92.46%
Originality
7.54%
Similarity
277
Sources
Doc vs Internet + Library
Web sources: 197 sources found
1. https://www.springer.com/cda/content/document/cda_downloaddocument/9783319024714-c2.pdf?S… 0.53% 2. https://www.scirp.org/(S(351jmbntvnsjt1aadkposzje))/reference/ReferencesPapers.aspx?Reference… 0.53% 3. http://drlchem.com/CHEM112/Lecture%203%20Intermolecular%20Forces.pdf 0.53% 4. https://quizlet.com/189525313/chem-180-ch-3-flash-cards 0.53% 5. https://www.intechopen.com/books/water-treatment/natural-zeolites-in-water-treatment-how-effectiv… 0.53%
6. https://quizlet.com/122687325/chem-ch-13-flash-cards 0.53%
7. http://www.mikeblaber.org/oldwine/chm1046/notes/Kinetics/Temprate/Temprate.htm 0.53%
8. http://file.scirp.org/Html/4-2580028_30450.htm 0.53%
9. http://agro.icm.edu.pl/agro/element/bwmeta1.element.agro-73987ffd-8e37-449d-b53b-83dd98a773c… 0.53% 10. http://repository.akfar-isfibjm.ac.id/557/1/Anisa.pdf 0.53%
11. https://link.springer.com/10.1007/s10562-017-2133-2 0.53%
12. http://westchemistry.weebly.com/uploads/6/6/9/9/6699537/chapter14notes.pdf 0.53% 13. https://www.chegg.com/homework-help/questions-and-answers/arrhenius-equation-shows-relation… 0.53% 14. https://www.saddleback.edu/faculty/cabel/Saddleback/Chem_1B_Schedule_files/Exp15-thermo.pdf 0.53% 15. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4604301 0.48% 16. http://docshare.tips/biotech_5752211ab6d87fee7c8b45a6.html 0.48% 17. https://pubs.rsc.org/en/content/articlehtml/2017/ra/c6ra27036k 0.48% 18. http://www.textureanalyser.co.uk/PublishedPapers.htm 0.48% 19. https://link.springer.com/referenceworkentry/10.1007%2F978-3-319-54528-8_12-1 0.45% 20. http://repository.unika.ac.id/729/7/10.70.0143%20Fransiska%20Nugrahani%20DAFTAR%20PUS… 0.45% 21. https://onlinelibrary.wiley.com/doi/full/10.1111/1541-4337.12185 0.45% 22. http://repository.unika.ac.id/view/subjects/G63.html 0.45%
23. https://bdnutritionhealth.ca/category/article 0.42%
24. https://www.imbe.fr/christopher-augur.html 0.42%
25. https://bdnutritionhealth.ca/blog 0.42%
26. https://www.uv.mx/mca/files/2016/08/CV-DraMJF.pdf 0.42%
27. https://vdocuments.mx/book-of-abstracts-krakow.html 0.42%
28. http://www.scialert.net/fulltext/?doi=ijp.2016.394.400 0.39% 29. http://repository.unika.ac.id/1945/6/10.70.0137%20Vincent%20Kevin%20Tejo%20DAFTAR%20P… 0.39% 30. https://scholar.google.com/citations?user=JOi7TBcAAAAJ 0.39% 31. http://www.academia.edu/10676323/Cometabolic_biodegradation_of_methyl_t_-butyl_ether_by_P… 0.39%
32. https://scialert.net/fulltext/?doi=ijp.2016.394.400 0.39%
35. https://digitum.um.es/jspui/bitstream/10201/175/5/GarciaAlonso2de2.pdf.txt 0.39%
36. http://www.mdpi.com/2077-0472/5/1/48/htm 0.36%
37. https://www.mdpi.com/2077-0472/5/1/48/htm 0.36%
38. https://fulltxt.org/article/258 0.36%
39. https://docplayer.es/9333298-Numero-9-marzo-2013-issn-2173-0903.html 0.36% 40. http://www.musictherapy.org/research/researchabstracts10 0.33% 41. https://link.springer.com/article/10.1007%2Fs11947-014-1464-x 0.33% 42. http://www.montana.edu/msse/cap-abstracts/2012-cap-abstracts.html 0.33% 43. http://lppm.ipb.ac.id/wp-content/uploads/2018/02/JIPI-22.2_2-1-1.pdf 0.33% 44. https://link.springer.com/article/10.1007/s00217-010-1416-2 0.33% 45. https://www.musictherapy.org/research/researchabstracts08 0.33% 46. https://www.sciencedirect.com/science/article/pii/S0360131510002289 0.33% 47. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3794819 0.33%
48. http://repository.unika.ac.id/15946 0.33%
49. http://diginole.lib.fsu.edu/islandora/search/catch_all_names_mt%3A(Gregory,%20%20Sarah%20… 0.33% 50. https://link.springer.com/article/10.1007/s13197-016-2345-2 0.33% 51. http://www.academia.edu/Documents/in/Teachers_self_efficacy_levels 0.33%
52. http://etheses.uin-malang.ac.id/5951 0.33%
53. https://www.ajol.info/index.php/ajb/article/viewFile/128904/118459 0.33% 54. https://link.springer.com/article/10.1007/s11947-017-2030-0 0.33%
55. http://repositori.usu.ac.id/handle/123456789/7785 0.33%
56. http://www.montana.edu/msse/cap-abstracts/2004-cap-abstracts.html 0.33% 57. https://eric.ed.gov/?q=homework&ff1=souEducational+Technology+%26+Society&id=EJ1137657 0.33% 58. http://diginole.lib.fsu.edu/islandora/search/catch_all_subjects_mt%3A%28Music%29?islandora_s… 0.33% 59. https://www.sciencedirect.com/science/article/pii/S1072751508000793 0.33%
60. http://www.mdpi.com/1420-3049/21/1/113/htm 0.3%
61. https://link.springer.com/article/10.1007/s11423-010-9174-1 0.3%
62. http://www.mdpi.com/1420-3049/21/7/901/htm 0.3%
63. https://dynamic-med.biomedcentral.com/articles/10.1186/1476-5918-2-4 0.3% 64. https://link.springer.com/article/10.1007/s10643-013-0596-3 0.3% 65. https://docplayer.net/46364650-Glucosinolates-during-preparation-of-brassica-vegetables-in-indon… 0.3% 66. https://digital.library.unt.edu/ark:/67531/metadc2508 0.3% 67. https://link.springer.com/chapter/10.1007%2F978-3-319-10677-9_6 0.3% 68. https://eric.ed.gov/?q=mathematics+in+free+play&pr=on&ff1=locTurkey 0.3% 69. https://vdocuments.site/the-journal-of-essential-oilpdf.html 0.3%
70. https://core.ac.uk/download/pdf/35401450.pdf 0.3%
71. https://journal.uc.ac.id/index.php/JEE/article/download/384/344 0.3% 72. https://eric.ed.gov/?q=strategy+training&ff1=eduGrade+2 0.3% 73. https://docplayer.es/18267084-Optimizacion-del-proceso-de-extraccion-supercritica-de-los-polifen… 0.3% 74. http://www.calderglen.s-lanark.sch.uk/wpChemistry/wp-content/uploads/2016/10/Unit-1-summary-… 0.3% 75. http://docshare.tips/beverage-quality-and-safety_5827e26bb6d87f25228b4a22.html 0.3% 76. https://docplayer.es/10484610-V-congreso-internacional-de-ciencia-y-tecnologia-de-los-alimentos-… 0.3% 77. https://link.springer.com/article/10.1007%2Fs10803-016-2768-7 0.3% 78. https://www.studymode.com/essays/Hourly-Rounding-49682052.html 0.3% 79. https://www.healio.com/nursing/journals/jcen/2017-10-48-10/{ea4b2d5f-09e1-43d2-9834-2df211dc4… 0.3%
81. https://www.organic-center.org/reportfiles/TasteReport.pdf 0.3% 82. https://eric.ed.gov/?q=student+motivation&ff1=eduGrade+5&ff2=eduGrade+3 0.3%
83. http://www.isackx.com/Sommelier/dieet2.htm 0.27%
84. https://saylordotorg.github.io/text_general-chemistry-principles-patterns-and-applications-v1.0/s15… 0.27% 85. https://academic.csc.edu/oer/chem1/course_text/chapter_11_text.docx 0.27% 86. https://www.intechopen.com/books/sustainable-supply-chain-management/performance-evaluatio… 0.27% 87. https://2012books.lardbucket.org/books/principles-of-general-chemistry-v1.0/s15-liquids.html 0.27% 88. https://www.intechopen.com/books/mycotoxin-and-food-safety-in-developing-countries/regulation-… 0.27% 89. https://2012books.lardbucket.org/books/principles-of-general-chemistry-v1.0m/s15-liquids.html 0.27% 90. https://en.wikibooks.org/wiki/Transportation_Deployment_Casebook/Automobile_Safety 0.27% 91. http://faculty.sdmiramar.edu/fgarces/ChemComon/Tutorial/Excel/Excel_Plotting/index.htm 0.27% 92. https://link.springer.com/chapter/10.1007/978-94-011-0015-1_10 0.27% 93. http://gjbss.org/wp-content/uploads/2016/12/Gjbss-vol6-Issue-1-Paper-4-Mani-Neethu.pdf 0.27% 94. http://fgbmfresources.blogspot.com/2011/04/religiousness-and-business-ethics-of.html 0.27% 95. https://www.intechopen.com/books/tribology-in-engineering/effect-of-abrasive-particle-size-on-abr… 0.27% 96. https://archive.org/stream/ERIC_EJ766062/ERIC_EJ766062_djvu.txt 0.27% 97. https://docplayer.nl/18546328-Wijn-en-de-dieetkeuken-le-vin-et-la-cuisine-de-regime-alimentaire.ht… 0.27%
98. https://en.wikipedia.org/wiki/Root_mean_square 0.27%
99. https://repository.ipb.ac.id/bitstream/handle/123456789/51323/I11ald_Daftar%20Pustaka.pdf?seq… 0.27% 100. https://onlinelibrary.wiley.com/doi/full/10.1002/star.201000099 0.24%
101. http://repository.unika.ac.id/15918 0.24%
102. http://repository.unika.ac.id/11686/7/12.70.0057%20Tjan%2C%20Ivana%20Chandra%20Purnam… 0.24% 103. https://repository.ipb.ac.id/bitstream/handle/123456789/52345/Daftar%20Pustaka_%20I11cla.pd… 0.24% 104. http://www.montana.edu/msse/cap-abstracts/2013-cap-abstracts.html 0.24% 105. https://link.springer.com/article/10.1007%2Fs11947-014-1253-6 0.24% 106. http://www.scielo.org.co/scielo.php?script=sci_arttext&pid=S0120-99652017000100100 0.24% 107. http://agussupriadi.yolasite.com/resources/Frozen%20Food%20Shelf%20Life.pdf 0.24% 108. http://www.scielo.br/scielo.php?script=sci_arttext&pid=S0101-20612014000400015 0.24% 109. https://link.springer.com/article/10.1007%2Fs11947-013-1244-z 0.24% 110. https://docplayer.fr/80556358-These-presentee-pour-l-obtention-du-diplome-de-doctorat-3-eme-cy… 0.24% 111. https://link.springer.com/article/10.1007%2Fs11947-012-1047-7 0.24% 112. http://www.icef11.org/content/papers/fpe/FPE825.pdf 0.24%
113. http://www.answers.com/Q/FAQ/3185-1275 0.24%
114. https://link.springer.com/article/10.1007%2Fs00217-011-1527-4 0.24%
115. http://eprints.vu.edu.au/view/year/2009.type.html 0.24%
116. https://patents.google.com/patent/US7767718B2/en 0.24%
127. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5017387 0.24%
128. http://europepmc.org/articles/PMC4831853 0.24%
129. https://www.precisionnutrition.com/all-about-organic-foods 0.24%
130. https://draxe.com/anti-aging-foods 0.24%
131. https://digitalcommons.wku.edu/ijesab/vol5/iss2/40 0.24%
132. https://www.tasc.tas.gov.au/students/courses/science/chm415115-1 0.24% 133. http://docshare.tips/80231766-mcat-chemistry-book-1_58ed636eee3435c17c99322e.html 0.24% 134. http://extravirginity.com/2013/10/science-cooking-olive-oil 0.24% 135. http://epubs.surrey.ac.uk/view/year/2009.default.html 0.24% 136. https://en.wikipedia.org/wiki/Prostate_cancer,_familial 0.24% 137. http://astarbiology.com/ocr_categories/module-2-foundations-in-biology 0.24%
138. https://onlineedu.neit.edu/en422/page/4 0.24%
139. https://www.mcgill.ca/isid/files/isid/seufert.pb13.pdf 0.24% 140. http://article.sapub.org/10.5923.j.food.20120204.02.html 0.24% 141. https://www.canada.ca/en/health-canada/programs/consultation-perfluorooctanoic-acid-pfoa-in-d… 0.24%
142. http://iopscience.iop.org/issue/1742-6596/739/1 0.24%
143. http://www.readbag.com/sta-uwi-resources-documents-uwi-faculty-report-08-09 0.24%
144. https://works.bepress.com/kelly_brooks 0.24%
145. http://ijhsnet.com/journals/ijhs/Vol_3_No_1_March_2015/14.pdf 0.24%
146. http://naturalsociety.com/microwaves 0.24%
147. http://www.answers.com/Q/List_4_factors_that_can_affect_enzyme_activity 0.24% 148. https://www.intechopen.com/books/aircraft-technology/adaptive-automation-and-the-third-pilot 0.24% 149. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3989295 0.24% 150. https://link.springer.com/chapter/10.1007%2F978-90-368-1878-0_5 0.24% 151. https://www.studymode.com/essays/Reaction-Of-Catalase-With-Hydrogen-Peroxide-396662.html 0.24%
152. https://www.eatthis.com/best-supplements 0.24%
153. http://eprints.vu.edu.au/view/year/2009.html 0.24%
154. http://www.answers.com/Q/How_do_enzyme_activators_affect_enzymes 0.24% 155. https://juravin.wordpress.com/2017/01/29/are-sweeteners-harmful-for-your-weight-loss 0.24% 156. http://repository.unika.ac.id/7349/7/11.70.0025%20Jong%20Epha%20Yosia%20-%20DAFTAR%… 0.24% 157. https://www.slideshare.net/zgoutham/effect-of-sugar-replacement-with-stevia-on-physical-and-se… 0.24% 158. http://fac.ksu.edu.sa/sites/default/files/9-jnfr_493.pdf 0.24% 159. https://onlinelibrary.wiley.com/doi/full/10.1111/j.1541-4337.2007.00024.x 0.24% 160. https://www.universeofmemory.com/the-goldlist-method-scientific-critique 0.24% 161. https://docplayer.cz/73021541-Univerzita-karlova-v-praze-prirodovedecka-fakulta-studijni-program… 0.24% 162. https://www.universeofmemory.com/tag/active-learning 0.24% 163. https://www.universeofmemory.com/tag/language-learning 0.24% 164. https://docplayer.es/6089883-Comparacion-de-la-produccion-de-compuestos-aromaticos-por-cep… 0.24% 165. https://docplayer.nl/9140254-Bioactieve-componenten-in-functie-van-genetische-diversiteit-oogs… 0.24% 166. https://link.springer.com/article/10.1007%2Fs11947-012-0986-3 0.24% 167. https://www.universeofmemory.com/category/languages 0.24% 168. https://portal.nifa.usda.gov/web/crisprojectpages/0187352-developing-value-added-agricultural-c… 0.24% 169. http://www.chemhume.co.uk/AS%20AQA%20CHEM/Physical/1.5%20%20Kinetics.pdf 0.24% 170. http://www.centraldatacore.com/reference/education/curriculum-year-11 0.24%
171. http://www.moringa4africa.com 0.24%
173. https://www.studymode.com/course-notes/Effect-Of-Temperature-On-The-Rate-1920165.html 0.24% 174. https://www.slideshare.net/iosrjce/measuring-the-dynamics-of-financial-deepening-and-economic… 0.24% 175. http://www.geogsci.com/EN/10.1007/s11442-018-1532-7 0.24% 176. http://agresearch.tennessee.edu/publication/major_pubs.asp?who=5473 0.24%
177. https://patents.google.com/patent/EP1641913A2/en 0.24%
178. https://stemcellsjournals.onlinelibrary.wiley.com/doi/abs/10.1634/stemcells.2004-0021 0.24% 179. https://stemcellsjournals.onlinelibrary.wiley.com/doi/full/10.1634/stemcells.2004-0021 0.24% 180. https://peereffects.com/blog/dentist/the-phenotype-psychotype-and-genotype-of-bruxism 0.24%
181. http://www.mdpi.com/1660-4601/14/9/1081/pdf 0.24%
182. https://onlinelibrary.wiley.com/doi/full/10.1111/j.1365-313X.2008.03446.x 0.24% 183. https://www.cambridge.org/core/journals/animal-science/article/protein-levels-in-diets-for-europea… 0.24%
184. https://www.ingentaconnect.com/content/jws 0.24%
185. https://onlinelibrary.wiley.com/doi/full/10.1111/j.1750-3841.2009.01294.x 0.24% 186. https://www.studymode.com/essays/How-Does-The-Concentration-Of-The-476645.html 0.24% 187. https://onlinelibrary.wiley.com/doi/full/10.1002/ffj.2082 0.24%
188. https://pubs.acs.org/doi/abs/10.1021/jf101077s 0.24%
189. https://www.iprjb.org/journals/index.php/IJEPM/article/view/286 0.24% 190. https://www.acefitness.org/certifiednewsarticle/1644/the-truth-about-stevia-the-so-called-quot-he… 0.24% 191. https://www.semanticscholar.org/paper/Food-Value-of-Mealworm-Grown-on-Acrocomia-aculeata-… 0.24% 192. https://www.sciencedirect.com/science/article/pii/S0002937802849857 0.24% 193. http://scholarship.claremont.edu/cgi/viewcontent.cgi?article=1160&context=cmc_theses 0.24% 194. https://patents.google.com/patent/US20060233765A1/en 0.24% 195. https://www.sciencedirect.com/science/article/pii/S0747563215000552 0.24%
196. https://open.library.ubc.ca/handle/2429/43904 0.24%
197. https://www.slideserve.com/erv/reaction-rates-equilibrium 0.24%
Web omitted sources: 21 sources found
1. http://repository.unika.ac.id/15922 10.54%
2. http://repository.unika.ac.id/712/1/10.70.0102%20Monica%20Ariyani%20Santoso%20COVER.pdf 5.49%
3. https://core.ac.uk/download/pdf/35393563.pdf 5.49%
4. http://repository.unika.ac.id/9130/6/12.70.0046%20Vina%20Anyerina%20DAFTAR%20PUSTAKA.… 2.08%
5. https://link.springer.com/chapter/10.1007%2F978-1-4614-3458-0_3 1.75%
6. https://www.mdpi.com/2076-3921/7/4/53/htm 1.01%
7. https://tryl2012.blogspot.com/2018/06/betalains-in-some-species-of.html 1.01%
8. https://repository.maranatha.edu/3433/9/0810214_References.pdf 0.8%
9. http://tpa.fateta.unand.ac.id/index.php/JTPA/article/view/81 0.77%
10. http://repository.unika.ac.id/7343/7/11.70.0014%20%20BENEDICTA%20M.W.%20-%20DAFTAR… 0.77%
11. https://onlinelibrary.wiley.com/doi/10.1002/9781118653975.refs01 0.77%
12. https://onlinelibrary.wiley.com/doi/abs/10.1111/j.1365-2621.1983.tb10754.x 0.74%
13. http://repository.unika.ac.id/5842/7/11.70.0054%20Febby%20Ernita%20Santoso%20DAFTAR%2… 0.74%
14. http://www.mdpi.com/1420-3049/19/11/17985/htm 0.65%
15. https://www.chem.fsu.edu/chemlab/chm1046course/activation.html 0.65%
16. http://etd.repository.ugm.ac.id/index.php?mod=download&sub=DownloadFile&act=view&typ=htm… 0.62%
17. https://link.springer.com/article/10.1007%2Fs13197-012-0741-9 0.62%
19. http://www.chemistry.wustl.edu/~edudev/LabTutorials/CourseTutorials/Tutorials/AirQuality/AirQua… 0.62%
20. http://europepmc.org/articles/PMC4190260 0.59%
21. https://quizlet.com/106417078/chem-102-l-flash-cards 0.56%
Library omitted sources: 59 sources found
3rd ISC (PP-03).pdf 100%
3rd ISC (PP-04).pdf 6.56%
3rd ISC (FNT-01).pdf 5.64%
3rd ISC (FNT-01).pdf 5.64%
3rd ISC (FDP-04).pdf 5.43%
3rd ISC (FDP-04).pdf 5.43%
3rd ISC (PP-02).pdf 5.37%
3rd ISC (PP-02).pdf 5.37%
3rd ISC (FNT-04).pdf 5.08%
3rd ISC (FNT-03).pdf 5.08%
3rd ISC (FNT-03).pdf 5.08%
3rd ISC (FNT-04).pdf 5.08%
2nd ISC (PP-28).pdf 2.4%
2nd ISC (PP-28).pdf 2.4%
03. WFE I-1.pdf 2.14%
06. WFE I-4.pdf 2.14%
04. WFE I-2.pdf 2.14%
03. WFE I-1.pdf 2.14%
06. WFE I-4.pdf 2.14%
04. WFE I-2.pdf 2.14%
IRFAN-3 JULI.docx 2.14%
Irfan Aditya Setyadji 15.I1.0152.docx 2.14%
Skripsi_Plagscan Yosia.docx 0.45%
14.I1.0086_Raissa Tiffani Harrijanto.doc 0.42%
14.I1.0086_Raissa Tiffani Harrijantoo.doc 0.42%
Jurnal Skripsi Susi FIX.docx 0.39%
14.i1.0024-Florentina Sonia Hadipranoto.docx 0.36%
14.i1.0024-Florentina Sonia Hadipranoto (revisi1).docx 0.36%
2nd ICSAF 2016 (Hal 201) (1).pdf 0.33%
2nd ICSAF 2016 (Hal 201).pdf 0.33%
Melita14.I1.0097.docx 0.33%
14.I1.0091-Sara Novita.docx 0.33%
14.I1.0109_Omita Saras P.docx 0.33%
14.i1.0107_ANASTASIAJEANICE.docx 0.33%
Skripsi_Ade Putra Haryono_13.70.0036.docx 0.33%
Jurnal Pengaruh Pra Perlakuan terhadap Kualitas Kunyit.pdf 0.33%
13.70.0067-Andreas Setiabudi.docx 0.33%
13.70.0152- Josephine Indriana Kusumo rev.docx 0.33%
manuscript - Momordica charantia.doc 0.33%
13.70.0152- Josephine Indriana Kusumo rev1.docx 0.33%
19. Kumis kucing - KEL19.docx 0.3%
9. proposal Laksmi - 2.3.18.docx 0.3%
108134_108106_CLOVE LEAVES HERBAL PAPER (Yosafat).docx 0.3%
14.I1.0045-ONNY SHELVIA.docx 0.27%
Kintika-Taufiq Kurniawan.docx 0.24%
Food Bioprocess Technology Impact of Green Tea Extract.pdf 0.24%
Green Tea Catechins During Food Processing and Storage.pdf 0.24%
William Krisriandi 14i10094.docx 0.24%
02_67_Ranto.pdf 0.24%
Katherine Kristalia-KP-15 MEI.docx 0.24%
14.I1.0001-Marcellina-26 FEB.docx 0.24%
CALVIN-TUGAS TAPE-2 APRIL.doc 0.24%
PULUNG-26 MARET-FIX.docx 0.24%
PULUNG-26 MARET.docx 0.24%
16.I3.0003-LAVERNCHY JOVANSKA-22 MARET.docx 0.24%
Nindita Niartika-18 FEB.docx 0.24%
Laporan KP - Agnesia Nugroho - 15.I2.0008-19 DES.docx 0.24%
DEGRADATION KI
DEGRADATION KINETICS OF RED BE
NETICS OF RED BEET (
ET (
Beta vulgariss
Beta vulgari
)
)
POWDER DURING
POWDER DURING THERMAL PROCE
THERMAL PROCESSING AND CHANG
SSING AND CHANGES OF pH
ES OF pH
Monica Ariyani Santoso
Monica Ariyani Santoso , Ellena Widuriati Dewi1)1), Ellena Widuriati Dewi , Victoria Kristina 1)1), Victoria Kristina Ananingsih
Ananingsih2)2), Alberta Rika Pratiwi, Alberta Rika Pratiwi2) 2)
1)Students of Food Technology Department, Faculty of Agricultural Technology,
Soegijapranata Catholic University
2)
Lecturer of Food Technology Department, Faculty of Agricultural Technology, Soegijapranata Catholic University
ABSTRACT ABSTRACT
Red beet (Beta vulgaris) is a source of antioxidants and often used as a natural colorant in the food processing. Antioxidant content in beets is called betalain, which are classified into betacyanin which is purplish red pigment and betaxhantin which is the yellow orange pigment. Antioxidants are compounds that can delay or prevent the oxidation of free radicals such as rancidity and discoloration but are very susceptible to degradation. Free radicals are highly reactive molecules because it has unpaired electrons in the outer orbital of the molecule so that it can react with the cells of the body that can cause disease. Antioxidant activity degradation is determined by calculating the activation energy. The activation energy is the minimum energy required to perform a reaction. The purpose of this study was to determine the effect of pH, temperature and heating duration on antioxidant degradation of red beet powder and to determine the degradation kinetics by calculating the value of the activation energy based on the decrease in antioxidant activity with Arrhenius model. Preparation of red beet powder is conducted by drying red beet using cabinet dryer. Methods of research conducted with three levels, i.e. pH 6, 7 and 8; with four different temperatures, i.e. 40 C, 60 C, 80 C and 90 C; and at five heating rate, i.e. 0, 5, 10, 15 and, 20 o o o o minutes. The analysis performed in this study is the analysis of antioxidant activity in red beet powder using DPPH method. The results were calculated using the Arrhenius equation. The results showed that lowest decreasing antioxidant activity is at pH 6, temperature 40 C and 0 minute; is o equal to 25.40% and the highest decreasing antioxidant activity is at pH 8, temperature 80 C and o 20 minutes is equal to 49.76%. The highest activation energy (Ea) is pH 6, at 0 minute that equal to 7303.85 J/mol. While the lowest Ea is pH 8 at 20 minutes that equal to 4230.16 J/mol.
Keywords:
Keywords: red beet powder, antioxidants, activation energy, degradation kinetics
1.INTRODUCT
1.INTRODUCTION ION cause carcinogenic effects. To reduce the use
Red beet (Beta vulgaris) is purplish red tuber of synthetic food colorant, we can use natural
and often used as natural food colorant so the food colorant, i. e. red beet. The benefits of
product can improve consumer attraction. this colorant are safe use and also contain
Nowadays, synthetic food colorant already high of antioxidants. Antioxidants found in
widely circulated in the community. red beet called betalain. Betalain classified
However, it becomes dangerous if we into two pigment, betaxhantin which has
consumed it for a long period because it will orange and yellow color and betacyanin
3rd International Student Conference
―Greening The Food Industry : Innovation for Sustainibility‖
which has purplish red color (Pitalua et al.,
2010).
Besides betalain, antioxidants contained in
red beet namely polyphenols, vitamins,
flavonoids and folic acid. Antioxidants work
to prevent the oxidation reactions of free
beverage processing using heat treatment,
antioxidants found in red beets will be
degraded. Stability of betalain influenced by
light, oxygen, water activity, pH and
temperature (Gaztonyi et al., 2001).
Temperature affects the rate of reaction as a
factor affecting the decline of the food
product quality. Higher temperature will
caused reaction rate become faster so the
antioxidant activity became decrease.
Betalain stable at temperatures below 40oC and in acidic conditions with a pH range of
4-6 (Fennema, 1997)
This research use three different pH
condition; pH 6 (acidic), 7 (neutral), and 8
(alkaline). Selection of the pH to determine
antioxidant degradation of red beet, because
not all products are produced under
conditions of acidic beverages. Based on that
statement, the research is conducted to
determine the degradation kinetics of the
antioxidant activity in red beet powder with a
combination of pH, temperature and heating
duration. Besides that, the Arrhenius
approach is used to calculate the changes of
activation energy.
2.MATERIALS A
2.MATERIALS AND METHODSND METHODS
2.1.Chemicals and To 2.1.Chemicals and Tools. ols.
The materials used in this study are red beet
powder, methanol, distilled water, DPPH
solution, 10% HCl solution and 10% NaOH
solution. The tools used in this study are the
cabinet dryer, blender, slicer, pH meter, a
closed reaction tube, water bath,
thermocouple and spectrophotometer. The
data analyses are conducted with Microsoft
Excel 2010 and MatLab 2010a.
2.2.Sample Prepar 2.2.Sample Preparation. ation.
Red beet is dried with dryer cabinet to form a
powder blend. The powder is dissolved, and
then treated with 3 levels of pH (pH 6, pH 7,
and pH 8). NaOH solution is added to make
alkaline condition; meanwhile HCl solution is
added to make acidic condition of sample.
Next, the sample are placed in a reaction tube
and heated in a water bath. Each pH level is
heated with four level of temperature (40 C, o 60oC, 80 C, and 90 C) for 0, 5, 10, 15 and, o o 20 minutes. Heating temperature of 40oC betalain pigments to represent optimum
degree of stabilization of pigment; and the
temperature of 60 C, 80 C and 90 C are o o o
3rd International Student Conference
―Greening The Food Industry : Innovation for Sustainibility‖
represent pasteurization temperature.
Combination treatment performed three
replications. Determination of antioxidant
activity is conducted with DPPH (2,
2-diphenyl-1-picrylhydrazyl) method.
2.3.Antioxidant Act
2.3.Antioxidant Activity oivity of the Red Beet f the Red Beet
Powder (DPP
Powder (DPPH method). H method).
A (t) = 1+ − C= −1
Description:
A: antioxidant activity degradation A0: antioxidant activity degradation at t=0 Af: antioxidant activity degradation at t time r : constant
t: heating duration (min)
0.5 gram sample was weighed and extracted
with 5 ml of methanol for 2 hours in the dark
control, use 0, 1 ml of methanol were reacted
with 3.9 DPPH solution and methanol used
as a blank solution.
Antioxidant activity is expressed as %
inhibition with the following equation:
%inhibition= {(AB-A )/AA B} x 100%
Description:
AA = sample absorbance at 30 minutes
AB= control absorbance (methanol+DPPH)
(Williams et al., 1995)
2.3.Logistic Equat 2.3.Logistic Equation. ion.
The mathematics equation model(logistic
equation) is used to determine degreeof
antioxidantactivity degradationwith
equation:
(Wolf & Venus, 1992 in Garnier et al., 1999;
modification).
2.4.Antioxidant Degr
2.4.Antioxidant Degradation Kinadation Kinetics of etics of
Red Beet Powd Red Beet Powder. er.
The determination of the Ea value using
Arrhenius equation, the data obtained by
analysis of linear equations where Ea is the
activation energy, whose value is assumed to
be constant at a certain temperature range, R
is the gas constant (8.314 J/mol K), T is the
temperature expressed in Kelvin (K).
Arrhenius equation can be seen as follows:
ln k = ln k 0–Ea/RT
Where the rate constant k showed a decrease
in quality, k is a constant (independent of 0
temperature), Ea is the activation energy, T is
absolute temperature (K), R is the gas
constant (8.314 J/mol.K) (Arpah, 2003).
3.RESULT
3.RESULTS AND DISCS AND DISCUSSION USSION
3.1.Antioxidant
3.1.Antioxidant Activity Activity DegradationDegradation of of
Red Beet Powd Red Beet Powder er
3rd International Student Conference
―Greening The Food Industry : Innovation for Sustainibility‖
Table 1
Table 1 .Antioxidant Activity Degradation in Red Beet Powder with Variation of pH, Temperature,
and the Heating Duration
Temperature Antioxidant Activity Degradation (%)
pH Heating Duration
(oC)
0 minute 5 minutes 10 minutes 15 minutes 20 minutes
6 40 25.40±0.64 28.32±1,55 32.53±1.00 35.53±0.96 36.11±0,59
60 27.77±0.29 29.53±0.99 34.30±2.72 35.85±2.62 36.79±2.35
80 34.28±0.90 36.02±0.35 39.32±1.50 39.40±1.13 41.42±2.54
90 37.76±0.21 42.11±0.75 43.98±1.70 45.02±1.82 45.52±0,47
7 40 29.05±0.20 32.86±0.76 32.95±1.18 36.03±1.20 37.58±3.38
60 30.30±0.47 33.04±0.45 35.63±1.61 37.64±1.62 40.19±2.38
80 34.20±0.88 37.45±0.36 39.21±1.94 41.77±1,49 42.72±1.03
90 39.86±0.61 43.90±1.52 44.46±0.76 45.13±0.79 46.51±0.94
8 40 29.71±0.98 32.59±1.56 35.38±2.06 37.89±3.29 39.71±2.61
60 31.13±0.41 35.09±1.96 39.11±2.84 41.20±0.46 42.50±1.43
80 36.44±0.59 36.53±0.66 43.00±1.21 45.79±0.15 47.59±0.38
90 41.05±0.89 44.98±1.79 46.01±0.15 47.33± 0.99 49.76±2.77
Description:
All values are mean ± SD (Standard Deviation)
From Table 1. there was gradual increase
antioxidant activity degradation from red beet
powder at pH 6 in temperatures ranging from
40oC to 90 C also in the heating duration is o started from minute 0 to minute 20. At pH 7
and pH 8 also showed similar results,
Antioxidant activity degradation will increase
with higher heating temperature and longer
heating duration. The higher degradation is at
pH 8 with a temperature of 90 C in the 20 o minutes. Fennema (1997) said that red beet
has a pH optimum between pH 4 and pH 6.
Therefore the antioxidant activity
degradation of red beet at pH 6 is lower than
at pH 7 and 8 because in pH 6 the sample is
at stable pH conditions. Acidic conditions
can increase antioxidant activity stability
because it can prevent oxidation so as to
minimize the damage of betalain pigments
(Ravichandran et al., 2013). Temperature of
40oC is the optimal temperature for the pigment stability so the damage or the
degradation is still relatively lower than the
higher temperature (Roy et al., 2004).
Heating causes degradation in the form of
betalain pigment degradation. This pigment
is converted into betalamic acid and
cycloDOPA 5-O-glycoside. The longer the
heating process, the pigment betalain
3rd International Student Conference
―Greening The Food Industry : Innovation for Sustainibility‖
breakdown process will be greater
(Delgado-Vargas et al., 2000).
(a)
(b)
(c)
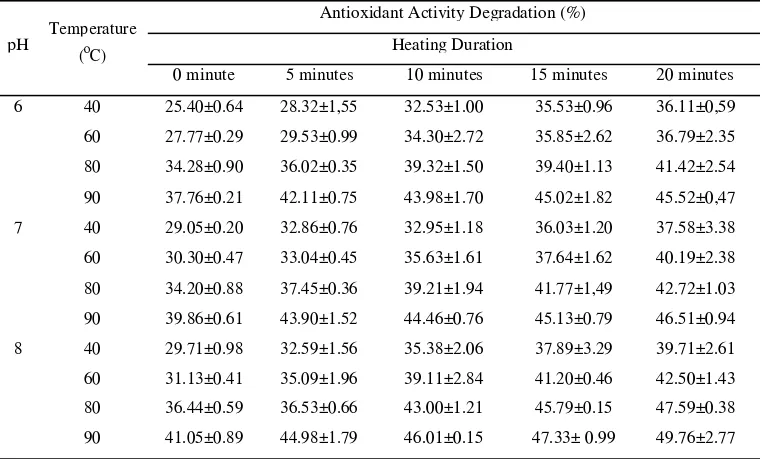
Figure
Figure 11. Decrease in antioxidant activity of
red beet powder on (a) pH 6, (b) pH 7, (c) pH
8.
In Figure 1. shows that the higher pH will
caused higher degradation of antioxidant
activity of red beet powder. On the research
from Stintzing and Carle (2004), it said that
pH is a factor determines the stability of
antioxidant activity. In addition to pH,
temperature and heating duration can also
cause antioxidant activity of red beet become
unstable. Temperature affects the stability of
the antioxidants in red beets. In theory of
Stintzing and Carle (2004), betalain stability
is affected by temperature, pH, moisture,
light and oxygen. At high temperature,
antioxidants in red beet powder will be
damaged.
Logistic model Logistic model
(a)
(b)
(c)
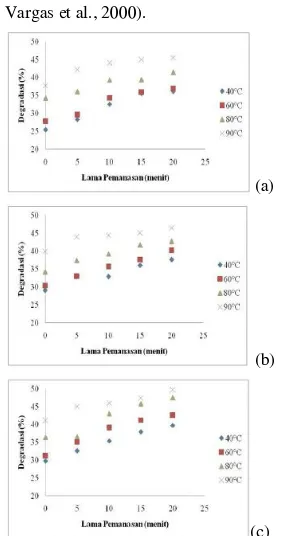
Figure 2.
Figure 2. Decrease antioxidant activity of red
beet powder to the logistic equation on (a)
pH 6, (b) pH 7, (c) pH 8
In Figure 2. we can be see that the antioxidant
activity degradation will increased with the longer heating duration. Highest antioxidant
activity degradation is on 90 C at 20 minutes o and the lowest antioxidant activity
degradation is on 40oC at
3rd International Student Conference
―GreeningThe Food Industry : Innovation for Sustainibility‖
5 minutes.
Table 2
Table 2 .Correlation Coefficient of Linear
Equations in Red Beet Powder
Temperature
Table 3 .Correlation Coefficient of
Logistic Equations in Red Beet Powder.
Temperature
Temperature pH 6pH 6pH 7pH 7 pH 8 pH 8
According to D'Amico et al. (2006), the
temperature and time of pasteurization for red
beet powder is 60 C for 18 minutes; while o according Klewicka & Czyzowska (2011)
using temperature 80 C for 10 minutes and o
90oC for 5 min. When the pasteurization temperature and time applied in the logistic
equation for each pH, the value of %
antioxidant activity degradation of red beet
powder can be seen in Table 4. From Table 4 it can be seen that the pasteurization time and
temperature are inversely related. The higher
the heating temperature, the shorter the time
the higher correlation coefficient, the
relationship between x and y will be higher
too (Labuza, 1978). Based on these results it
can be concluded that the antioxidant activity
degradation of red beet powder tends to use
the principle of logistic equation, where the
initial heating of antioxidant degradation
begins to rise higher and then increases the
longer the increase be fixed at some point.
Timuneno (2008) states that the logistic
equation is showed that at a certain point
decrease in antioxidant activity reaches
equilibrium, so that the graph will be a
Temperature/duration % degradation
of pasteurization pH 6 pH 7 pH 8
3.2.Antioxidant Degradation Degradation Kinetics Kinetics of of
Red Beet Powd Red Beet Powder er
Degradation kinetics of the antioxidant of red
beet powder was analyzed using the
Arrhenius equation. The trick is plotting ln k
(%antioxidants degradation) with 1/T
(temperature heating) for each heating time
and pH conditions in order to obtain a linear
equation. From this linear equation obtained
value gradient/slope equal to the activation
energy that divided with the gas constant
3rd International Student Conference
―Greening The Food Industry : Innovation for Sustainibility‖
values (Table 5).
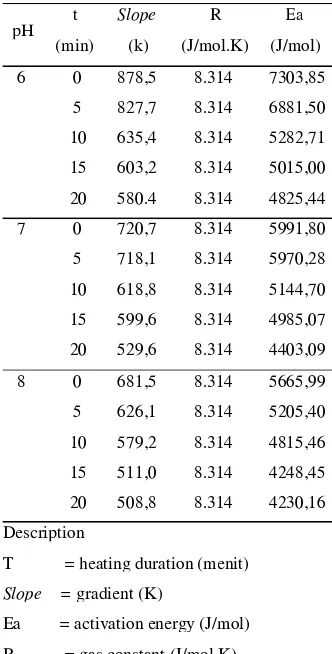
Table 5.
Table 5. Activation Energy Values at Each
pH
T = heating duration (menit)
Slope = gradient (K)
Ea = activation energy (J/mol)
R = gas constant (J/mol.K)
From the Table 5 it can be seen that the
activation energy of each sample is
proportional to the value of degradation
quality constant. The highest activation
energy is red beet powder at pH 6 were
heated for 0 minutes; while the lowest
activation energy is red beet extract at pH 8
were heated for 20 minutes. According
Azeredo (2009), degradation of the pigment
betalain will caused the value of the
activation energy decreases. This means that
the greater the degree of degradation of
betalain pigments, the value of the activation
energy decreases.
The activation energy the minimum amount
of energy supplied to a reaction (Hariyadi,
2004). From the theory of Robertson (1993),
states that the smaller the activation energy,
the faster the product will be severely
degrades. To calculate the activation energy,
make a graph of ln k with 1/T, can be seen in
Figure 3. it can be seen that the obtained
linear equation y=mx+b. The slope of the
equation above represents the value (-Ea/R)
with constant gas of 8,314 J/mol.K. In the
linear equation y=-878,5x+1.433 (R =0.995) 2 obtained Ea value of 7303.85 J/mol.
CONCLUSION CONCLUSION
•Decrease in the antioxidant activity of
extracts of red beet will increase when
the temperature and heating time
increased. Decrease in antioxidant
activity in red beet powder will increase
when conditioned in neutral and alkaline
pH.
•The best temperature of pasteurization is
at a temperature of 60 C while the o pasteurization temperature which causes a decrease in antioxidant activity was
highest in the temperature of 90oC. •The higher the temperature of
3rd International Student Conference
―Greening The Food Industry : Innovation for Sustainibility‖
pasteurization, the heating duration will
be lower, stable antioxidant activity
carried out at a temperature of 60 C at o 18 minutes pasteurization, pasteurization
is done at a temperature of 80 C at 10 o minutes and at a temperature of 90 C at o 5 minutes.
REFERENC REFERENCES ES
Arpah.(2003).PenentuanKadaluarsa
Pangan. Buku 2: Penuntut Praktikum. Institut Pertanian Bogor. Bogor.
Azeredo, H. M. C. (2009).Betalains:
Properties, Sources, Applications, and Stability – A Review.International
Journal of FoodScienceand Technology 44: 2365 2376. –
D'Amico, D. J.; T. M. Silk; J. Wu; & M. Guo. (2006). Inactivation of
Microorganisms in Milk and Apple Cider Treated with Ultrasound. J. Food Prot 69: 556-563.
Delgado-Vargas, F. & O.Paredes-López.
(2003). Natural Colorants for Food and Nutraceutical Uses. CRC Press. Boca Raton.
Fennema, O. R. (1997). Food Chemistry: SecondEdition,Revisedand
Expanded. Marcel Dekker, Inc. New York.
Gasztonyi, M. N.; H. Dadood; M. Takacs & P.Biacs. (2001). Comparison of Red
Beet (Beta vulgaris var conditiva) Varietas on the Basic of Their Pigment Components. Journal of the Science of Food and Agriculture. 81(9): 932-933. Hariyadi, P. (2004). Prinsip Penetapan dan
Pendugaan Masa Kadaluarsa dan Upaya-Upaya Memperpanjang Masa Simpan. Institut Pertanian Bogor. Bandung.
Klewicka, E. & A. Czyżowska. (2011). Biological Stability of Lactofermented Beetroot Juice During Refrigerated Storage. Pol. J. Food Nutr. Sci. 4:251-256.
Labuza, T. P. (1982). Shelf Life Dating of Foods. Food and Nutrition Press Inc.
Connecticut.
Pitalua E; M. Jimenez; E. J. Vernon-Carter; C.I. Beristain. (2010). Antioxidative
Activity of Microcapsules with Beetroot Juice Using Gum Arabic As Wall Material. J. Food And Bioproducts Processing 88: 253-258
Ravichandran, K.; N. M. M. T. Saw; A. A. A. Mohdaly; A. M. M. Gabr; A. Kastell; H. Riedel; Z. Cai; D. Knorr; & I.
Smetanska. (2013). Impact of Processing of Reed Beet on Betalain Content and Antioxidant Activity. Journal of Food Research International 50:670-675
Robertson, G. L. (1993). Food Packaging: Principles and Practice. Marcel Dekker
Inc. New York.
Roy, K.; S. Gullapalli; U. R. Chaudhuri & R. Chakraborthy. (2004). The Use of a Natural Colorant Based on Betalain in the Manufacture of Sweet Products in India. Journal of Food science and Technology 39: 1087 1091 –
Stintzing,F. C. & R. Carle. (2004). Functional Properties of Anthocyanins and Betalains in Plants, Food, and in Human Nutrition. Trends in Food Science and Technology 15: 19 38 –
Timuneno,H. M.; R. H. S. Utomo; & Widowati. (2008). Model Pertumbuhan Logistik dengan Waktu Tunda . Jurnal Matematika 11: 43-51
Williams,W. B.; M. E. Cuveller; & C.
Berset. (1995) Use of a Free Radical Method to Evaluate Antioxidant Activity. Lebensm-wiss Vol. 28. pp.25-30.
3rd International Student Conference
―Greening The Food Industry : Innovation for Sustainibility‖
Windono, T.; S. Soediman; U. Yudawati; E. Ermawati; Srielita; T. I.Erowati.
(2001). Uji Peredam Radik Bebas al terhadap 1, 1-Diphenyl-2-
Picrylhydrazyl (DPPH) dari Ekstrak Kulit Buah dan BijiAnggur (Vitis vinifera L.) Probolinggo Biru dan Bali. Artocarpus 1: 34-43
Wolf, K. H. & J. Venus. (1992). Description of the Delayed Microbial Growth by an Extend Logistic Equation.Dalam
Garnier, P. M.; R. Auria; C. Augur; & S.Revah. (1999). Cometabolic
Biodegradation of Methyl T-Butyl Ether by Pseudomonas aeruginosa Grown on Pentane. Appl Microbiol Biotechnol 51: 498-503
3rd International Student Conference
―Greening The Food Industry : Innovation for Sustainibility‖