similar to the positions of the TATA-boxes identified in the promoters of the two MsEnod12
genes [2]. Further upstream at position -99/-118 a
sequence element was identified that is ho-mologous to the binding site for the transcription factor ENBP1 that was identified in the promoter of the PsEnod12b gene [5]. The palindromic motif
characteristic of this binding site (TTATT-N7--12
-AATAA, [14]) is present at a similar position appr. 120 bp upstream from the start codon in different Enod12 promoters (Fig. 4). Interestingly, the terminal base of the AATAA subelement that is conserved in all Enod12 promoters isolated so far is changed to AATAT in the VfEnod12
Fig. 4. Comparison of the binding site for the transcription factor ENBP1 found in the promoters of different Enod12 genes. The sequences of the binding sites for the transcription factor ENBP1 found in the promoters of different Enod12 genes are compared with the conserved termini being under-lined. The positions of these elements relative to the transla-tional start are given on the right.
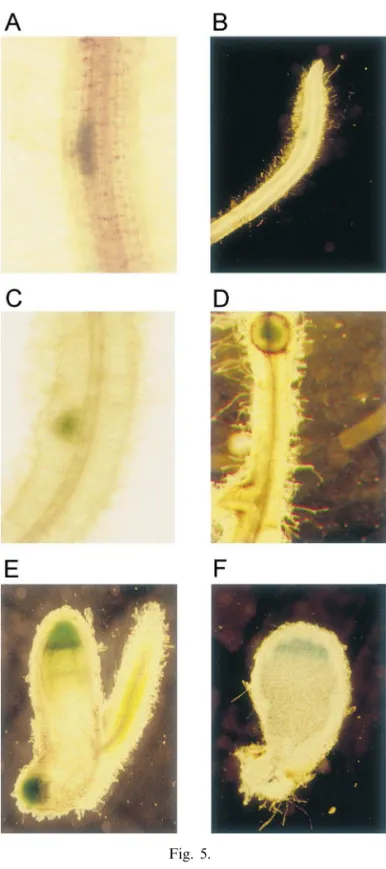
not observed in non-infected parts of the trans-genic root system including the epidermis and the meristem (Fig. 5B, E). The activity of the VfEnod12 promoter in outer cortical cells that probably contain infection threads conforms to the localization of PsEnod12a transcripts [31]. Re-porter gene expression in root inner cortical cells conforms to results for Enod12 promoters from M. sati6a [2], M. truncatula [26] and P. sati6um [31]. In contrast to the MsENOD12a promoter [3],
Fig. 5.
moter (Fig. 4). Apart from the homologies to the transcription factor binding site, the sequence of
the VfEnod12 promoter is significantly
ho-mologous to the sequences of other Enod12 gene
promoters fromM. sati6a and P. sati6um [1,13,26]
in the region of appr. 200 bp upstream of the start codon with the homologies decreasing in the up-stream regions (data not shown). We concluded that the Enod12 promoter from broad bean con-tained an altered binding site for the transcription factor ENBP1.
3.4. The -692/-41 VfEnod12 promoter containing an altered ENBP1 binding site is sufficient to acti6ate gene expression in early stages of nodule de6elopment and in mature root nodules
The -692/-41 promoter region of the VfEnod12
gene containing the ENBP1 binding site altered in the terminal position and the putative TATA-box
was cloned in front of the gusAint reporter gene
[38] and the resulting fusion was expressed in
transgenic roots and nodules ofV.hirsutausing an
no expression of the VfENOD12 promoter was seen in emerging lateral roots.
VfEnod12 promoter activity in the prefixing zone II of mature nodules corresponds to the
results obtained in V. faba by tissue-printing (Fig.
3) and resembles the expression of Enod12 pro-moters from other legumes [2,14]. Vijn et al. [42] demonstrated that a 200 bp region of the PsEnod12 promoters is sufficient for an expression in nodules. Christiansen et al. [5] identified the transcription factor ENBP1 that interacted with a specific region of the PsEnod12b promoter. Re-cently, Hansen et al. [14] showed by mutational analysis that the terminal AATAA subelement of
the TTATT-N9-AATAA binding site is essential
for expression of the PsEnod12b promoter in
transgenic hairy roots and nodules of V. hirsuta.
In these experiments, the AATAA element was changed to CCGCC. The expression properties of the PsEnod12b [14] and the VfEnod12 (Fig. 5) promoters in transgenic systems are very similar and therefore should allow a comparison of the results obtained. Our results on the expression of
the VfEnod12 promoter in transgenic V. hirsuta
hairy roots show that the AATAA element can tolerate a limited amount of variation and that such variation exists in a native Enod12 promoter. These data might provide a starting point for additional mutational analyses to elucidate the requirements for a functional ENBP1 binding site.
Acknowledgements
This work was supported by a grant from the
‘Studienstiftung des Deutschen Volkes’ to Gerald Schro¨der.
References
[1] L.A. Allison, G.B. Kiss, P. Bauer, M. Poiret, M. Pierre, A. Savoure, E. Kondorosi, A. Kondorosi, Iden-tification of two alfalfa early nodulin genes with ho-mology to members of the pea Enod12 gene family, Plant Mol. Biol. 21 (1993) 375 – 380.
[2] P. Bauer, M.D. Crespi, J. Szecsi, L.A. Allison, M. Schultze, P. Ratet, E. Kondorosi, A. Kondorosi, Al-falfa Enod12 genes are differentially regulated during nodule development by Nod factors and Rhizobium invasion, Plant Physiol. 105 (1994) 585 – 592.
[3] P. Bauer, P. Ratet, M.D. Crespi, M. Schultze, A. Kon-dorosi, Nod factors and cytokinins induce cortical cell division, amyloplast deposition and MsEnod12a ex-pression patterns in alfalfa roots, Plant J. 10 (1996) 91 – 105.
[4] N.J. Brewin, Development of the legume root nodule, Annu. Rev. Cell Biol. 7 (1991) 191 – 226.
[5] H. Christiansen, A.C. Hansen, I. Vijn, N. Pallisgaard, K. Larsen, W.C. Yang, T. Bisseling, K.A. Marcker, E.O. Jensen, A novel type of DNA-binding protein interacts with a conserved sequence in an early nodulin ENOD12 promoter, Plant Mol. Biol. 32 (1996) 809 – 821.
[6] G. Csanadi, J. Szecsi, P. Kalo, P. Kiss, G. Endre, A. Kondorosi, E. Kondorosi, G.B. Kiss, ENOD12, an early nodulin gene, is not required for nodule forma-tion and efficient nitrogen fixaforma-tion in alfalfa, Plant Cell 6 (1994) 201 – 213.
[7] A.J. Delauney, D.P.S. Verma, Cloned nodulin genes for symbiotic nitrogen fixation, Plant Mol. Biol. Rep. 6 (1988) 279 – 285.
[8] R.F. Fisher, S.R. Long, Rhizobium-plant signal ex-change, Nature 357 (1992) 655 – 660.
[9] H.J. Franssen, I. Yiyn, W.C. Yang, T. Bisseling, De-velopmental aspects of the Rhizobium-legume symbio-sis, Plant Mol. Biol. 19 (1992) 89 – 107.
[10] A.M. Frischauf, H. Lehrach, A. Poustka, N. Murray, Lambda replacement vectors carrying polylinker se-quences, J. Mol. Biol. 170 (1983) 827 – 842.
[11] M. Fru¨hling, H. Roussel, V. Gianinazzi-Pearson, A. Pu¨hler, A.M. Perlick, The Vicia faba leghemoglobin gene VfLb29 is induced in root nodules and in roots colonized by the arbuscular mycorrhizal fungus Glo-mus fasciculatum, Mol. Plant-Microbe Interact. 10 (1997) 124 – 131.
[12] R. Geurts, H. Franssen, Signal transduction in Rhizo-bium-induced nodule formation, Plant Physiol. 112 (1996) 447 – 453.
[13] F. Govers, H. Harmsen, R. Heidstra, P. Michielsen, M. Prins, A. van Kammen, T. Bisseling, Characteriza-tion of the pea ENOD12B gene and expression analy-ses of the two ENOD12 genes in nodule, stem and flower tissue, Mol. Gen. Genet. 228 (1991) 160 – 166. Fig. 5. Activity of the VfEnod12 promoter in transgenic V.
hirsuta roots and nodules. A 652 bp fragment of the VfEnod12 promoter ranging from position −41 to position
−692 relative to the translational start was fused to the
[14] A.C. Hansen, H. Busk, A. Marcker, K.A. Marcker, E.O. Jensen, VsENBP1 regulates the expression of the early nodulin PsENOD12B, Plant Mol. Biol. 1999 (1999) 495 – 506.
[15] R. Heidstra, T. Bisseling, Nod factor-induced host re-sponses and mechanisms of Nod factor perception, New Phytol. 133 (1996) 25 – 43.
[16] J.C. Hong, R.T. Nagao, J.L. Key, Characterization of a proline-rich cell wall protein gene family of soybean. A comparative analysis, J. Biol. Chem. 265 (1990) 2470 – 2475.
[17] B. Horvath, R. Heidstra, M. Lados, M. Moerman, H.P. Spaink, J.C. Prome´, A. van Kammen, T. Bisseling, Lipo-oligosaccharides of Rhizobium induce infection-re-lated early nodulin gene expression in pea root hairs, Plant J. 4 (1993) 727 – 733.
[18] T.V. Huynh, R.A. Young, R.W. Davis, Construction and screening cDNA libraries in lambda gt10 and lambda gt11, in: D.M. Glover (Ed.), DNA cloning, a practical approach, vol. 1, IRL Press Limited, Oxford, 1985, pp. 56 – 110.
[19] H. Ku¨ster, H.-J. Quandt, I. Broer, A.M. Perlick, A. Pu¨hler, The promoter of the Vicia faba L. VfENOD-GRP3 gene encoding a glycine-rich early nodulin medi-ates a predominant gene expression in the interzone II – III region of transgenic Vicia hirsuta root nodules, Plant Mol. Biol. 29 (1995) 759 – 772.
[20] H. Ku¨ster, M. Fru¨hling, A. Pu¨hler, A.M. Perlick, The modular nodulins Nvf-28/32 of broad bean (Vicia faba L.): alternative exon combinations account for different modular structures, Mol. Gen. Genet. 252 (1996) 648 – 657.
[21] M. Lo¨bler, A.M. Hirsch, A gene that encodes a proline-rich nodulin with limited homology to PsEnod12 is expressed in the invasion zone of Rhizobium meliloti-in-duced alfalfa root nodules, Plant Physiol. 103 (1993) 21 – 30.
[22] P. Mylona, K. Pawlowski, T. Bisseling, Symbiotic nitro-gen fixation, Plant Cell 7 (1995) 869 – 885.
[23] K. Pawlowski, Nodule-specific gene expression, Phys. Plantarum 99 (1997) 617 – 631.
[24] A.M. Perlick, A. Pu¨hler, A survey of transcripts ex-pressed specifically in root nodules of broadbean (Vicia faba L.), Plant Mol. Biol. 22 (1993) 957 – 970.
[25] A.M. Perlick, M. Fru¨hling, G. Schro¨der, S.C. Frosch, A. Pu¨hler, The broad bean gene VfNOD32 encodes a nodulin with sequence similarities to chitinases that is homologous to (a/b) barrel-type seed proteins, Plant Physiol. 110 (1996) 147 – 154.
[26] M. Pichon, E.P. Journet, A. Dedieu, F. de Billy, G. Truchet, D.G. Barker, Rhizobium meliloti elicits tran-sient expression of the early nodulin gene ENOD12 in the differentiating root epidermis of transgenic alfalfa, Plant Cell 4 (1992) 1199 – 1211.
[27] M. Pichon, E.P. Journet, F. de Billy, A. Dedieu, T. Huguet, G. Truchet, D.G. Barker, ENOD12 gene ex-pression as a molecular marker for comparing Rhizo-bium-dependent and -independent nodulation in alfalfa, Mol. Plant-Microbe Interact. 7 (1994) 740 – 747. [28] U. Priefer, Genes involved in lipopolysaccharide
produc-tion and symbiosis are clustered on the chromosome of
Rhizobium leguminosarum biovar viciae VF39, J. Bacte-riol. 171 (1989) 6161 – 6168.
[29] H-J. Quandt, A. Pu¨hler, I. Broer, Transgenic root nod-ules of Vicia hirsuta: A fast and efficient system for the study of gene expression in indeterminate-type nodules, Mol. Plant-Microbe Interact. 6 (1993) 699 – 706. [30] J. Sambrook, E.F. Fritsch, T. Maniatis, Molecular
cloning: A laboratory manual, 2nd, Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York, 1989.
[31] B. Scheres, C. Van De Wiel, A. Zalensky, B. Horvath, H. Spaink, H. Van Eck, F. Zwartkruis, A.M. Wolters, T. Gloudemans, A. van Kammen, T. Bisseling, The ENOD12 gene product is involved in the infection pro-cess during the pea-Rhizobium interaction, Cell 60 (1990a) 281 – 294.
[32] B. Scheres, F. van Engelen, E. van der Knaap, C. van de Wiel, A. van Kammen, T. Bisseling, Sequential induc-tion of nodulin gene expression in the developing pea nodule, Plant Cell 2 (1990b) 687 – 700.
[33] G. Schro¨der, M. Fru¨hling, A. Pu¨hler, A.M. Perlick, The temporal and spatial transcription pattern in root nod-ules of Vicia faba nodulin genes encoding glycine-rich proteins, Plant Mol. Biol. 33 (1997) 113 – 123.
[34] M. Schultze, A. Kondorosi, What makes nodulation signals host-plant specific?, Trends Microbiol. 3 (1995) 370 – 372.
[35] A.M. Showalter, Structure and function of plant cell wall proteins, Plant Cell 5 (1993) 9 – 23.
[36] R. Simon, U.B. Priefer, A. Pu¨hler, A broad host range mobilization system for in vivo genetic engineering: Transposon mutagenesis in gram negative bacteria, Bio-technology 1 (1983) 784 – 791.
[37] R. Staden, The current status and portability of our sequence handling software, Nucl. Acids Res. 14 (1986) 217 – 231.
[38] G. Vancanneyt, R. Schmidt, A. O’Connor-Sanchez, L. Willmitzer, M. Rocha-Sosa, Construction of an intron-containing marker gene: Splicing of the intron in trans-genic plants and its use in monitoring early events in Agrobacterium-mediated plant transformation, Mol. Gen. Genet. 220 (1990) 245 – 250.
[39] A. van Kammen, Suggested nomenclature for plant genes involved in nodulation and symbiosis, Plant Mol. Biol. Rep. 2 (1984) 43 – 45.
[40] D.P.S. Verma, C.-A. Hu, M. Zhang, Root nodule devel-opment: origin, function and regulation of nodulin genes, Phys. Plantarum 85 (1992) 253 – 265.
[41] I. Vijn, W.C. Yang, N. Pallisgard, E. Ostergaard Jensen, A. van Kammen, T. Bisseling, VsENOD5, VsENOD12 and VsENOD40 expression during Rhizobium-induced nodule formation on Vicia sativa roots, Plant Mol. Biol. 28 (1995a) 1111 – 1119.
[42] I. Vijn, H. Christiansen, P. Lauridsen, I. Kardailsky, H.J. Quandt, I. Broer, J. Drenth, E. Ostergaard Jensen, A. van Kammen, T. Bisseling, A 200 bp region of the pea ENOD12 promoter is sufficient for nodule-specific and nod factor induced expression, Plant Mol. Biol. 28 (1995b) 1103 – 1110.
[44] R.C. Wilson, F. Long, E.M. Maruoka, J.B. Cooper, A new proline-rich early nodulin from Medicago truncat-ula is highly expressed in nodule meristematic cells, Plant Cell 6 (1994) 1265 – 1275.
[45] C. Yanisch-Perron, J. Vieira, J. Messing, Improved M13 phage cloning vectors and host strains: nucleotide
se-quences of the M13mp18 and pUC19 vectors, Gene 33 (1985) 103 – 119.
[46] J. Zimmermann, H. Voss, C. Schwager, J. Stegemann, H. Erfle, K. Stucky, T. Kristensen, W. Ansorge, A simplified protocol for fast plasmid DNA sequencing, Nucl. Acids Res. 18 (1990) 1067.