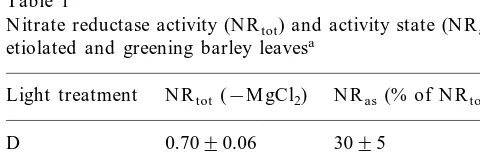Phytochrome and post-translational regulation of nitrate reductase
in higher plants
K laus-J. Appenroth
a,*, R ezarta M ec¸o
a, Vincent Jourdan
b, Cathrine Lillo
baDepartment of Plant Physiology, Uni6ersity of Jena, Dornburger S traße 159, D -07743Jena, Germany bS ta6anger College, Box 2557Ullandhaug,4091S ta6anger, N orway
R eceived 1 M arch 2000; received in revised form 9 M ay 2000; accepted 14 June 2000
Abstract
The possible influence of phytochrome on the activity state of nitrate reductase (N R ) was investigated in etiolated plants where the expression of the N R gene is known to be under the control of phytochrome. Activity state is defined as N R activity assayed in the presence of M g2+as percentage of N R activity measured in the absence of M g2+. This measurement is assumed to reflect
non-phosphorylated N R as percentage of total N R . Beside etiolated barley and maize leaves, a photosynthetic mutant of L emna
aequinoctialis was investigated and compared with the wild type. The increase of N R activity following a red light pulse, mediated
via phytochrome, was confirmed in all etiolated plant species investigated as well as in both strains of L . aequinoctialis cultivated in glucose-containing medium. The effect of continuous red light surpassed the effect of a single red light pulse in each case. H owever, the results did not show any stimulating effect of phytochrome on the activity state caused by post-translational modulation. The activity state was strongly increased by continuous red light in the wild type of L . aequinoctialis but not in the photosynthetic mutant. These results show that the phytochrome system is not important for the post-translational regulation of N R . © 2000 Elsevier Science Ireland Ltd. All rights reserved.
Keywords:Barley; L emna aequinoctialis; M aize; N itrate reductase; Phytochrome 14-3-3
www.elsevier.com/locate/plantsci
1. Introduction
N itrate reductase (N R ) (EC 1.6.6.1) is subjected to complex regulation on the transcriptional, translational and post-translational levels [1]. Both photosynthetically active light and light acting through phytochrome are known to influence the N R expression [2 – 5]. Phytochrome-mediated ef-fects of light on N R are evident in etiolated leaves. Pulses of red light are known to induce an increase in N R activity that is caused by higher N R mR N A levels and by an increased concentration of N R protein. This has been interpreted in terms
of phytochrome involvement in the regulation of gene expression of N R in etiolated higher plants [6 – 13]. Stimulating effects of light on N R expres-sion can also be observed in green tissues. In a previous study, the regulation of N R in etiolated turions was compared with that in green vegeta-tive fronds of the duckweed S pirodela polyrhiza [10]. It has been shown that in the green fronds the role of photoreceptor has mainly been overtaken by chlorophyll (acting via photosynthesis), whereas phytochromes play only a marginal role. In etiolated turions, however, N R content and activity are regulated by the ordinary low fluence response of phytochrome [7,8]. In all other plants except S pirodela and barley [6], the regulation of N R by phytochrome has been studied only in etiolated or non-photosynthetic tissue (e.g. roots, [11]). R egulation of N R by light was also investi-gated in the phytochrome-deficient aurea mutant
A bbre6iations: cR , continuous red light; D M O, 5,5-dimethyl-2,4
oxazolidinedione; N R , nitrate reductase; N Ras, activity state of N R ;
N Rtot, total N R activity; R p, red light pulse.
D edicated to the 70th birthday of Prof. H ans M ohr
* Corresponding author. Tel.: +49-3641-949233; fax: + 49-3641-949232.
E -mail address: [email protected] (K .-J. Appenroth).
and wild strain of tomato [13,14]. In etiolated tomato tissue, the regulation of N R by phy-tochrome is quite obvious. The results in green tomato tissue, however, do not sort out the pho-toreceptor involved. The shared action of photore-ceptors in etiolated and green plants of S .
polyrhiza [10] may represent a general rule.
D uring the past decade, the post-translational regulation of N R has been intensively studied. As a result, two steps have been established to take part in the regulation of N R : phosphoryla-tion/dephosphorylation and binding of inhibiting proteins [15 – 18]. Several groups have shown that (the small) changes in N R concentration cannot account for the variations in N R activity observed during approximately 30 min following a light/
dark or dark/light shift [19 – 22]. The involvement of phosphorylation and inhibiting proteins can easily be confirmed by assaying N R activity under selective assay conditions: In the presence of M g2+ and inhibiting proteins, the activity of
the phosphorylated form of N R is low or not detectable, whereas ethylenediamine tetraacetic acid (ED TA) leads to a rapid activation of the enzyme. N R assayed in the presence of ED TA (N Rtot) is proportional to the N R steady-state
protein concentration regardless of the effective activity under in vivo conditions. The assay in the presence of M g2+ gives an estimation of the
in-situ N R activity [23]. Comparison of N R activities in assays with and without M g2+ allows one to
estimate the so-called N R activity state (N Ras),
which is defined as the part of N R not affected by inhibiting proteins (i.e. non-phosphorylated N R (N Rnp)) expressed as the percentage of N Rtot
(N Rnp+phosphorylated N R ), i.e. N Ras=100×
N Rnp/N Rtot. It is well documented that the
post-translational regulation of N R activity depends on light, and that the N Rasin green tissue is regulated
by photosynthesis. The N Ras is high under
photo-synthetic conditions and decreases under non-photosynthetic conditions, e.g. in darkness, in the presence of photosynthetic inhibitors or under low CO2 pressure [24]. This suggests that, in a green
tissue, the influence of phytochrome on the N Ras
is either small or not detectable. H owever, a possi-ble role of phytochrome as the photoreceptor con-trolling N Ras in non-photosynthetic tissue (similar
to the regulation of N Rtot) has not been tested.
Therefore, we examined if N Ras, reflecting the
post-translational regulation of N R , is influenced by light absorbed by the phytochrome system in several etiolated higher plant tissues.
2. Materials and methods
2.1. L ight treatments and ex traction of N R
2.1.1. Etiolated lea6es of barley
Barley seeds (H ordeum 6ulgare cv. K innan) were sown in vermiculite at 20°C in darkness and wa-tered with H oagland solution containing 15 mM K N O3 [25]. Six days after sowing, the seedlings
were subjected to different light treatments: pulses of red light (2 mmol m−2s−1) for 5 min at 0, 4, 8,
12, 16 and 20 h, or continuous white light (50
mmol m−2 s−1). Leaves were harvested 24 h after
the start of the treatments. Leaves (1 g) were ground in a mortar with 4 ml of 50 mM H epes – K OH (pH 7.5), 5 mM cysteine, 1 mM ED TA, 7.5
mM leupeptin, 0.1 mM phenylmethylsulfonyl
fluoride, 1 mM F AD and 1 mM N a2M oO4. The
extract was used immediately to assay the N R activity in the presence (6 mM ) or absence of M gCl2.
F or acid loading (see Section 3.1), barley leaves were cut into 2 mm pieces and vacuum infiltrated in 50 mM M ops (pH 6.5), 10 mM 5,5-dimethyl-2,4 oxazolidinedione (D M O) for 2×20 s (to get D M O into the tissue), then flushed with air for 15 s to avoid anaerobic conditions, and left in dark-ness for 30 min before the extraction and the assay of N R .
2.1.2. Etiolated lea6es of maize
Seeds of maize (Z ea mays cv. agio, Van der H ave) were soaked in distilled water for 24 h and grown on moist filter paper for 9 days at 18.09 0.1°C in darkness [26]. Etiolated, primary leaves were excised and floated on distilled water. The sample was irradiated with a red light pulse (R p) of 3 min, 500mmol m−2s−1, continuous red (cR )
of 12 mmol m−2 s−1 or white light of 50 mmol
m−2 s−1. The leaves were kept for 2 h on water
and then transferred to K N O3 solution (60 mM )
mM ED TA-N a2 and 3 mM cysteine [27]. The
homogenate was divided into two parts and one part was supplemented with M gCl2 to a final
concentration of 6 mM . F ollowing centrifugation (3 min, 15 800×g), the supernatant was used for
the N R assay.
2.1.3. L emna aequinoctialis
L emna aequinoctialis, strain 6746, a mutant not
performing photosynthesis (see R ef. [28]), was cul-tivated in a carbohydrate containing medium: 50 mM glucose was added to the nutrient medium described elsewhere [29]. The plants, cultivated in white light under axenic conditions, were dark-adapted for 72 h and kept in complete darkness or in cR (12 mmol m−2 s−1) for a further 48 h, or
they were transferred to the darkness after a R p (3 min, 500 mmol m−2 s−1) before enzyme activity
was assayed. Pilot experiments had shown that this time was required to obtain the maximum effect of light on the enzyme activity. The results were compared with those obtained for the wild type of
L . aequinoctialis, strain 1073, which was cultivated
and treated in the same way as the mutant. One hour before the enzyme extraction, N aH CO3 (10
mM ) was added as a source of CO2necessary for
assimilation. N R extraction was carried out as described previously [10], except that protease in-hibitors 0.02 mM leupeptin, 1 mM PM SF , and 0.2 mM pefabloc (M erck, D armstadt, G ermany) were
added. In experiments without glucose, plants were kept for 48 h in inorganic solution before enzyme activity was assayed. 3-(3,4-D ichlorophenyl)-1,1-dimethylurea (D CM U ) (final concentration, 10
mM ) was dissolved in acetone and applied together
with 0.5% (vol./vol.) solvent immediately before the onset of light treatment.
2.2. A ssay of N R
N R was assayed as described previously in the presence or absence of 6 mM M gCl2, and the rate
of nitrite formation was determined [10]. Total nitrate reductase activity (N Rtot) represents the
N R activity in the absence of M g2+. Activity
states (N Ras) were calculated as N R activity
mea-sured in the presence of M g2+ as percentage of the
N R activity measured in the absence of M g2+
(N Rtot).
3. Results and discussion
3.1. Etiolated barley lea6es
Etiolated barley seedlings were treated with R p according to a previously optimized scheme known to enhance the N Rtot activity [25]. In the work
presented in this paper, we additionally tested the N R activity in the presence of M g2+ to determine
the N Ras. The low N Ras in etiolated seedlings
suggested that most of the N R was present in its phosphorylated form and inhibited by 14-3-3 proteins. The experiments confirmed that R p influ-enced the N Rtotactivity (cf. [6,25]). In contrast, the
N Ras was not influenced (Table 1); i.e. following
the red pulse, there was an insignificant increase in the percentage of the active form of the enzyme. It was previously shown for protoplasts of green barley leaves [30] that weak acids that penetrated the tissue activated N R . Acid loading (treatment with D M O) of the leaves in continuous darkness for 30 min induced the high N Ras (Table 1).
Therefore, etiolated barley leaves did have the potential for the activation of N R , i.e. phos-phatases involved in the activation of N R ap-peared to be present. Lack of phytochrome effects on N Ras is thus not caused by absence of these
activating factors, but phytochrome does not influ-ence the balance of activating and inactivating factors like, for instance, pH .
Table 1
N itrate reductase activity (N Rtot) and activity state (N Ras) in
etiolated and greening barley leavesa
N Ras(% of N Rtot)
N Rtot (−M gCl2)
Light treatment
D 0.7090.06 3095 3895 1.3490.11
R p
D , D M O 0.7890.04 7998 cW 2.6990.11 7895
aCaryopses were germinated in darkness (D ) for 6 days,
and then treated either with red light pulses (R p) or continu-ous white light (cW), or kept in darkness. Samples were tested for N R activity in the presence of 6 mM M gCl2 or without
M gCl2in the assay mixture. Etiolated leaves were also treated
with 10 mM D M O to test the capacity of the N R -activating system. The presented enzyme activities are given as nmol s−1
(g fresh weight)−19S.E. The activity states is calculated as
percentage of the N R activity measured in the presence of M g2+of the N R activity measured in the absence of M g2+
(N Rtot). All values are averages of three independent
Table 2
N itrate reductase activity (N Rtot) and activity state (N Ras) in
etiolated and greening leaves of maizea
N Ras(% of N Rtot)
N Rtot (−M gCl2)
Light treatment
D 0.2890.01 5692 4493 R p 0.5490.01
4893 0.4290.02
R p–F R p
8692 cR 1.1390.06
aSeeds were germinated for 9 days in darkness. Primary
leaves were excised and either kept in complete darkness or transferred to continuous red light. Alternatively, light pulses were given and leaves were kept thereafter in darkness. F or further explanations, see Table 1. D ata presented are means of six independent experiments. F R p, far red light pulse.
not increase the N Ras. On the contrary, there
seemed to be a decrease in the percentage of the unblocked, active enzyme. This decrease, however significant, was not very pronounced. It can be concluded that phytochrome does not influence the 14-3-3 inhibition in etiolated maize leaves.
A mechanism of phytochrome-mediated, post-translational modification of N R has been pro-posed previously in etiolated maize leaves [31]. This mechanism, however, is apparently different from the mechanism of the 14-3-3 inhibition and up to now unknown in other plant species.
3.3. Fronds of the duckweed L . aequinoctialis
In the wild type of L . aequinoctialis, strain 1073, N Rtot was increased both by a single light pulse
and, to a larger extent, by cR (Table 3). This effect of cR was completely prevented by the application of the photosynthesis inhibitor, D CM U during the light treatment. The effect of a single R p, however, was not influenced by the same concentration of D CM U (data not shown). This demonstrates the importance of photosynthesis for the regulation of N Rtot in green tissue as shown for the closely
related species S . polyrhiza [10]. The N Rasin
dark-ness was high in the presence of glucose in the nutrient solution. Previous experiments with bar-ley leaves have demonstrated that the N Ras is
increased by short-term (30 min) treatments with glucose or sucrose [24]. This indicates that the N Ras is increased by carbohydrate treatment, as it
is known for the increase of N Rtot in A rabidopsis thaliana [32]. The results presented in this paper
show a stimulating effect of the long-term treat-When continuous, photosynthetically active
light was given for 24 h, the N Rtot activity
in-creased by a factor of 4. Concomitantly, the N Ras
in this de-etiolating tissue increased from 30 to 78%. This confirmed the previous observation that photosynthesis favours both high N Rtot and high
N Ras [30].
3.2. M aize lea6es
The influence of phytochrome on N Rtot in
etio-lated maize leaves is demonstrated in Table 2. These results are in agreement with those pub-lished previously for etiolated maize leaves [9,26,27] and roots [11]. The existence of the 14-3-3 inhibition was confirmed by the data presented in Table 2. The N Ras increased in cR that, most
probably, was caused by the effect of photosynthe-sis in the de-etiolating excised leaves. Application of R p, or R p followed by far red light pulse, did
Table 3
N itrate reductase activity (N Rtot) and activity state (N Ras) in green, vegetative fronds of L . aequinoctialis were investigated a
Photosynthetic mutant Wild type
Light treatment
N Rtot (−M g) N Ras(% of N Rtot) N Rtot (−M g) N Ras(% of N Rtot)
7896 7194
D 0.2090.01 0.1790.02
n.d. n.d.
3993 D−glucose 0.01190.001
0.4490.04 6795 0.2890.03 6399 R p
0.5690.04 9297
cR 0.5190.03 7696
7393 0.4990.02
6793 0.1990.01
cR+D CM U
aThe influence of light treatment on the wild type (strain 1073) was compared with the photosynthetic mutant (strain 6746).
The fronds were cultivated in the glucose-containing medium (50 mM ) or kept for 48 h in glucose-free medium (−glucose). F ollowing application of light pulses, the plants were kept in darkness, or transferred to continuous red light. Control plants were kept in complete darkness. D CM U (10 mM ) was applied during the light treatment. n.d., not determined. F or further
ment with glucose (50 mM ) on the N Ras in L . aequinoctialis (Table 3). N Ras was unaffected by
the single R p, but was increased to more than 90% in cR . This demonstrates that light may be effec-tive in increasing the N Ras, even in the presence of
glucose.
L . aequinoctialis, strain 6746, was used for the
present investigation, because this strain represents a photosynthetic mutant. The R ieske protein does not accumulate and, as a consequence, the cyt – b6f
complex is not formed [28]. This photosynthetic mutant offers the opportunity to investigate the regulation of nitrate reductase in green tissue in light without the contribution of photosynthesis. N Rtot was stimulated with a single R p. This
stimu-lation was somewhat smaller than in the wild type for reasons that are not clear. Surprisingly, N Rtot
was more strongly enhanced in cR compared with a single R p. In contrast to the wild type, however, the photosynthesis inhibitor, D CM U had no effect on N Rtot in the mutant. This result confirms that
photosynthesis is not involved in the increase of N Rtot in cR in the photosynthesis mutant of L . aequinoctialis. We suggest that the increase of
N Rtot in the mutant by cR is mediated by the high
irradiance response of phytochrome. This would mean that the action of phytochrome in the green tissue of the wild strain is blocked by an up to now unknown mechanism and this is in agreement with our previous conclusion that N Rtot in green
tissue is largely regulated by photosynthesis [10]. By application of R p, the question was ad-dressed whether the N Ras could be increased by
light treatment of the mutant mediated via phy-tochrome (Table 3). Although N Rtot was increased
65% by a single R p, the N Ras was not influenced
by the same light treatment. The N Ras in the
photosynthetic mutant was also unaffected by cR irradiation, in contrast to the response of the wild type. These results exclude a possible influence of phytochrome on the N Ras.
3.4. Etiolated white mustard, turions and green
fronds of the great duckweed
Other higher plants (S inapis alba, S . polyrhiza) were also tested according to previously estab-lished schemes [2,7,8,10], and effects of phy-tochrome on N Rtot in etiolated plants were
confirmed. H owever, generally, only very small effects on N Ras were observed by light acting
through the phytochrome system (data not presented).
4. General conclusions
In etiolated tissue, N Rtot (reflecting total N R
protein) was increased following single R p and/or cR in all species tested. This is consistent with the concept of phytochrome regulation of N R activity. In contrast, N Ras was not much influenced by the
red light pulse treatment. A very small increase was observed for barley (Table 1) and, in other species, even small decreases were observed (Ta-bles 2 and 3). In conclusion, these experiments do not support the involvement of the phytochrome system in the post-translational regulation of N R by phosphorylation and by the 14-3-3 binding in etiolated plants.
Acknowledgements
The technical assistance of G . Lenk and the experimental support by C. D ietel, U niversity of Jena, are gratefully acknowledged. We thank D ozent D r H . G abrys, U niversity of K rakow, and Prof. R . Oelmu¨ller, U niversity of Jena, for sup-porting this research.
References
[1] W.H . Campbell, N itrate reductase structure, function and regulation: bridging the gap between biochemistry and physiology, Annu. R ev. Plant Physiol. Plant M ol. Biol. 50 (1999) 277 – 303.
[2] H . M ohr, A. N eininger, B. Seith, Control of nitrate reductase and nitrite reductase gene expression by light, nitrate and a plastidic factor, Bot. Acta 105 (1992) 81 – 89.
[3] N .M . Crawford, H .N . Arst Jr, The molecular genetics of nitrate assimilation in fungi and plants, Annu. R ev. G enet. 27 (1993) 115 – 146.
[4] S.C. H uber, M . Bachmann, J.L. H uber, Post-transla-tional regulation of nitrate reductase activity: a role for Ca2+ and 14-3-3 proteins, Trends Plant Sci. 1 (1996)
432 – 438.
[5] C. M eyer, M . Caboche, M anipulation of nitrogen metabolism, in: K . Lindsey (Ed.), Transgenic Plant R e-search, H arwood Academic, Amsterdam, The N ether-lands, 1998, pp. 125 – 133.
[7] K .-J. Appenroth, H . Augsten, H . M ohr, Photophysiology of turion germination in S pirodela polyrhiza (L.) Schlei-den. X. R ole of nitrate in the phytochrome-mediated response, Plant Cell Environ. 15 (1992) 743 – 748. [8] K .-J. Appenroth, R . Oelmu¨ller, C. Schuster, H . M ohr,
R egulation of transcript level and synthesis of nitrate reductase by phytochrome and nitrate in turions of
S pirodela polyrhiza (L.) Schleiden, Planta 188 (1992)
587 – 593.
[9] N . R aghuram, M .R . Chandok, S.K . Sopory, Light regu-lation of nitrate reductase gene expression in maize involves a G -protein, M ol. Cell Biol. R es. Commun. 2 (1999) 86 – 90.
[10] C. Lillo, F . Provan, K .-J. Appenroth, Photoreceptors involved in the regulation of nitrate reductase in S pirodela
polyrhiza, J. Photochem. Photobiol. B: Biol. 44 (1998)
205 – 210.
[11] H . Augsten, D . M ichel, Effects of irradiation with light of different wavelengths on nitrate reductase activity in roots of Z ea mays L., Z. Pflanzenphysiol. 102 (1981) 1 – 10. [12] K . Venkateshwar, R . Sharm, R etention of photoinduction of cytosolic enzymes in aurea mutant of tomato (L
ycop-ersicon esculentum ), Plant Physiol. 105 (1994) 643 – 650.
[13] T.W. Becker, C. F oyer, M . Caboche, Light-regulated expression of the nitrate-reductase and nitrite-reductase genes in tomato and in the phytochrome-deficient mutant of tomato, Planta 188 (1992) 39 – 47.
[14] A. M igge, G . M eya, E. Carryol, B. H irel, T.W. Becker, Coaction of light and the nitrogen substrate in controlling the expression of the tomato genes encoding nitrite reductase and nitrate reductase, J. Plant Physiol. 151 (1997) 151 – 158.
[15] W.M . K aiser, E. Brendle-Behnisch, R apid modulation of spinach leaf nitrate reductase activity by photosynthesis. I. M odulation in vivo by CO2availability, Plant Physiol.
96 (1991) 363 – 367.
[16] M . Bachmann, J.L. H uber, G .S. Athwal, K . Wu, R .J. F erl, S.C. H uber, 14-3-3 proteins associate with the regulatory phosphorylation site of spinach leaf nitrate reductase in an isoform-specific manner and reduce dephosphorylation of Ser-543 by endogenous protein phosphatases, F EBS Lett. 398 (1996) 26 – 30.
[17] G . M oorhead, P. D ouglas, N . M orrice, M . Scarabel, A. Aitken, C. M acK intosh, Phosphorylated nitrate reductase from spinach leaves is inhibited by 14-3-3 proteins and activated by fusicoccin, Curr. Biol. 6 (1996) 1104 – 1113. [18] E. Pigaglio, N . D urand, C. M eyer, A conserved acidic motif in the N -terminal domain of nitrate reductase is necessary for the inactivation of the enzyme in the dark by phosphorylation and 14-3-3 binding, Plant Physiol. 119 (1999) 219 – 229.
[19] J.L. R emmler, W.H . Campbell, R egulation of corn leaf nitrate reductase. II. Synthesis and turnover of the en-zymes activity and protein, Plant Physiol. 80 (1986) 442 – 447.
[20] C. Lillo, D iurnal variations of corn leaf nitrate reduc-tase: an experimental distinction between transcriptional and post-translational control, Plant Sci. 73 (1991) 149 – 154.
[21] M . K ojima, S.-J. Wu, H . F ukui, T. Sugimoto, T. N an-mori, Y. Oji, Phosphorylation/dephosphorylation of K o-matsuna (Brassica campestris) leaf nitrate reductase in vivo and in vitro in response to environmental light conditions: effects of protein kinase and protein phos-phatase inhibitors, Physiol. Plant. 93 (1995) 139 – 145. [22] W.-R . Scheible, A. G onzalez-F ontes, R . M orcuende, M .
Lauerer, M . G eiger, J. G laab, A. G ojon, E.-D . Schulze, M . Stitt, Tobacco mutants with a decreased number of functional nia genes compensate by modifying the diur-nal regulation of transcription post-translatiodiur-nal modifi-cation and turnover of nitrate reductase, Planta 203 (1997) 304 – 319.
[23] W.M . K aiser, S.C. H uber, Correlation between apparent activation state of nitrate reductase (N R ), N R hysteresis and degradation of N R protein, J. Exp. Bot. 48 (1997) 1367 – 1374.
[24] F . Provan, C. Lillo, Photosynthetic post-translational activation of nitrate reductase, J. Plant Physiol. 154 (1999) 605 – 609.
[25] C. Lillo, A. H enriksen, Comparative studies of diurnal variations of nitrate reductase activity in wheat, oat and barley, Physiol. Plant. 62 (1984) 89 – 94.
[26] A.K . Sharma, N . R aghuram, M .R . Chandok, R . D as, S.K . Sopory, Investigations on the nature of the phy-tochrome-induced transmitter for the regulation of ni-trate reductase in etiolated leaves of maize, J. Exp. Bot. 45 (1994) 485 – 490.
[27] A.K . Sharma, S.K . Sopory, Effect of ammonium, su-crose and light on the regulation of nitrite reductase activities in maize, Photochem. Photobiol. 39 (1984) 491 – 493.
[28] B.D . Bruce, R . M alkin, Biosynthesis of the chloroplast cytochrome b6f complex: studies in a photosynthetic mutant of L emna, Plant Cell 3 (1991) 203 – 212. [29] K .-J. Appenroth, S. Teller, M . H orn, Photophysiology
of turion formation and germination in S pirodela
polyrhiza, Biol. Plant. 38 (1996) 95 – 106.
[30] C. Lillo, L.H . Smith, H .G . N immo, M .B. Wilkins, R eg-ulation of nitrate reductase and phosphoenolpyruvate carboxylase activities in barley leaf protoplasts, Planta 200 (1996) 181 – 185.
[31] M .R . Chandok, S.K . Sopory, Phosphorylation/ dephos-phorylation steps are key events in the phytochrome-me-diated enhancement of nitrate reductase mR N A levels and enzyme activity in maize, M ol. G en. G enet. 251 (1996) 599 – 608.
[32] C.L. Cheng, G .N . Acedo, M . Christinsin, M .A. Con-kling, Sucrose mimics the light induction of A rabidopsis nitrate reductase gene transcription, Proc. N atl. Acad. Sci. U SA 89 (1992) 1861 – 1864.