Recurring Complications of Pregnancy
JOHN C. SMULIAN, MD, MPH
Guest Editor
T A B L E O F C O N T E N T S
Introduction John C. Smulian ... 125 Fetal Growth Restriction and Subsequent Pregnancy Risks Wendy L. Kinzler and
Lillian Kaminsky ... 126
Preeclampsia Recurrence and Prevention Gary A. Dildy III, Michael A. Belfort,
and John C. Smulian ... 135
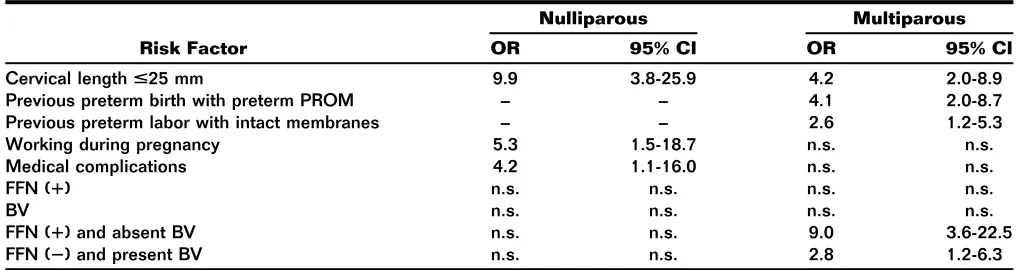
Recurrent Preterm Birth Shali Mazaki-T ovi, Roberto Romero, Juan Pedro Kusanovic, Offer Erez, Beth L. Pineles, Francesca Gotsch, Pooja Mittal, Nandor Gabor T han,
Jimmy Espinoza, and Sonia S. Hassan ... 142
Postpartum Hemorrhage: A Recurring Pregnancy
Complication Michelle A. Kominiarek and Sarah J. Kilpatrick ... 159
Thromboembolism in Pregnancy: Recurrence and Its
Prevention Andra H. James, Chad A. Grotegut, Leo R. Brancazio,
and Haywood Brown ... 167
Recurrent Gestational Diabetes: Risk Factors, Diagnosis, Management, and
Implications Joseph N. Bottalico ... 176 After Shoulder Dystocia: Managing the Subsequent Pregnancy and
Delivery Edith D. Gurewitsch, T ara L. Johnson, and Robert H. Allen ... 185 Epidemiologic Approaches for Studying Recurrent Pregnancy Outcomes:
Challenges and Implications for Research Cande V. Ananth ... 196
SEMINARS IN
PERINATOLOGY
SEMINARS IN
PERINATOLOGY
T O P I C S
F O R
2 0 0 6
O P T I M I Z I N G C A R E A N D O U T C O M E S F O R L A T E P R E T E R M
( N E A R - T E R M ) I N F A N T S : P A R T 1
Tonse N. K. Raju, MD
O P T I M I Z I N G C A R E A N D O U T C O M E S F O R L A T E P R E T E R M
( N E A R - T E R M ) I N F A N T S : P A R T 2
Tonse N. K. Raju, MD
A D V A N C E S I N N E O N A T O L O G Y : S E L E C T E D P R O C E E D I N G S O F T H E
I N T E R N A T I O N A L P E R I N A T A L C O L L E G I U M
William Oh, MD, and Harry Bard, MD
B P D : S T A T E O F T H E A R T
Vineet Bhandari, MD, DM
C E S A R E A N D E L I V E R Y O N M A T E R N A L R E Q U E S T
Catherine Y. Spong, MD, and Uma M. Reddy, MD, MPH
I N H E R I T E D R E S P I R A T O R Y D I S O R D E R S O F T H E N E O N A T E
Lawrence M. Nogee, MD, and Aaron Hamvas, MD
T O P I C S
F O R
2 0 0 7
N E O N A T A L I N F E C T I O N S
Robert S. Baltimore, MD, and Hal B. Jensen, MD
T H E E V I D E N C E B A S E S U P P O R T I N G N U T R I T I O N A L
P R A C T I C E S F O R V E R Y L O W B I R T H W E I G H T I N F A N T S
Richard A. Ehrenkranz, MD, and Brenda B. Poindexter, MD, MS
R E C U R R I N G C O M P L I C A T I O N S O F P R E G N A N C Y
John C. Smulian, MD, MPH
C O A G U L A T I O N D I S O R D E R S I N T H E P E R I N A T A L
P E R I O D
Michael J. Paidas, MD
P A I N
K. J. S. Anand, MD, and Richard W. Hall, MD
O R G A N T R A N S P L A N T A T I O N A N D R E P R O D U C T I O N
Introduction
. . .Whoever wishes to foresee the future must consult the past; for human results ever resemble those of preceding times.
Niccoló Machiavelli (The Discourses,1517)
P
regnancy is associated with many unique health prob-lems that occur at no other time in life and in no specialty other than Obstetrics. Complications can be medical or sur-gical and may affect the mother, the baby, or both. What is even more unique is that we have the opportunity to follow women through successive pregnancies, each of which is at risk for either occurrence or recurrence of these complica-tions. Whereas prediction of complications for nulliparous women with no pregnancy track record is notoriously diffi-cult, it is becoming clear that a history of a pregnancy plication is the greatest predictor of a future pregnancy com-plication. It is the role of research in complication recurrence to grow our understanding of the heterogeneity of pregnan-cy-associated diseases and give insight into etiologies and risk. This will ultimately lead to better clinical prediction, counseling, and management. As clinicians, we should not manage these at-risk women the same as women who have never had a pregnancy or who have had previous normal pregnancy outcomes. We also should avoid the practice of “anecdotal medicine,” where wide variations in clinical care can be driven by either insufficient experience or the trauma of a previous adverse event.This issue ofSeminars in Perinatologyis organized around the theme of recurrent pregnancy complications. There are eight articles by recognized experts, of which seven are
de-voted to some of the most significant pregnancy complica-tions encountered by clinicians. (There clearly are many more pregnancy complications with a tendency to recur, which deserve the same careful scrutiny as those reviewed here.) Because recurrence research is complicated and re-quires different thinking about epidemiologic and statistical methodologies, there is included an important eighth article that addresses challenges specific to the study of recurrent pregnancy complications.
The selected topics covered in this issue illustrate how important the concept of recurrence is to Obstetrics. These articles review what is known about the epidemiology of recurrence for each specific complication, proposed etiologic pathways, prevention options, and management recommen-dations for successive pregnancies. Each article also is meant to highlight knowledge gaps and areas with a pressing need for research. Unfortunately, our knowledge gaps about re-currence are wide for many of these conditions.
It is my hope that this issue ofSeminars in Perinatologywill provide information to directly help with the clinical care of our highest risk women. I also hope that these articles will stimulate further research on the recurrence of complications in successive pregnancies and promote collaborations be-tween epidemiologists and clinicians to advance our under-standing of this very important area of medicine.
John C. Smulian, MD, MPH Guest Editor
Volume 31, Number 3
June 2007
125
Fetal Growth Restriction and
Subsequent Pregnancy Risks
Wendy L. Kinzler, MD, and Lillian Kaminsky, MD
Fetal growth restriction can result from a variety of intrinsic or extrinsic insults, resulting from maternal, fetal, and placental factors. Determining the underlying cause of poor fetal growth can be difficult but is essential for assessing potential risks for future pregnancies. Importantly, recurrence risks greatly depend on these underlying conditions. Understand-ing these risks can allow more appropriate patient counselUnderstand-ing and may influence manage-ment strategies to optimize future pregnancies.
Semin Perinatol 31:126-134 © 2007 Elsevier Inc. All rights reserved.
KEYWORDSfetal growth restriction, recurrence risks
N
ormal fetal growth is dependent on complex interac-tions between the fetal, placental, and maternal units. Poor fetal growth can result from a variety of intrinsic or extrinsic insults. Determining the underlying cause can be difficult but is essential for assessing potential risks for future pregnancies.Small for gestational age (SGA) infants are defined as those born with a weight⬍10th percentile for gestational age. Fetal growth restriction (FGR) can be defined as an abnormal growth trend, which is less than the genetic growth potential of the individual fetus and which is always pathological. This pathological FGR may affect only 3% to 5% of births. Impor-tantly, not all SGA babies have FGR since some may be con-stitutionally small. Conversely, a fetus still may be growth restricted if it is above the 10th percentile in weight if it is substantially smaller than its growth potential would predict. It is important to accurately determine as best as possible whether a previous pregnancy was complicated by patho-logic FGR or merely a constitutionally SGA infant. Informa-tion that might be helpful for establishing a diagnosis of fetal growth restriction in a previous pregnancy is listed inTable 1. Once the diagnosis of FGR is established, an assessment of subsequent pregnancy risk can be performed based on the etiology of the initial case.
Etiology-Specific Risks for FGR
There are multiple factors which can adversely influence fetal growth. In broad terms, contributing factors can be intrinsic to the fetus, they can be specific to the uteroplacental unit, or they may be the result of underlying maternal conditions. Determining which of these was responsible for FGR can be the most difficult but the most useful part of the evaluation during a woman’s subsequent pregnancy. Prior medical records, including prenatal labs and notes, previous ultra-sound reports, maternal and infant hospital records, and pla-cental pathology, should be requested and carefully re-viewed, with particular attention being made to the following etiologic pathways.Fetal Causes
Chromosomal aberrations are a well-established cause of fe-tal growth restriction and are estimated to be responsible for up to 20% of cases.1Early onset of growth restriction, the presence of polyhydramnios, and the presence of structural malformations all increase the likelihood of a chromosomal abnormality. Triploidy is most common in severe cases of FGR when⬍26 weeks gestation, and Trisomy 18 is the most common aneuploidy noted with severe FGR after 26 weeks gestation.1 However, other trisomies, deletions/additions, and ring chromosomes have all been associated with various degrees of fetal growth abnormalities. Fetal aneuploidy is not the only chromosomal abnormality to be associated with poor fetal growth. Confined placental mosaicism is present when the cytogenetics of the placental mass are different than the cytogenetics of the fetus. It occurs either as the result of a meiotic rescue of a trisomic embryo or due to a mitotic Division of Maternal Fetal Medicine, Department of Obstetrics, Gynecology
and Reproductive Sciences, UMDNJ-Robert Wood Johnson Medical School, New Brunswick, NJ.
Address reprint requests to Wendy L. Kinzler, MD, Department of Obstet-rics, Gynecology and Reproductive Sciences, Clinical Academic Build-ing, 2nd Floor, 125 Paterson Street, New Brunswick, NJ 08901. E-mail: kinzlewe@umdnj.edu
postzygotic error.2It has been found in approximately 15% of intrauterine growth restriction cases, compared with⬍2% of appropriately grown fetuses. The recurrence risk of aneu-ploidy is approximately 1%, but a recurrence risk for con-fined placental mosaicism has not been established.
Nonaneuploid genetic syndromes have also been associ-ated with fetal growth restriction but may be more difficult to identify without a known family history and/or detailed ge-netic evaluation. Lower birth weights are also often identified in infants born with structural malformations, especially when cardiac, even in the presence of a normal fetal karyo-type.3 Uniparental disomy (UPD) is the inheritance of two homologous chromosomes from only one parent. There are clinical syndromes which are known to be the result of UPD (Silver Russell Syndrome, for example) and have significant growth restriction as part of their phenotype.4Although an association with poor fetal growth has been established, UPD is an uncommon finding in most cases.5The risk for FGR recurrence from these causes is dependent on the specific associated condition.
Congenital infections, namely rubella, cytomegalovirus, toxoplasmosis, herpes simplex, and varicella, have been as-sociated with FGR.6-9Despite this clear association, the pro-portion of growth restriction attributed to congenital infec-tion is low (5%),10and they are not expected to recur.
For a variety of reasons, fetuses within multiple gestations have an increased incidence of growth restriction. The inci-dence of growth restriction in twins is 15% to 25%,11-13 mak-ing multiple gestations responsible for approximately 5% of all cases of FGR. There is a significant increase in the rate of fetal growth abnormalities in direct relationship to the num-ber of fetuses present.14This may be related to a reduction in cell size or a relative reduction in nutrient supply as the nutritional demands of the fetuses increase. This form of FGR is usually mild. Nevertheless, other contributing factors may include an increased incidence of placental and umbilical cord abnormalities (velamentous insertions), a greater likeli-hood of structural malformations and vascular anastomoses in monozygotic multiples, and an increased incidence of
ma-ternal complications linked with poor fetal growth. There are no data to assess recurrence risks for FGR when the index case is a multiple gestation. However, the risks in a subse-quent singleton pregnancy are likely to be increased mini-mally, if at all.
Placental Factors
Umbilical cord and placental abnormalities are frequently identified in pregnancies complicated by poor fetal growth. A single umbilical artery is present in approximately 0.4% of pregnancies15and has been associated with fetal structural malformations, particularly cardiac. In cases of an isolated two-vessel cord, FGR is estimated to be up to twice as com-mon as in pregnancies with three-vessel cords.16 Velamen-tous cord insertions, which enter the fetal membranes instead of the placental parenchyma, have a reported incidence of 1% to 2%.15They have been associated with higher rates of low birth weight (OR 2.3), small-for-gestational age infants (OR 1.5), and preterm delivery (OR 2.1)17compared with umbil-ical cords normally inserted into the placenta. This suggests that at least some of these low-birth-weight and SGA births have FGR. Circumvallate placentation is defined as an ele-vated edge of the placenta⬎50% of the circumference, and has been associated with poor fetal growth and prematuri-ty.18These factors are generally considered to have low re-currence rates.
Placental bleeding at any trimester places a pregnancy at increased risk for adverse outcomes. The incidence of first trimester intrauterine hematoma is reported to be 3% in the general population with increased risks of hypertensive dis-orders of pregnancy (2- to 4-fold), abruption (5-fold), pre-term delivery (2-fold), and FGR (2.4-fold).19 Abruption at term is also significantly associated with FGR (2-fold in-creased risk), stillbirth, preterm delivery, and pregnancy-in-duced hypertension.20,21Placental bleeding at any gestational age in a pregnancy complicated by FGR may be a clinical manifestation of a chronic placental disorder that can recur. Although the majority of low-birth-weight neonates deliv-ered from pregnancies complicated by placenta previa are small due to preterm gestational age at birth, at least one report estimated that as much as 3.7% of FGR is attributable to placenta previa.22
Microscopically, many placental lesions have been identi-fied in cases of abnormal fetal growth. Placental infarctions have been associated with FGR and abnormal fetal blood flow.23Placental examination from term pregnancies compli-cated by idiopathic FGR also found a high incidence of in-farction (24%).24The placenta can also be the target of im-mune-mediated injury, such as occurs with massive fibrin deposition and chronic villitis.
Massive perivillous fibrin deposition, previously referred to as maternal floor infarct, is a significant placental lesion characterized by a heavy deposition of fibrin in the decidua basalis and extending into the intervillous space, preventing appropriate maternal–fetal exchange of nutrients. It is be-lieved to be the result of an immune-mediated maternal re-sponse and has been associated with high rates of poor fetal
Table 1 Information Useful for Establishing a Diagnosis of
FGR in a Previous Pregnancy
● Previous birth weight and gestational age to determine birth weight centile
● Antepartum complications (bleeding, multiple gestation, congenital abnormalities, preeclampsia)
● Maternal pre-pregnancy BMI and weight gain during pregnancy
● Social history (tobacco, alcohol, or illicit drug use) ● Medication exposure
● Prenatal fetal growth trends and ultrasound reports ● Medical complications (chronic hypertension, IDDM,
SLE, anemia, asthma, etc.) ● Birth weights of other pregnancies ● Maternal birth weight
● Neonatal complications (respiratory, metabolic, congenital abnormalities, NICU admission, and length of stay)
outcomes, including a 15% to 40% fetal death rate, a 30% to 60% preterm birth rate, and a 50% to 100% rate of FGR.25,26 Another potential immune-mediated lesion is chronic vil-litis of unknown etiology (VUE). Chronic vilvil-litis has been found in approximately 30% of pregnancies complicated by FGR.24,27Other, rare placental abnormalities include placen-tal mesenchymal dysplasia, which is a placenta vascular mal-formation that is often confused clinically with a partial hy-datiform mole. This abnormality can predispose to placental thrombosis. In one series, 50% of the cases were associated with FGR and 43% of the pregnancies were complicated by a fetal death.28
Maternal Factors
Multiple maternal factors have been implicated in poor fetal growth. Careful review of the maternal history for potential contributing factors can sometimes identify nutritional dis-orders, anemia and maternal hypoxia-related conditions, en-vironmental exposures (particularly tobacco or cocaine use), and conditions with maternal vascular disease. Any review should include a family history, as it has been shown that familial factors influence the risk of SGA births.29
Malnutrition is an uncommon, but nonetheless important, risk factor for poor fetal growth, especially in developing countries. The effect of starvation was best studied in a Dutch cohort during the famine of 1944 to 1945. Pregnant women under such severe nutritional deprivation experienced weight loss and a drop in birth weight by 250 to 300 g.30 Women with eating disorders have also been shown to have higher rates of SGA births.31Correction of such nutritional deficiency is likely to reduce recurrence risk.
Maternal conditions leading to hypoxemia can lead to a reduction in fetal growth potential. Studies have inconsis-tently linked maternal anemia, including sickle cell anemia, and low birth weight.32-34Chronic respiratory disease, such as asthma, can also lead to decreased fetal growth through fetal hypoxia.35 Likewise, women with congenital cyanotic heart disease and those living at high altitude are at higher risk for lower birth weight babies.36,37
Maternal substance abuse, including tobacco, alcohol, and cocaine, is an important preventable cause of poor fetal growth. The mechanism may involve direct toxic damages of these substances as well as associated comorbidities, such as inadequate nutrition, maternal infections, etc. Smoking is associated with an increased risk of delivering a low-birth-weight or SGA infant in a dose-dependent fashion.38,39It has been estimated that approximately 20% of low-birth-weight and SGA births are attributable to maternal smoking.40Bada and coworkers estimated that, if smoking could be com-pletely prevented during pregnancy, 13.8% of FGR cases would be eliminated.41FGR is one of the major features of fetal alcohol syndrome, which is also associated with facial dysmorphia and central nervous system disorders.42The ad-verse effects of prenatal cocaine exposure on fetal growth43as well as on placental abruption, preterm birth, and intrauter-ine fetal demise have been well established. Various thera-peutic drugs, such as warfarin, folic acid antagonists, and
anticonvulsants, can also be associated with FGR. The risk for recurrence of FGR for these exposures is likely linked to their continued presence or their discontinuation.
Clinical maternal vascular disease secondary to chronic hypertension, renal disease, diabetes mellitus, and collagen vascular disease, especially when complicated by preeclamp-sia, is the most common cause of impaired fetal growth, accounting for nearly a third of FGR cases.2Both preeclamp-sia and FGR may share a similar pathophysiology involving abnormal placentation leading to placental insufficiency.44 Chronic hypertension is associated with a two- to threefold increase in the rate of FGR, an association mostly due to the presence of superimposed preeclampsia.45 In fact, when growth restriction cases due to preeclampsia, smoking, and malnutrition are excluded, diabetes and hypertension may no longer be independent risk factors.46The association of chronic hypertension and poor fetal growth may also be par-tially mediated by the effect of antihypertensive medications, such as beta-blockers.47The risk of FGR in pregnancies af-fected by pregestational diabetes is dependent on the severity and duration of the disease.45 The incidence of FGR in chronic renal disease has been reported to be as high as 23%.48 Patients with systemic lupus have an eightfold in-creased risk for developing FGR,45,49-51with the highest risks noted in women diagnosed before pregnancy and those with active renal and central nervous system involvement.49-51 These risk factors will persist in subsequent pregnancies.
Inherited thrombophilias are a group of genetic conditions that increase the risk of thromboembolic disease. They in-clude Factor V Leiden (FVL), prothrombin G20210A muta-tion, protein C deficiency, protein S deficiency, antithrombin III deficiency, and MTHFR mutation, as well as other, less studied, familial conditions. Theoretically, placental throm-bosis in patients with inherited thrombophilias may lead to an increased risk for FGR, although the published data are conflicting. A number of studies have demonstrated an asso-ciation between maternal inherited thrombophilias and FGR.52-55 However, other studies were unable to show an association.56-59Based on the conflicting current data, inher-ited thrombophilia in isolation does not seem to be a major risk factor for most cases of FGR. However, the contributing role of thrombophilias in combination with other risk factors, including previous FGR, may be important. Antiphospho-lipid antibody syndrome (with or without underlying sys-temic lupus) is an acquired thrombophilia, which has been linked to several recurrent adverse pregnancy outcomes, in-cluding FGR, fetal death, and recurrent miscarriages.60,61
Evaluation Options to
Assess FGR Etiologies and Risks
Once the diagnosis of FGR has been confirmed and the above etiologies have been evaluated by history and examination, there may continue to be gaps in knowledge that may have a significant impact on further management. Some of these gaps, however, can be filled with the addition of a few simple steps. If not already done, consider having the previous pla-cental slides reviewed for histologic abnormalities by a
natal pathologist. If there is evidence of a prior abruption, or placental thromboses and/or infarctions are noted, it is im-portant to assess for maternal risk factors that may contribute to coagulation abnormalities. Testing for acquired and inher-itable thrombophilias is recommended in these cases, partic-ularly when the growth restriction is severe, associated with preterm delivery, or noted in the presence of a family history of thromboembolic disease. Specific evaluations would in-clude maternal testing for lupus anticoagulant, anticardio-lipin antibodies (IgG and IgM), and2-glycoprotein-1 anti-bodies. It is important to remember that 10% to 15% of women with systemic lupus erythematosus will have second-ary antiphospholipid antibody syndrome and should be tested if their antiphospholipid status is not already known.62 Genetic thrombophilia testing should include an evaluation for deficiencies in protein C, protein S, and antithrombin III and the presence of the Factor V Leiden and prothrombin gene mutations. If immune-mediated placental dysfunction is suspected, testing for the antiphospholipid antibody syn-drome and screening for other autoimmune disorders are warranted. There is insufficient information to support test-ing the affected offsprtest-ing for the presence of thrombophilias at this time.
If the prior child was found to have structural malforma-tions (prenatally or postnatally diagnosed), and/or there have been concerns about development delay, genetic counseling and possible evaluation by a pediatric geneticist is recom-mended. Review of prior records, including autopsy reports or infant discharge summaries, can be very helpful, as parents may not be fully aware of known or suspected diagnoses. If there has been a documented or suspected case of aneu-ploidy, parental karyotypes should be considered. This is particularly important for cases of aneuploidy other than the autosomal trisomies thought to be the result of meiotic non-disjunction, such as unbalanced translocations and ring chromosomes. A formal genetics evaluation is also essential in detecting and counseling about nonaneuploid genetic syn-dromes.
In women with an underlying medical condition, assessing the severity of the disorder can also provide insight into the ongoing obstetrical risks. This would include determining the presence of renal dysfunction in women with systemic lupus or other autoimmune processes and evaluating the degree of vascular and/or end-organ disease in women with long-standing diabetes or chronic hypertension.
Assess Risks for a Subsequent Pregnancy
The diagnosis and/or risk factors that have been identified in the above steps will allow appropriate counseling regarding the risk(s) to subsequent pregnancies (Table 2). It is clear that the data on etiology-specific FGR recurrence risks are sparse. Recently, it has been recognized that women with a history of FGR are at risk for multiple adverse pregnancy outcomes in subsequent pregnancies that include recurrent FGR, pre-eclampsia, and abruption.63These risks are particularly rele-vant in the setting of an acquired or inherited thrombophil-ia.64-68The recurrence rate of adverse pregnancy outcomes in
women with untreated inherited or acquired thrombophilia is approximately 66% to 83%.63,69 A cohort study of 491 patients with a history of adverse pregnancy outcomes has demonstrated that the presence of a maternal thrombophilia is associated with fetal loss after 14 weeks (OR 3.4, 95% CI 1.9-6.1), abruption (OR 3.6, 95% CI 1.4-9.1), and pre-eclampsia (OR 3.2, 95% CI 1.2-8.6).70Women who deliv-ered infants⬍3rd percentile may be three times more likely to have initial or recurrent preeclampsia in the next preg-nancy compared with those who deliver infants⬎10th per-centile.71They may also be at increased risk of fetal demise, especially when the previous fetal growth abnormalities were identified at early gestational ages.72Interestingly, of the 23% of women experiencing recurrent SGA in one study, the poor fetal growth was not more severe the second time.73
Previous abruption is a risk factor for FGR, as well as other adverse obstetrical outcomes. Recurrent placental abruption has been observed in 22% of subsequent pregnancies.74After an abruption, the risk of a SGA infant is 18.5%, the risk of a spontaneous preterm birth is 36%, and the risk of pregnancy-induced hypertension is 6%.75 A previous SGA birth in-creases the risk of abruption by 1.6-fold and by 2.5- to 6.0-fold if there is preexisting chronic hypertension or diabetes.76 If a first delivery has been complicated by a preterm birth, SGA infant, or perinatal death, the risk of an abruption in the second pregnancy is 7/1000 if no abruption was present in the initial pregnancy and 33/1000 if there was a prior abrup-tion.77
If there has been a history of FGR and placental infarction, the risk of recurrent growth restriction is high. It has been estimated to be 61% when two or more prior pregnancies have been similarly affected.78 Recurrent villitis has been noted in 17% of gestations, and has been associated with FGR, pregnancy loss, preterm delivery, and postnatal death. There is an even higher rate of recurrence of massive inter-villous fibrin deposition and poor fetal growth (67%).79 Pla-cental mesenchymal dysplasia is a rare finding with an un-likely risk of recurrence.
Prevention of Recurrent FGR
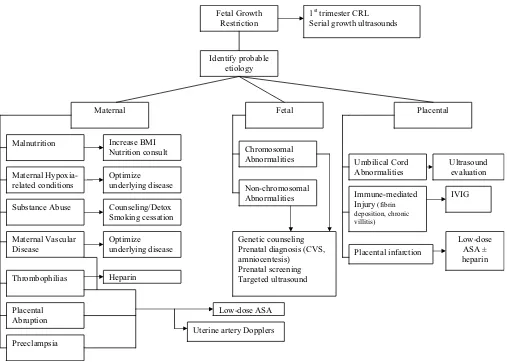
The importance of a thorough evaluation for potential etiol-ogies in evaluating a woman’s risk of adverse obstetrical out-comes as the result of a prior FGR birth cannot be overem-phasized. A comprehensive assessment will lead to appropriate counseling and, in many cases, will allow the implementation of targeted risk-specific strategies to reduce recurrence (Fig. 1).
If there is an opportunity for preconceptional care, it should focus on the elimination of known maternal expo-sures (cocaine, smoking, alcohol), folic acid supplementation to reduce the risk of congenital malformations, and the opti-mization of maternal medical conditions. Low prepregnancy body mass index (BMI) is a risk factor for FGR. If a woman continues to have a suboptimal BMI in the next pregnancy, this risk persists. However, an increase in maternal BMI be-tween pregnancies has been associated with a decreased SGA risk.80Although prospective trials of weight gain are lacking,
patients should be counseled about this potentially modifi-able risk factor. Other nutritional supplements, such as fish oil, are unproven.81 In cases of underlying medical condi-tions (diabetes, systemic lupus, hypertension, asthma), the woman’s health should be optimized before subsequent pregnancies and actively managed during the pregnancy in a multidisciplinary fashion.
If assisted reproduction is required, there should be cau-tious use of ovulation induction agents and attention paid to the number of embryos transferred as it relates not only to the chance of successful implantation, but also to the risks of multifetal gestations.
Any pregnancy at risk for a fetal growth abnormality should be screened in the first trimester to establish early and accurate gestational dating. Subsequent assessments of fetal growth trends will depend heavily on accurate dating. When possible, a crown–rump length in the first trimester is best. If a second trimester ultrasound alone is available due to a delay in presenting for prenatal care, the transcerebellar diameter should be measured, as it provides the most accurate dating in the 2nd and even 3rd trimesters.82 For women at the highest risk for FGR, serial fetal growth ultrasounds should be considered at approximately 4- to 6-week intervals to assess fetal growth trends.
In those couples at risk for having an aneuploid conceptus,
several options for screening and prenatal diagnosis are avail-able. In couples at high risk of recurrent autosomal trisomies or in those in which a balanced translocation has been iden-tified, in vitro fertilization with preimplantation genetic di-agnosis can be offered. Others may opt for assisted reproduc-tion with the use of a gamete donor. Once a pregnancy is achieved, early prenatal diagnosis with either chorionic vil-lous sampling or amniocentesis is available. For women un-decided about invasive testing, prenatal screening should be offered. First trimester combined nuchal translucency and biochemical screening is an excellent start, with a subsequent detailed sonographic fetal anatomy survey. For some non-aneuploid genetic syndromes, genetic testing is available. It is important to involve a provider specialized in genetics to assist in coordinating this testing and also to aid in targeting the fetal ultrasound.
Detailed sonography should also assess umbilical cord ab-normalities, such as single umbilical artery. Placental cord insertion should be visualized to rule out a velamentous cord insertion. The placental cord insertion can be identified in 99% of cases with gray scale and color Doppler ultrasound,83 with identification of a velamentous insertion having a sen-sitivity of 100%, specificity of 99.8%, a positive predictive value of 83%, and a negative predictive value of 100%.84 Other placental anomalies, such as placenta previa and
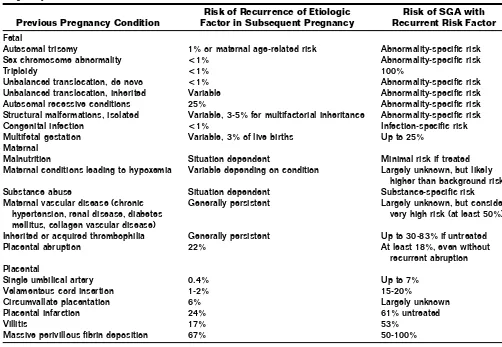
cir-Table 2 Recurrence Risks of Etiologies and FGR Based on Etiology of the Poor Fetal Growth in the Previous
Pregnancy1,3,10-13,16-18,20,21,24,25,27,28,45,48,52-55
Previous Pregnancy Condition
Risk of Recurrence of Etiologic Factor in Subsequent Pregnancy
Risk of SGA with Recurrent Risk Factor
Fetal
Autosomal trisomy 1% or maternal age-related risk Abnormality-specific risk
Sex chromosome abnormality <1% Abnormality-specific risk
Triploidy <1% 100%
Unbalanced translocation, de novo <1% Abnormality-specific risk
Unbalanced translocation, inherited Variable Abnormality-specific risk
Autosomal recessive conditions 25% Abnormality-specific risk
Structural malformations, isolated Variable, 3-5% for multifactorial inheritance Abnormality-specific risk
Congenital infection <1% Infection-specific risk
Multifetal gestation Variable, 3% of live births Up to 25%
Maternal
Malnutrition Situation dependent Minimal risk if treated
Maternal conditions leading to hypoxemia Variable depending on condition Largely unknown, but likely higher than background risk
Substance abuse Situation dependent Substance-specific risk
Maternal vascular disease (chronic hypertension, renal disease, diabetes mellitus, collagen vascular disease)
Generally persistent Largely unknown, but consider very high risk (at least 50%)
Inherited or acquired thrombophilia Generally persistent Up to 30-83% if untreated
Placental abruption 22% At least 18%, even without
recurrent abruption Placental
Single umbilical artery 0.4% Up to 7%
Velamentous cord insertion 1-2% 15-20%
Circumvallate placentation 6% Largely unknown
Placental infarction 24% 61% untreated
Villitis 17% 53%
Massive perivillous fibrin deposition 67% 50-100%
cumvallate placentation, should be noted. The sonographic diagnosis of placental abruption relies on the identification of a thickened placenta, a hematoma (retroplacental, subchori-onic, or preplacental),85 or the presence of intraamniotic blood.
Uterine artery Doppler velocimetry has been utilized as a screening tool for pregnancies at high risk of complications from ischemic placental disease. At 20 weeks’ gestation, bi-lateral diastolic notching and mean resistance index of
⬎90th%ile had a positive predictive value of 57% for severe
preeclampsia⫾FGR and a 93% positive predictive value for mild or severe disease.86Even in women with previous ad-verse pregnancy outcomes on low-dose aspirin therapy, the presence of a diastolic notch at 23 weeks gestation was asso-ciated with a higher rate of vascular complications such as preeclampsia and FGR (31% versus 5%).87
There may be a modest benefit of low-dose aspirin use in pregnancies at high risk for poor fetal growth from specific maternal or placental conditions. Although a benefit has not been demonstrated by all studies,88many have demonstrated a significant reduction in the risk of FGR among high-risk women treated with low-dose aspirin.89,90A meta-analysis of low-dose aspirin use suggested an 18% reduction in FGR, but a much greater effect (OR 0.35) when therapy was instituted before 17 weeks gestation. Given the excellent safety profile
of low-dose aspirin use in pregnancy, it seems reasonable to offer this to women at significant risk of recurrent FGR, start-ing as early in the pregnancy as possible. It has been found to be especially beneficial in those with a history of recurrent FGR and placental infarction, reducing the incidence of FGR from 61% in the untreated to 13% in the treated pregnan-cies.78
In the setting of antiphospholipid antibody syndrome (ei-ther primary or secondary), women should receive low-dose aspirin and prophylactic heparin during pregnancy. Several randomized control trials have investigated this issue and have demonstrated improved outcomes compared with pla-cebo or with aspirin alone.91-94The optimal management of pregnancies complicated by recurrent chronic villitis or mas-sive fibrin deposition has not been definitively determined. Although low-dose aspirin and/or heparin have been utilized with some benefit,79these lesions are not the result of a co-agulation abnormality and may be best treated with intrave-nous immunoglobulin95due to the suspected immune etiol-ogy.
In high-risk women with inherited thrombophilias and adverse pregnancy events, heparin has been used to improve obstetrical outcomes. Heparin prophylaxis (compared with aspirin) has been shown to improve live birth rates (86% versus 29%) and reduce the incidence of FGR (10% versus Identify probable
etiology
Maternal Fetal Placental
Maternal Hypoxia-related conditions
Maternal Vascular Disease
Substance Abuse
Thrombophilias
Chromosomal Abnormalities
Non-chromosomal Abnormalities
Umbilical Cord Abnormalities Malnutrition
Immune-mediated Injury (fibrin deposition, chronic villitis)
Increase BMI Nutrition consult
Optimize underlying disease
Counseling/Detox Smoking cessation
Optimize underlying disease
Low-dose ASA Heparin
Fetal Growth Restriction
1st trimester CRL Serial growth ultrasounds
Ultrasound evaluation
IVIG
Genetic counseling Prenatal diagnosis (CVS, amniocentesis)
Prenatal screening Targeted ultrasound
Placental Abruption
Preeclampsia
Uterine artery Dopplers
Placental infarction
Low-dose ASA ± heparin
Figure 1 Management of subsequent pregnancy based on presumed etiology of FGR.
30%) in women heterozygous for either FVL or prothrombin G20210A mutation or with protein S deficiency.96Other tri-als examining the use of heparin and/or low-dose aspirin for prevention of recurrent adverse pregnancy outcomes in women with genetic thrombophilias had relatively small numbers of subjects, were observational, included heteroge-neous groups of patients, had various dosages of heparin, and often used historical controls.63,69,97-99 Nevertheless, these studies suggest that there may be some benefit to using hep-arin in selected circumstances.
Targeted Areas for Future Research
Despite the vast amount of research that has been done on FGR, there are many knowledge gaps that persist, particu-larly in the areas of defining etiology-specific risks and inter-ventions for preventing recurrence. The heterogeneity of the condition has hindered our ability to determine optimal treatments for individual cases. In addition, our understand-ing of the complex biologic– environmental interactions and genetic susceptibilities that exist is limited. Future research should attempt to use risk-specific inclusion criteria and should take into consideration the array of adverse outcomes that can result from similar underlying conditions (ie, isch-emic placental disease).
Conclusions
In summary, poor fetal growth can result from a myriad of fetal, placental, and maternal conditions. Since many of these factors can persist throughout subsequent pregnancies, women should be counseled and managed appropriately to minimize future adverse outcomes. It is also important to recognize that risk factors for FGR overlap the risk factors for many other obstetrical concerns, such as recurrent miscar-riage, preeclampsia, abruption, and fetal death. Therefore, future pregnancies should be given close attention to the development of these possible events.
References
1. Snijders RJ, Sherrod C, Gosden CM, et al: Fetal growth retardation: associated malformations and chromosomal abnormalities. Am J Ob-stet Gynecol 168:547-555, 1993
2. Creasy RLR: Maternal–Fetal Medicine. Philadelphia, PA, Saunders, 2005
3. Khoury MJ, Erickson JD, Cordero JF, et al: Congenital malformations and intrauterine growth retardation: a population study. Pediatrics 82: 83-90, 1988
4. Hannula K, Lipsanen-Nyman M, Kristo P, et al: Genetic screening for maternal uniparental disomy of chromosome 7 in prenatal and postna-tal growth retardation of unknown cause. Pediatrics 109:441-448, 2002
5. Kotzot D, Lurie IW, Mehes K, et al: No evidence of uniparental disomy 2, 6, 14, 16, 20, and 22 as a major cause of intrauterine growth retar-dation. Clin Genet 58:177-180, 2000
6. Brown ZA, Vontver LA, Benedetti J, et al: Effects on infants of a first episode of genital herpes during pregnancy. N Engl J Med 317:1246-1251, 1987
7. Meyberg-Solomayer GC, Fehm T, Muller-Hansen I, et al: Prenatal ul-trasound diagnosis, follow-up, and outcome of congenital varicella syn-drome. Fetal Diagn Ther 21:296-301, 2006
8. Donner C, Liesnard C, Content J, et al: Prenatal diagnosis of 52
preg-nancies at risk for congenital cytomegalovirus infection. Obstet Gynecol 82:481-486, 1993
9. Daffos F, Forestier F, Capella-Pavlovsky M, et al: Prenatal management of 746 pregnancies at risk for congenital toxoplasmosis. N Engl J Med 318:271-275, 1988
10. Khan NA, Kazzi SN: Yield and costs of screening growth-retarded in-fants for torch infections. Am J Perinatol 17:131-135, 2000
11. Arbuckle TE, Wilkins R, Sherman GJ: Birth weight percentiles by ges-tational age in Canada. Obstet Gynecol 81:39-48, 1993
12. Houlton MC, Marivate M, Philpott RH: The prediction of fetal growth retardation in twin pregnancy. Br J Obstet Gynaecol 88:264-273, 1981 13. Secher NJ, Kaern J, Hansen PK: Intrauterine growth in twin pregnan-cies: prediction of fetal growth retardation. Obstet Gynecol 66:63-68, 1985
14. Sherer DM, Divon MY: Fetal growth in multifetal gestation. Clin Obstet Gynecol 40:764-770, 1997
15. Bjoro K Jr: Vascular anomalies of the umbilical cord. I. Obstetric im-plications. Early Hum Dev 8:119-127, 1983
16. Predanic M, Perni SC, Friedman A, et al: Fetal growth assessment and neonatal birth weight in fetuses with an isolated single umbilical artery. Obstet Gynecol 105:1093-1097, 2005
17. Heinonen S, Ryynanen M, Kirkinen P, et al: Perinatal diagnostic eval-uation of velamentous umbilical cord insertion: clinical, Doppler, and ultrasonic findings. Obstet Gynecol 87:112-117, 1996
18. Rolschau J: Circumvallate placenta and intrauterine growth retarda-tion. Acta Obstet Gynecol Scand Suppl 72:11-14, 1978
19. Nagy S, Bush M, Stone J, et al: Clinical significance of subchorionic and retroplacental hematomas detected in the first trimester of pregnancy. Obstet Gynecol 102:94-100, 2003
20. Ananth CV, Berkowitz GS, Savitz DA, et al: Placental abruption and adverse perinatal outcomes. J Am Med Assoc 282:1646-1651, 1999 21. Sheiner E, Shoham-Vardi I, Hallak M, et al: Placental abruption in term
pregnancies: clinical significance and obstetric risk factors. J Matern Fetal Neonatal Med 13:45-49, 2003
22. Ananth CV, Demissie K, Smulian JC, et al: Relationship among placenta previa, fetal growth restriction, and preterm delivery: a population-based study. Obstet Gynecol 98:299-306, 2001
23. Laurini R, Laurin J, Marsal K: Placental histology and fetal blood flow in intrauterine growth retardation. Acta Obstet Gynecol Scand 73:529-534, 1994
24. Salafia CM, Vintzileos AM, Silberman L, et al: Placental pathology of idiopathic intrauterine growth retardation at term. Am J Perinatol 9:179-184, 1992
25. Bane AL, Gillan JE: Massive perivillous fibrinoid causing recurrent placental failure. Br J Obstet Gynaecol 110:292-295, 2003
26. Mandsager NT, Bendon R, Mostello D, et al: Maternal floor infarction of the placenta: prenatal diagnosis and clinical significance. Obstet Gy-necol 83:750-754, 1994
27. Bjoro K Jr, Myhre E: The role of chronic non-specific inflammatory lesions of the placenta in intra-uterine growth retardation. Acta Pathol Microbiol Immunol Scand [A] 92:133-137, 1984
28. Pham T, Steele J, Stayboldt C, et al: Placental mesenchymal dysplasia is associated with high rates of intrauterine growth restriction and fetal demise: a report of 11 new cases and a review of the literature. Am J Clin Pathol 126:67-78, 2006
29. Svensson AC, Pawitan Y, Cnattingius S, et al: Familial aggregation of small-for-gestational-age births: the importance of fetal genetic effects. Am J Obstet Gynecol 194:475-479, 2006
30. Stein AD, Ravelli AC, Lumey LH: Famine, third-trimester pregnancy weight gain, and intrauterine growth: the Dutch Famine Birth Cohort Study. Hum Biol 67:135-150, 1995
31. Kouba S, Hallstrom T, Lindholm C, et al: Pregnancy and neonatal outcomes in women with eating disorders. Obstet Gynecol 105:255-260, 2005
32. Murphy JF, O’Riordan J, Newcombe RG, et al: Relation of haemoglobin levels in first and second trimesters to outcome of pregnancy. Lancet 1:992-995, 1986
33. Steer P, Alam MA, Wadsworth J, et al: Relation between maternal
haemoglobin concentration and birth weight in different ethnic groups. Br Med J 310:489-491, 1995
34. Lu ZM, Goldenberg RL, Cliver SP, et al: The relationship between maternal hematocrit and pregnancy outcome. Obstet Gynecol 77:190-194, 1991
35. Sheiner E, Mazor M, Levy A, et al: Pregnancy outcome of asthmatic patients: a population-based study. J Matern Fetal Neonatal Med 18: 237-240, 2005
36. Jensen GM, Moore LG: The effect of high altitude and other risk factors on birthweight: independent or interactive effects? Am J Public Health 87:1003-1007, 1997
37. Galan HL, Rigano S, Radaelli T, et al: Reduction of subcutaneous mass, but not lean mass, in normal fetuses in Denver, Colorado. Am J Obstet Gynecol 185:839-844, 2001
38. Hammoud AO, Bujold E, Sorokin Y, et al: Smoking in pregnancy re-visited: findings from a large population-based study. Am J Obstet Gynecol 192:1856-1862, discussion 1862-1863, 2005
39. Spinillo A, Capuzzo E, Nicola SE, et al: Factors potentiating the smok-ing-related risk of fetal growth retardation. Br J Obstet Gynaecol 101: 954-958, 1994
40. U.S. Department of Health and Human Services. Women and Smoking: A Report of the Surgeon General. Public Health Service, Office of the Surgeon General, 2001
41. Bada HS, Das A, Bauer CR, et al: Low birth weight and preterm births: etiologic fraction attributable to prenatal drug exposure. J Perinatol 25:631-637, 2005
42. Floyd RL, O’Connor MJ, Sokol RJ, et al: Recognition and prevention of fetal alcohol syndrome. Obstet Gynecol 106:1059-1064, 2005 43. Bada HS, Das A, Bauer CR, et al: Gestational cocaine exposure and
intrauterine growth: maternal lifestyle study. Obstet Gynecol 100:916-924, 2002
44. Ness RB, Sibai BM: Shared and disparate components of the pathophys-iologies of fetal growth restriction and preeclampsia. Am J Obstet Gy-necol 195:40-49, 2006
45. Bernstein PS, Divon MY: Etiologies of fetal growth restriction. Clin Obstet Gynecol 40:723-729, 1997
46. Villar J, Carroli G, Wojdyla D, et al: Preeclampsia, gestational hyper-tension and intrauterine growth restriction, related or independent conditions? Am J Obstet Gynecol 194:921-931, 2006
47. Abalos E, Duley L, Steyn DW, et al: Antihypertensive drug therapy for mild to moderate hypertension during pregnancy. Cochrane Database of Systematic Reviews 2001:CD002252
48. Stettler RW, Cunningham FG: Natural history of chronic proteinuria complicating pregnancy. Am J Obstet Gynecol 167:1219-1224, 1992 49. Yasmeen S, Wilkins EE, Field NT, et al: Pregnancy outcomes in women
with systemic lupus erythematosus. J Matern Fetal Med 10:91-96, 2001
50. Rahman P, Gladman DD, Urowitz MB: Clinical predictors of fetal out-come in systemic lupus erythematosus. J Rheumatol 25:1526-1530, 1998
51. Julkunen H, Jouhikainen T, Kaaja R, et al: Fetal outcome in lupus pregnancy: a retrospective case-control study of 242 pregnancies in 112 patients. Lupus 2:125-131, 1993
52. Kupferminc MJ, Rimon E, Ascher-Landsberg J, et al: Perinatal outcome in women with severe pregnancy complications and multiple throm-bophilias. J Perinat Med 32:225-227, 2004
53. Kupferminc MJ, Many A, Bar-Am A, et al: Mid-trimester severe intra-uterine growth restriction is associated with a high prevalence of thrombophilia. Br J Obstet Gynaecol 109:1373-1376, 2002 54. Martinelli P, Grandone E, Colaizzo D, et al: Familial thrombophilia and
the occurrence of fetal growth restriction. Haematologica 86:428-431, 2001
55. Howley HE, Walker M, Rodger MA: A systematic review of the associ-ation between factor V Leiden or prothrombin gene variant and intra-uterine growth restriction. Am J Obstet Gynecol 192:694-708, 2005 56. Infante-Rivard C, Rivard GE, Yotov WV, et al: Absence of association of
thrombophilia polymorphisms with intrauterine growth restriction. N Engl J Med 347:19-25, 2002
57. Salomon O, Seligsohn U, Steinberg DM, et al: The common
prothrom-botic factors in nulliparous women do not compromise blood flow in the feto-maternal circulation and are not associated with preeclampsia or intrauterine growth restriction. Am J Obstet Gynecol 191: 2002-2009, 2004
58. Franchi F, Cetin I, Todros T, et al: Intrauterine growth restriction and genetic predisposition to thrombophilia. Haematologica 89:444-449, 2004
59. Infante-Rivard C, Rivard GE, Guiguet M, et al: Thrombophilic poly-morphisms and intrauterine growth restriction. Epidemiology 16:281-287, 2005
60. Yasuda M, Takakuwa K, Tokunaga A, et al: Prospective studies of the association between anticardiolipin antibody and outcome of preg-nancy. Obstet Gynecol 86:555-559, 1995
61. Levine JS, Branch DW, Rauch J: The antiphospholipid syndrome. N Engl J Med 346:752-763, 2002
62. Alarcon-Segovia D: Clinical manifestations of the antiphospholipid syndrome. J Rheumatol 19:1778-1781, 1992
63. Paidas MJ, Ku DH, Arkel YS: Screening and management of inherited thrombophilias in the setting of adverse pregnancy outcome. Clin Peri-natol 31:783-805, 2004
64. Alfirevic Z, Roberts D, Martlew V: How strong is the association be-tween maternal thrombophilia and adverse pregnancy outcome? A sys-tematic review. Eur J Obstet Gynecol Reprod Biol 101:6-14, 2002 65. Robertson L, Wu O, Langhorne P, et al: Thrombophilia in pregnancy: a
systematic review. Br J Haematol 132:171-196, 2006
66. Kupferminc MJ, Eldor A, Steinman N, et al: Increased frequency of genetic thrombophilia in women with complications of pregnancy. N Engl J Med 340:9-13, 1999
67. Lim W, Crowther MA, Eikelboom JW: Management of antiphospho-lipid antibody syndrome: a systematic review. J Am Med Assoc 295: 1050-1057, 2006
68. ACOG Practice Bulletin #68: Antiphospholipid syndrome. Obstet Gy-necol 106:1113-1121, 2005
69. Stella CL, Sibai BM: Thrombophilia and adverse maternal-perinatal outcome. Clin Obstet Gynecol 49:850-860, 2006
70. Roque H, Paidas MJ, Funai EF, et al: Maternal thrombophilias are not associated with early pregnancy loss. Thromb Haemost 91:290-295, 2004
71. Rasmussen S, Irgens LM, Albrechtsen S, et al: Predicting preeclampsia in the second pregnancy from low birth weight in the first pregnancy. Obstet Gynecol 96:696-700, 2000
72. Salihu HM, Sharma PP, Aliyu MH, et al: Is small for gestational age a marker of future fetal survival in utero? Obstet Gynecol 107:851-856, 2006
73. Kuno N, Itakura A, Kurauchi O, et al: Decrease in severity of intrauter-ine growth retardation in subsequent pregnancies. Int J Gynaecol Ob-stet 51:219-224, 1995
74. Furuhashi M, Kurauchi O, Suganuma N: Pregnancy following placental abruption. Arch Gynecol Obstet 267:11-13, 2002
75. Rasmussen S, Albrechtsen S, Dalaker K: Obstetric history and the risk of placenta previa. Acta Obstet Gynecol Scand 79:502-507, 2000 76. Rasmussen S, Irgens LM, Dalaker K: A history of placental dysfunction
and risk of placental abruption. Paediatr Perinat Epidemiol 13:9-21, 1999
77. Rasmussen S, Irgens LM, Albrechtsen S, et al: Women with a history of placental abruption: when in a subsequent pregnancy should special surveillance for a recurrent placental abruption be initiated? Acta Ob-stet Gynecol Scand 80:708-712, 2001
78. Wallenburg HC, Rotmans N: Prevention of recurrent idiopathic fetal growth retardation by low-dose aspirin and dipyridamole. Am J Obstet Gynecol 157:1230-1235, 1987
79. Fuke Y, Aono T, Imai S, et al: Clinical significance and treatment of massive intervillous fibrin deposition associated with recurrent fetal growth retardation. Gynecol Obstet Invest 38:5-9, 1994
80. Cheng CJ, Bommarito K, Noguchi A, et al: Body mass index change between pregnancies and small for gestational age births. Obstet Gy-necol 104:286-292, 2004
81. Olsen SF, Secher NJ, Tabor A, et al: Randomised clinical trials of fish oil
supplementation in high risk pregnancies. Fish Oil Trials In Pregnancy (FOTIP) Team. Br J Obstet Gynaecol 107:382-395, 2000
82. Chavez MR, Ananth CV, Smulian JC, et al: Fetal transcerebellar diam-eter measurement with particular emphasis in the third trimester: a reliable predictor of gestational age. Am J Obstet Gynecol 191:979-984, 2004
83. Sepulveda W, Rojas I, Robert JA, et al: Prenatal detection of velamen-tous insertion of the umbilical cord: a prospective color Doppler ultra-sound study. Ultraultra-sound Obstet Gynecol 21:564-569, 2003 84. Nomiyama M, Toyota Y, Kawano H: Antenatal diagnosis of
velamen-tous umbilical cord insertion and vasa previa with color Doppler im-aging. Ultrasound Obstet Gynecol 12:426-429, 1998
85. Nyberg DA, Cyr DR, Mack LA, et al: Sonographic spectrum of placental abruption. AJR Am J Roentgenol 148:161-164, 1987
86. Chan FY, Pun TC, Lam C, et al: Pregnancy screening by uterine artery Doppler velocimetry: which criterion performs best? Obstet Gynecol 85:596-602, 1995
87. Haddad B, Uzan M, Breart G, et al: Uterine Doppler wave form and the prediction of the recurrence of pre-eclampsia and intra-uterine growth retardation in patients treated with low-dose aspirin. Eur J Obstet Gy-necol Reprod Biol 62:179-183, 1995
88. Low-dose aspirin in prevention and treatment of intrauterine growth retardation and pregnancy-induced hypertension. Italian study of as-pirin in pregnancy. Lancet 341:396-400, 1993
89. Ebrashy A, Ibrahim M, Marzook A, et al: Usefulness of aspirin therapy in high-risk pregnant women with abnormal uterine artery Doppler ultrasound at 14-16 weeks pregnancy: randomized controlled clinical trial. Croat Med J 46:826-831, 2005
90. Uzan S, Beaufils M, Breart G, et al: Prevention of fetal growth
retarda-tion with low-dose aspirin: findings of the EPREDA trial. Lancet 337:1427-1431, 1991
91. Kutteh WH: Antiphospholipid antibody-associated recurrent preg-nancy loss: treatment with heparin and low-dose aspirin is superior to low-dose aspirin alone. Am J Obstet Gynecol 174:1584-1589, 1996 92. Rai R, Cohen H, Dave M, et al: Randomised controlled trial of aspirin
and aspirin plus heparin in pregnant women with recurrent miscarriage associated with phospholipid antibodies (or antiphospholipid antibod-ies). Br Med J 314:253-257, 1997
93. Farquharson RG, Quenby S, Greaves M: Antiphospholipid syndrome in pregnancy: a randomized, controlled trial of treatment. Obstet Gy-necol 100:408-413, 2002
94. Empson M, Lassere M, Craig JC, et al: Recurrent pregnancy loss with antiphospholipid antibody: a systematic review of therapeutic trials. Obstet Gynecol 99:135-144, 2002
95. Chang P, Millar D, Tsang P, et al: Intravenous immunoglobulin in antiphospholipid syndrome and maternal floor infarction when stan-dard treatment fails: a case report. Am J Perinatol 23:125-129, 2006 96. Gris JC, Mercier E, Quere I, et al: Low-molecular-weight heparin versus
low-dose aspirin in women with one fetal loss and a constitutional thrombophilic disorder. Blood 103:3695-3699, 2004
97. Kupferminc MJ, Fait G, Many A, et al: Low-molecular-weight heparin for the prevention of obstetric complications in women with thrombo-philias. Hypertens Pregnancy 20:35-44, 2001
98. Brenner B, Hoffman R, Blumenfeld Z, et al: Gestational outcome in thrombophilic women with recurrent pregnancy loss treated by enox-aparin. Thromb Haemost 83:693-697, 2000
99. Grandone E, Brancaccio V, Colaizzo D, et al: Preventing adverse ob-stetric outcomes in women with genetic thrombophilia. Fertil Steril 78:371-375, 2002
Preeclampsia Recurrence and Prevention
Gary A. Dildy III, MD,* Michael A. Belfort, MD, PhD,
†and John C. Smulian, MD, MPH
‡Women with a previous pregnancy complicated by preeclampsia have an increased risk for recurrence in subsequent pregnancies. For severe preeclamptic women in an initial preg-nancy, recurrence rates for any type of preeclampsia are very high, approaching 50% in some studies. Significant maternal and fetal complications are more common in recurrent preeclampsia compared with an initial episode. For women who have experienced a pregnancy complicated by preeclampsia, a systematic evaluation for underlying risk factors may identify a specific pathway suitable for a specific intervention. Although some progress has been made in developing potential therapeutic options to prevent preeclampsia recur-rence, there is a great need for better data to determine who will benefit most from any specific therapy.
Semin Perinatol 31:135-141 © 2007 Elsevier Inc. All rights reserved.
KEYWORDSpreeclampsia, eclampsia, risk factors, recurrence, prevention, HELLP syndrome
P
reeclampsia complicates approximately 5% to 10% of nulliparous pregnancies1and is consistently among the top three causes of maternal death in both developed and developing countries.2-4Two-thirds of cases will be mild and the other third severe in degree.1Preeclampsia is considered a disease of nulliparous women, as it is twice as common in primigravidas as it is in women who have previously given birth.5It is well known that women with a previous preg-nancy complicated by preeclampsia have an increased risk for recurrence in subsequent pregnancies. For severe pre-eclamptic women in an initial pregnancy, recurrence rates for any type of preeclampsia are very high, approaching 50% in some studies. Significant maternal and fetal complications are more common in recurrent preeclampsia compared with an initial episode. Thus, accurate and thorough counseling regarding recurrence risks and potential preventive measures will assist women and their caregivers to make important decisions pertaining to future childbearing.Preeclampsia Risk Factors
Many factors for preeclampsia have been described in the obstetrical literature, and the majority will persist in subse-quent pregnancies (Table 1).6 Preeclampsia tends to be a disease of first pregnancy in women with no other obvious risk factors; however, underlying medical conditions with vascular or renal implications (diabetes mellitus, chronic hy-pertension) and conditions with increased trophoblast mass (multifetal gestation or hydrops fetalis) substantially increase the risk. As preeclampsia is likely a syndrome of multiple etiologies and many underlying factors persist across preg-nancies, a significant risk factor for future preeclampsia is a prior history of preeclampsia.
The Epidemiology of
Preeclampsia Recurrence
A number of studies have examined the risk for preeclampsia recurrence in subsequent pregnancies, and all have indicated a significantly increased risk (Table 2). The highest risks for recurrence are found most consistently when the initial case was preterm, severe, or complicated by eclampsia, HELLP (hemolysis, elevated liver enzymes, and low platelet count) syndrome, or fetal growth restriction. However, good data are still relatively sparse because definitions for preeclampsia often vary from study to study.
Campbell and coworkers studied a population of pregnant women (n ⫽ 29,851) whose first recorded pregnancy oc-curred between the years 1967 and 1978 in Aberdeen, Scot-land and had⬎2 subsequent pregnancies during that same *Department of Obstetrics and Gynecology, LSU Health Sciences Center,
New Orleans, LA.
†Department of Obstetrics and Gynecology, University of Utah Health Sci-ences Center, Salt Lake City, UT.
‡Division of Maternal Fetal Medicine, Department of Obstetrics, Gynecology and Reproductive Sciences, UMDNJ-Robert Wood Johnson Medical School, New Brunswick, NJ.
Address reprint requests to Gary A. Dildy III, MD, Maternal Fetal Medicine Center, St. Mark’s Hospital, 1140 East 3900 South, Suite 390, Salt Lake City, Utah 84124. E-mail: Gary.Dildy@HCAhealthcare.com
135
time period (n⫽6637).7Women were categorized as nor-motensive (68.0%), mildly preeclamptic (26.3%), protein-uric preeclamptic (5.6%), and eclamptic (0.2%). They found that the overall incidence of preeclampsia in a second preg-nancy was less than that in a first pregpreg-nancy, but was
depen-dent on the outcome of the first pregnancy. If the first preg-nancy was complicated simply by proteinuric preeclampsia, the incidence in the second pregnancy was 7.5%, whereas those who were normotensive in the first pregnancy had a low rate of proteinuric preeclampsia in the second pregnancy
Table 1 The Strength of the Association of Selected Risk Factors for Preeclampsia*
Risk Factor Associated with Preeclampisa Reference OR (95% CI)
Preeclampsia in a previous pregnancy Hnat18 3.88 (2.98-5.05)
Duckitt48 7.19 (5.85-8.83)
First pregnancy Conde-Agudelo49 2.38 (2.28-2.49)
Duckitt48 2.91 (1.28-6.61)
Multifetal gestation Sibai50 2.62 (2.03-3.38)
Conde-Agudelo49 2.10 (1.90-2.32)
Duckitt48 2.93 (2.04-4.21)
Chronic hypertension Conde-Agudelo49 1.99 (1.78-2.22)
Gestational diabetes Conde-Agudelo49 1.93 (1.66-2.25)
Pregestational diabetes Duckitt48 3.56 (2.54-4.99)
Vascular and connective tissue disease Stamilio51 6.9 (1.1-42.3)
Nephropathy
Urinary tract infection Abi-Said52 4.23 (1.27-14.06)
Antiphospholipid antibody syndrome Robertson53 2.73 (1.65-4.51)
Duckitt48 9.72 (4.34-21.75)
Genetic factors (eg, thrombophilias) Robertson53
Factor V Leiden heterozygosity 2.19 (1.46-3.27)
Prothrombin heterozygosity 2.54 (1.52-4.23)
MTHFR homozygosity 1.37 (1.07-1.76)
Hyperhomocysteinemia 3.49 (1.21-10.11)
Obesity (BMI >35 kg/m2) Sibai1 3.38 (1.91-6.00)
Maternal age>35 years Conde-Agudelo49 1.67 (1.58-1.77)
Family history of preeclampsia Duckitt48 2.90 (1.70-4.93)
Fetal malformation Conde-Agudelo49 1.26 (1.16-1.37)
Abnormal maternal serum markers (AFP, hCG, uE3, Inhibin A)
Dugoff54
Inhibin A>2.0 MOM 2.39 (1.75-3.26)
2 abnormal markers 3.65 (2.79-4.78)
African-American race Tucker55 1.2 (0.8-1.7)
Abbreviations: AFP, alpha fetoprotein; HCG, human chorionic gonadotropin; uE3, unconjugated estriol. *Presented as odds ratio (OR) and 95% confidence intervals (CI).
Table 2 Summary of Studies that Present the Risk for Recurrence of Preeclampsia
Author Study Population Rate of Recurrence
Campbell7 Preeclampsia (nⴝ279) Preeclampsia 7.5%
Sibai9 Second trimester severe preeclampsia (nⴝ169) Any preeclampsia 65%
<28 weeks 21% 28-36 weeks 21% 37-40 weeks 24% van Rijn8 Preeclampsia with delivery
<34 weeks Preeclampsia 25%
Sullivan12 HELLP (nⴝ161) Preeclampsia 43%
HELLP 27%
Sibai11 HELLP (nⴝ192) Preeclampsia 19%
HELLP 3%
Chames13 HELLP with delivery<28 weeks (nⴝ62) Preeclampsia 55%
HELLP 6%
Adelusi14 Eclampsia (nⴝ64) Eclampsia 16%
Sibai16 Eclampsia (nⴝ366) Preeclampsia 22%
Eclampsia 2%
Trogstad17 Preeclampsia singleton (nⴝ19,960) Preeclampsia 14.1%
Preeclampsia twins (nⴝ325) Preeclampsia 6.8%
of 0.7%. However, women who had proteinuric preeclamp-sia in conjunction with a low-birth-weight (⬍2500 g) infant in their first pregnancy had double the incidence of protein-uric preeclampsia in their second pregnancy (11.9% versus 6.6%), compared with similar women with a normal infant birth weight during first pregnancy.
Van Rijn and coworkers studied primiparous women who delivered between 1993 and 2002 at the University Medical Center Utrecht in The Netherlands who had a history of early onset preeclampsia resulting in delivery before 34 weeks of gestation.8Preeclampsia recurred in 25% (30/120) of women in their second pregnancy. Five percent delivered before 34 weeks of gestation and 17% between 34 and 37 weeks of gestation.
Sibai and colleagues9reported subsequent pregnancy out-comes in women with severe second trimester preeclampsia. Of these 125 women, 108 had 169 subsequent pregnancies. For the subsequent pregnancies, approximately one-third were normotensive and two-thirds were complicated by pre-eclampsia. Of the women with preeclampsia, approximately one-third developed a recurrence at⬍28 weeks, one-third at 28 to 36 weeks, and one-third at 37 to 40 weeks.
Preeclampsia
Recurrence After HELLP Syndrome
Sibai and coworkers10described a retrospective analysis of 112 women with HELLP syndrome from 1977 to 1985. In this series, 38 women had 49 subsequent pregnancies.10One patient (2.6%) had recurrent HELLP syndrome in 2 subse-quent pregnancies, both complicated by abruption and fetal death. These investigators extended their series to the years 1977 to 1992, with follow up on 341 patients, of which 152 had subsequent pregnancies.11 Maternal complications in-cluded preeclampsia (19%), recurrent HELLP syndrome (3%), and placental abruption (2%). Perinatal complications included preterm birth (21%), intrauterine growth restric-tion (12%), and perinatal death (4%). Those with preexisting chronic hypertension had higher rates of preeclampsia (75%) but no significant increase in recurrent HELLP syndrome (5%). Perinatal complications such as preterm birth (80%), intrauterine growth restriction (45%), placental abruption (20%), and perinatal death (40%) were significantly higher among chronic hypertensives with previous HELLP syn-drome. Interestingly, all of the above conditions, including preeclampsia, preterm birth, fetal growth restriction, placen-tal abruption, and perinaplacen-tal death, are considered to have overlapping etiologic mechanisms that relate to abnormal placentation. Thus, a pregnancy with preeclampsia in an ini-tial pregnancy appears to be at risk for these other pregnancy complications and should be managed accordingly.
A retrospective study of 481 women with HELLP syn-drome between the years 1980 and 1991 analyzed 161 of 195 subsequent pregnancies in 122 patients.12Of these 161 preg-nancies, 43% had preeclampsia and 27% had HELLP syn-drome. A previous delivery⬍32 weeks of gestation was a risk factor for recurrence of prematurity at a similar gestational age secondary to preeclampsia in 61% of cases.
Chames and coworkers reported the outcomes of subse-quent pregnancies in women with a history of HELLP syn-drome for which delivery occurred at⬍28 weeks of gesta-tion.13Women were delivered in Memphis, TN (1984-1998) and Lexington, KY (1994-1998). Data were available in 69 patients; there were 76 subsequent pregnancies among 48 women, of which 62 progressed beyond 20 weeks of gesta-tion. Preeclampsia developed in 55% (34), of which 7 were mild and 27 were severe. Recurrent HELLP occurred in 6%. There were no cases of eclampsia; however, significant peri-natal complications were frequent. Preterm birth (⬍37 weeks of gestation) occurred in 53%. Newborns were growth restricted in 27%, and the overall perinatal mortality rate was 11%. Women with chronic hypertension had greater overall morbidity.
Preeclampsia Recurrence After Eclampsia
Eclampsia recurrence is to some extent dependent on ade-quacy of prenatal care and peripartum practices, including methods to control hypertensive crisis and prevention of eclamptic seizures vis-a-vis magnesium sulfate. Adelusi and Ojengbede14reported a prospective study of 64 eclamptics from Ibadan, Nigeria of whom 16% experienced recurrent eclampsia despite the benefit of antenatal care. Chesley’s seminal account of eclampsia recurrence during the early 20th century reported a recurrence risk range of 0% to 21% from published series, with an approximately 5% risk for viable gestations.15
Sibai and coworkers studied 223 women whose pregnan-cies were complicated by eclampsia between the years 1977 and 1989, with an average follow up of 7.2 years.16Of the 366 subsequent pregnancies, 22% were complicated by pre-eclampsia and 1.9% by pre-eclampsia. Within the nulliparous group, women who had eclampsia before 37 weeks of gesta-tion in the index pregnancy had significantly higher inci-dences of preeclampsia and poor perinatal outcome in sub-sequent pregnancies, compared with those who had eclampsia at or beyond 37 weeks of gestation. Of the normo-tensive women, 10% had chronic hypertension on follow up. The highest incidence of chronic hypertension was in those with eclampsia at⬍30 weeks of gestation (18%) and the lowest incidence (5%) in those with eclampsia at⬎37 weeks of gestation.
Preeclampisa Recurrence
After Multiple Gestations
Although multiple gestations are considered at risk for pre-eclampsia, it is not clear whether women developing this complication are clearly at risk for recurrence in the same degree as for singleton pregnancies. Only 1 study has ad-dressed this issue. Trogstad and coworkers examined the Norway birth registry from 1967 to 1998, including 550,218 women.17For women with a previous singleton pregnancy complicated by preeclampsia, the recurrence rate was 14.1% compared with the recurrence rate for twins of 6.8%, which was much closer to the population risk. This suggests that different potential etiologies for preeclampsia present