PLEASE SCROLL DOWN FOR ARTICLE
This article was downloaded by: [TÜBİTAK EKUAL]
On: 26 January 2011
Access details: Access Details: [subscription number 772815469]
Publisher Taylor & Francis
Informa Ltd Registered in England and Wales Registered Number: 1072954 Registered office: Mortimer House,
37-41 Mortimer Street, London W1T 3JH, UK
Archives Of Phytopathology And Plant Protection
Publication details, including instructions for authors and subscription information: http://www.informaworld.com/smpp/title~content=t713454295
Identification and pathogenicity of Rhizoctonia species isolated from bean
and soybean plants in Samsun, Turkey
Ismail Erpera; Ibrahim Ozkocb; Gürsel Hatat Karacac
a Plant Protection Department, Agriculture Faculty, Ondokuz Mayis University, Samsun, Turkey b Biological Sciences Department, Faculty of Arts and Sciences, Samsun, Turkey c Plant Protection Department, Agriculture Faculty, Süleyman Demirel University, Isparta, Turkey
Online publication date: 25 January 2011
To cite this Article Erper, Ismail , Ozkoc, Ibrahim and Karaca, Gürsel Hatat(2011) 'Identification and pathogenicity of
Rhizoctonia
species isolated from bean and soybean plants in Samsun, Turkey', Archives Of Phytopathology And Plant Protection, 44: 1, 78 — 84To link to this Article: DOI: 10.1080/03235400903395427
URL: http://dx.doi.org/10.1080/03235400903395427
Full terms and conditions of use: http://www.informaworld.com/terms-and-conditions-of-access.pdf
This article may be used for research, teaching and private study purposes. Any substantial or systematic reproduction, re-distribution, re-selling, loan or sub-licensing, systematic supply or distribution in any form to anyone is expressly forbidden.
Identification and pathogenicity of
Rhizoctonia
species isolated from
bean and soybean plants in Samsun, Turkey
Ismail Erpera*, Ibrahim Ozkocband Gu¨rsel Hatat Karacac
aPlant Protection Department, Agriculture Faculty, Ondokuz Mayis University, Samsun, Turkey;bBiological Sciences Department, Faculty of Arts and Sciences, Samsun, Turkey;cPlant Protection Department, Agriculture Faculty, Su¨leyman Demirel University, Isparta, Turkey
(Received 11 September 2009; final version received 24 September 2009)
A total of 434 isolates ofRhizoctoniabelonging to 10 anastomosis groups were obtained from the roots and rhizosphere soils of bean and soybean plants grown in Samsun, Turkey. AG-4 was found to be the most common group on bean and soybean plants and AG-5, AG-6, binucleate AG-A, AG-B andR. zeaewere other groups isolated from the both plant species. AG-1, AG-7 and AG-K from bean and AG-E from soybean were other groups obtained in the study. The pathogenicity tests on bean and soybean seedlings showed that the highest disease severities were caused by AG-4 isolates, whereas AG-1 and AG-6 isolates were moderately pathogenic. BinucleateRhizoctoniaAG-B isolates were also moder-ately pathogenic, while other binucleate Rhizoctonia were found to be weakly pathogenic.Rhizoctonia zeaeisolates caused moderate disease symptoms on bean, but soybean plants were slightly affected by this group of isolates. This is the first reported observation ofR. solaniAG-6 and AG-7 and binucleateRhizoctonia AG-B on bean, andR. solaniAG-5 and AG-6 and binucleateRhizoctoniaAG-A, AG-B and AG-E on soybean, in Turkey.
Keywords:Rhizoctonia;Phaseolus vulgarisL.;Glycine maxL.; anastomosis group; pathogenicity
Introduction
Bean (Phaseolus vulgaris L.) and soybean (Glycine max L.) are the major legume crops grown in Samsun, Turkey. Especially, bean is very common in the province, and with its 105.436 tones production Samsun comes first in our country. Soybean is the second important legume plant in the province with 11.598 tones production (Anonymous 2003). Because of the warm and humid weather conditions of the province, mainly fungal diseases can cause significant yield losses. Among those, damping off and root rot is very common on legume crops, andRhizoctoniagroup fungi were found to be one of the main agents of the disease (Ecevit et al. 1988). These fungi are among the soil-borne pathogens, which attacks a wide range of plants and are distributed all over the world (Ogoshi 1996). R. solani Ku¨hn [teleomorph: Thanatephorus cucumeris (Frank) Donk.] and binucleateRhizoctonia
(teleomorph: Ceratobasidium Rogers) are divided into anastomosis groups (AGs) based on hyphal anastomosis reactions between isolates. R. solani is composed of
*Corresponding author. Email: [email protected]
ISSN 0323-5408 print/ISSN 1477-2906 online
Ó2011 Taylor & Francis DOI: 10.1080/03235400903395427 http://www.informaworld.com
13 AGs designated as AG-1 to AG-13 (Sneh et al. 1991; Carling et al. 2002; Woodhall et al. 2007). Only 16 Binucleate Rhizoctonia AGs are currently known (Sharon et al. 2008). Rhizoctonia zeae and Rhizoctonia oryzae were accepted as anamorphs ofWaitea circinata(Leiner and Carling 1994).
Among Rhizoctonia group fungi, R. solani is the most common and virulent species and can cause several types of damage, including hypocotyl rot, root rot and web blight on bean (Galindo et al. 1983; Sumner 1985), and damping-off, root rot, stem rot, and foliar blight on soybean (Sinclair and Backman 1989). AG-4 is the major AG worldwide, causing root rot on bean. However, other AGs (AG-1, AG-1-IB, AG-2-1, AG-2-2 and AG-5) have also been reported on bean (Muyolo et al. 1993; Echavez-Badel et al. 2000). In Turkey, someR. solani(AG-1, AG 2-1, AG 2-2,
AG-3, AG-4, AG-5, AG-9, AG-10 and AG-11) and binucleate Rhizoctonia AGs
(AG-A, AG-E, AG-F, AG-G, AG-I and AG-K) have previously been recognized on bean (Demirci and Do¨ken 1995; Karaca et al. 2002; Eken and Demirci 2004).
R. solaniAGs reported on soybean were AG-1IA, AG-1IB, AG-1IC, AG-1, AG-2, AG-2-2, AG-3, AG-4 and AG-5 (Bolkan and Ribeiro 1985; Sneh et al. 1991; Nelson et al. 1996).
The aim of the present study was to determine the anastomosis groupings and pathogenicity ofRhizoctoniaisolates obtained from the roots and rhizosphere soils of bean and soybean that are the main legume crops grown in Samsun province.
Materials and methods
Collection, isolation and identification
Rhizoctoniaisolates were obtained from the roots and rhizosphere soils of bean and soybean plants, showing root rot disease symptoms in Samsun province, Turkey. Isolations from bean and soybean plants were made from discoloured or necrotic lesions on root and hypocotyl tissues. Affected tissues were washed under running tap water, and the surface was disinfected in 1% sodium hypochlorite (NaOCl) for 2–3 minutes and four segments per plant were plated on acidified water agar in a petri dish (2% water agar amended with 3 ml of 10% lactic acid per litre). Three replicate isolations were made for each field sample. Immediately after the soil samples were brought to the laboratory, each composite sample was filled in three sterile plastic pots and watered to field capacity with sterile water. Three internodal segments of sterile oat straw about 2–3 cm long were then inserted vertically to each plastic pot. Pots were covered with plastic sheet to prevent the water loss. After incubation at room temparature for 3 days, straw segments were removed from the soil and washed under tap water. They were surface sterilized with 1% NaOCl, rinsed with sterile distilled water, blotted dry and placed on acidified water agar as in plant root samples (Ogoshi et al. 1990). After 2 or 3 days incubation at (22+2)8C, hyphal tips were transferred to PDA (Oxoid) for further examination. Isolates were stored in tubes with oat grains atþ48C.
Rhizoctonia isolates were identified on the basis of characteristics of their vegetative hyphae; hyphal diameter and nuclear condition (Bandoni 1979), and hyphal anastomosis with known tester isolates. A modification of the method of Kronland and Stanghellini (1988) was used to identify AGs. Tester isolates of
R. solani(AG-1, AG-2-1, AG-2-2, AG-3, AG-4, AG-5, AG-6, AG-7, AG-8, AG-9, AG-10 and AG-11) were provided by Dr. Ogoshi, Hokkaido University, Japan, Dr. D.E. Carling, University of Alaska Fairbanks, USA and Dr. S.M. Neate, CSIRO,
Archives of Phytopathology and Plant Protection 79
Division of Soils, Australia. Tester isolates of binucleateRhizoctonia(AG-A, AG-B, AG-C, AG-D, AG-E, AG-F, AG-G, AG-H, AG-I, AG-K, AG-L, AG-O, AG-P, AG-Q and AG-S) were provided by Dr. Ogoshi, Hokkaido University, Japan. Tester isolates of subspecies ofW. circinata(W. circinatavar.zeae, W. circinatavar.oryzae) were provided by Dr. Hyakumachi, Gifu University, Japan.
Determination of pathogenicity
Pathogenicity of 21 selected isolates (Table 2) was determined on bean (cv. Lodi) and soybean (cv. Nova) seedlings. Inoculum was prepared on the moistened sterile oat grains (with 250mg/ml chloramphenicol) in tests tubes, which were autoclaved twice at 24 h intervals, inoculated with plugs of mycelium from cultures grown on PDA and incubated at 258C for 10 days (Martin 1988).
For seedling tests, plants were grown in plastic pots (1 l) containing sterilized mixture of sandy loam soil:manure:washed sand (2:2:1,v/v/v), in a greenhouse at 17–258C. Four seeds were sown to a depth of 2 cm in each pot. Plants were inoculated by placing 5 colonized oat grains in contact with each seedling. In control treatments, sterile oat grains were used. After 4 weeks from inoculation, plants were harvested, washed and disease severity ratings were made by using 1–5 scale modified from Muyolo et al. (1993), where 1, healthy seedling; 2, very little superficial lesions on roots and hypocothyls; 3, deep and large lesions on the roots or on the hypocothyl; 4, severe root rot, lesions surrounding hypocothyl, partially restricted root length and 5, complete root rot. Four replicate pots were used for each treatment. Disease severity data were subjected to analysis of variance (SPSS version 11.0) and separated using Duncan’s multiple range test (P¼0.05).
Results
Species and AGs of Rhizoctonia isolates
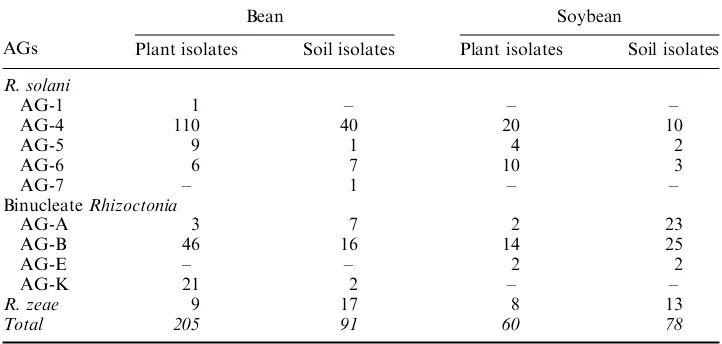
A total of 434Rhizoctonia isolates belonging to 10 AGs were obtained from roots and rhizosphere soil of bean and soybean plants in Samsun. Of 296 bean isolates, 205 were obtained from plant root samples and the remaining 91 were from rhizosphere soil. Number of soybean isolates were less than bean and 60 of the total 138 soybean isolates were recovered from plant roots and 78 of them were from soil samples. Among all bean isolates, 59% wereR. solani, 32% were binucleateRhizoctoniaand 9% wereR. zeae, whereas 36% wereR. solani, 49% were binucleateRhizoctoniaand 15% wereR. zeae, for those of soybean isolates. Isolates ofR. solaniobtained from bean root and rhizosphere soils were distinguished in five AGs: AG-1 (0.6%), AG-4 (85.7%), AG-5 (5.7%), AG-6 (7.4%) and AG-7 (0.6%). Binucleate Rhizoctonia
isolates recovered from bean were grouped in three AGs: AG-A (10.5%), AG-B (65.2%) and AG-K (24.3%). Isolates ofR. solani collected from soybean root and rhizosphere soils were distinguished in three AGs: AG-4 (61.2%), AG-5 (12.2%) and AG-6 (26.6%), and isolates of binucleate Rhizoctonia were grouped in three AGs: AG-A (36.8%), AG-B (57.3%) and AG E (5.9%) (Table 1).
Pathogenicity of the Rhizoctonia isolates
As a result of the pathogenicity test, it was found that the differences among the virulence of isolates ofR. solani, binucleateRhizoctoniaandR. zeaewere statistically
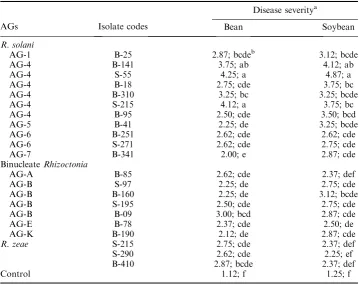
significant. Virulence differences also existed among the isolates from the same AG (Table 2). R. solaniAG-4 isolates B-141 and S-55 were found to be the most virulent isolates both on bean and soybean seedlings, whereas S-215 isolate from the same AG was highly virulent only on bean seedlings.R. solaniisolates belonging to AG-1 (B-25) and AG-6 (B-251 and S-271) were found to be moderately pathogenic on both plants. AG-7 (B-341) and AG-5 (B-41) were weakly pathogenic on bean cultivar tested, but same isolates were moderately virulent on soybean. Binucleate
Rhizoctonia AG-B isolates were also moderately pathogenic on bean (B-09) and soybean (B-160), although the majority of the binucleate Rhizoctonia were weakly pathogenic on both plants.R. zeaeisolates were found to be moderately or weakly pathogenic on bean and soybean plants.
Discussion
In this study, different AGs ofR. solani(AG-1, AG-4, AG-5, AG-6 and AG-7) and binucleateRhizoctonia(AG-A, AG-B, AG-E and AG-K) andR. zeaewere obtained from bean and soybean plants and rhizosphere soils in Samsun. In a previous study carried out in Samsun, AG-2-2, AG-4 and AG-5 were found on bean (Karaca et al. 2002). However, AG-1, AG-2-1, AG-3, AG-4, AG-5, AG-9, AG-10 and AG-11 of R. solani and AG-A, AG-E, AG-F, AG-G, AG-I and AG-K of binucleate
Rhizoctoniawere previously detected on bean in different regions of Turkey (Tuncer and Erdiller 1990; Demirci and Do¨ken 1995; Eken and Demirci 2004). There is rather less work on soybean in our country and only AG-4 was obtained from this plant (Tuncer and Erdiller 1990). In the present study, AG-6 and AG-7 ofR. solani
and AG-B of binucleate Rhizoctonia isolated from bean, and AG-5 and AG-6 of
R. solani and AG-A, AG-B and AG-E of binucleate Rhizoctonia isolated from soybean were reported for the first time in Turkey.R. zeaefrom bean and soybean determined in this study was recently reported by the same authors (Erper et al. 2005).
As a result of the pathogenicity tests, it was found that the differences among the virulence ofRhizoctoniaisolates belonging to same or different AGs or species were
Table 1. Number of plant and soil isolates ofRhizoctoniaspp. and AGs isolated from bean and soybean in Samsun, Turkey.
AGs
Bean Soybean
Plant isolates Soil isolates Plant isolates Soil isolates
R. solani
AG-1 1 – – –
AG-4 110 40 20 10
AG-5 9 1 4 2
AG-6 6 7 10 3
AG-7 – 1 – –
BinucleateRhizoctonia
AG-A 3 7 2 23
AG-B 46 16 14 25
AG-E – – 2 2
AG-K 21 2 – –
R. zeae 9 17 8 13
Total 205 91 60 78
Archives of Phytopathology and Plant Protection 81
statistically significant. It was found that the most virulent isolates were from AG-4 group. Likely, Bolkan and Ribeiro (1985) determined that AG-4 was highly virulent on soybean hypocotyls, and Phillips (1991) reported that AG-4 isolates caused pre-emergence death and hypocotyl lesions on bean and soybean plants. In this study, AG-5 isolate was found to be weakly pathogenic on bean, but caused moderate symptoms on soybean. Similiarly, Nelson et al. (1996) reported that AG-5 caused damping off on soybean, but its virulence was rather lower. Also, Ogoshi (1996) suggested that AG-5 isolates were weakly pathogenic or not pathogenic on plants. As in AG-5,R. solaniisolate belonging to AG-7 was also weakly pathogenic on bean and moderately pathogenic on soybean, whereas AG-6 was moderately pathogenic on both plants. AG-7 was thought to be a group with limited pathogenicity, but it was isolated from soybean plants (Rothrock et al. 1993). AG-6 was reported as agents of the crater disease of wheat (Carling et al. 1996) and root canker of lucerne (Anderson et al. 2004), and there was also evidence that it was pathogenic on potato, barley, lettuce, cauliflower and radish (Carling et al. 1999). In the present study, it was found that the virulence of R. zeae isolates was lower on soybean, whereas they were moderately pathogenic on bean. Some R. zeae isolates have been reported as pathogenic (Leiner and Carling 1994) or weakly pathogenic (Demirci 1998) on different plants, but it was found to be non pathogenic on soybean (Ploetz et al. 1985).
Table 2. Pathogenicity ofRhizoctoniaspecies on bean and soybean seedlings.
AGs Isolate codes
Disease severitya
Bean Soybean
R. solani
AG-1 B-25 2.87; bcdeb 3.12; bcde
AG-4 B-141 3.75; ab 4.12; ab
AG-4 S-55 4.25; a 4.87; a
AG-4 B-18 2.75; cde 3.75; bc
AG-4 B-310 3.25; bc 3.25; bcde
AG-4 S-215 4.12; a 3.75; bc
AG-4 B-95 2.50; cde 3.50; bcd
AG-5 B-41 2.25; de 3.25; bcde
AG-6 B-251 2.62; cde 2.62; cde
AG-6 S-271 2.62; cde 2.75; cde
AG-7 B-341 2.00; e 2.87; cde
BinucleateRhizoctonia
AG-A B-85 2.62; cde 2.37; def
AG-B S-97 2.25; de 2.75; cde
AG-B B-160 2.25; de 3.12; bcde
AG-B S-195 2.50; cde 2.75; cde
AG-B B-09 3.00; bcd 2.87; cde
AG-E B-78 2.37; cde 2.50; de
AG-K B-190 2.12; de 2.87; cde
R. zeae S-215 2.75; cde 2.37; def
S-290 2.62; cde 2.25; ef
B-410 2.87; bcde 2.37; def
Control 1.12; f 1.25; f
aDisease severity was assigned to each plant on a scale of 1–5, in which 1, healthy seedling; 5, complete
root rot.
b
Within columns, means followed by the same letters are not significantly differ from each other according to the Duncan’s multiple range test (P¼0.05).
BinucleateRhizoctoniaAG-B isolate B-09 and B-160 were found to be moderately pathogenic on bean and soybean, respectively. Other binucleateRhizoctoniaisolates were weakly pathogenic. Some binucleateRhizoctoniaisolates have been reported as pathogenic, non-pathogenic or weakly pathogenic on cultivated plants (Sumner 1985; Yuen et al. 1994). Ploetz et al. (1985) reported that AG-E was pathogenic on young soybean plants. AG-K was found to be weakly pathogenic on wheat and barley (Demirci 1998), and it was also previously isolated from bean hypocotyls, but its virulence was not mentioned (Demirci and Do¨ken 1995).
This group of fungi can cause economic yield losses on plants in Samsun, where vegetable growing is common. Hence, suitable cultural practices, such as the use of resistant cultivars and the improvement of soil conditions, will be useful. It is known that chemical control is not so efficient against soil-borne pathogens like
Rhizoctoniagroup fungi. Therefore, it will be useful to determine biocontrol agents that could be effective againtsR. solaniand to evaluate their efficiency byin vivoand
in vitrotrials.
References
Anderson JR, Bentley S, Irwin JAG, Mackie JM, Neate S, Pattemore JA. 2004. Characterization ofRhizoctonia solaniisolates causing root canker of lucerne in Australia. Australasian Plant Pathology. 33(2):241–247.
Anonymous. 2003. Agricultural structure (production, prize, value). Republic of Turkey, Ankara (Turkey): State Institute of Statistics.
Bolkan HA, Ribeiro WRC. 1985. Anastomosis groups and pathogenicity ofRhizoctonia solani isolates from Brazil. Plant Dis. 69:599–601.
Bandoni RJ. 1979. Safranin-O as a rapid nuclear stain for fungi. Mycologia. 63:873–874. Carling DE, Baird RE, Gitaitis RD, Brainard KA, Kuninaga S. 2002. Characterization of
AG-13, a newly reported anastomosis group of Rhizoctonia solani. Phytopathology. 92:893–899.
Carling DE, Meyer L, Brainard KA. 1996. Crater disease of wheat caused byRhizoctonia solaniAG-6. Plant Dis. 80:1429.
Carling DE, Pope EJ, Brainard KA, Carter DA. 1999. Characterization of mycorrhizal isolates ofRhizoctonia solanifrom an orchid, including AG-12, a new anastomosis group. Phytopathology. 89:942–946.
Demirci E. 1998.Rhizoctoniaspecies and anastomosis groups isolated from barley and wheat in Erzurum, Turkey. Plant Pathology. 47:10–15.
Demirci E, Do¨ken MT. 1995. Anastomosis groups ofRhizoctonia solaniKu¨hn and binucleate Rhizoctoniaisolates from various crops in Tu¨rkiye. J Turk Phytopathology. 24(2):57–62. Ecevit O, Du¨ndar F, Turan A. 1988. Bafra ovasındaki o¨nemli bitki hastalıkları ve sulamanın yaygınlas¸tırılması ile ortaya c¸ıkabilecek sorunlar ve c¸o¨zu¨m yolları. Bafra Ovası Tarım Sempozyumu OMU¨. Yayınları, No: 40; p. 281–317.
Echavez-Badel R, Gomez-Galue JE, Alameda-Lozada M. 2000. Characterization of Rhizoctoniaspp. isolates collected fromPhaseolus vulgarisin Puerto Rico. J Agric Univ Puerto Rico. 84(1–2):79–86.
Eken C, Demirci E. 2004. Anastomosis groups and pathogenicity ofRhizoctonia solaniand binucleate Rhizoctonia isolates from bean in Erzurum, Turkey. J Plant Pathology. 86(1):49–52.
Erper I, Karaca G, Ozkoc¸ I. 2005. First report of root rot of bean and soybean caused by Rhizoctonia zeaein Turkey. Plant Dis. 89(2):203.
Galindo JJ, Abawi GS, Thurston HD, Galvez G. 1983. Source of inoculum and development of bean web blight in Costa Rica. Plant Dis. 67:1016–1021.
Karaca GH, Ozkoc¸ I, Erper I. 2002. Determination of the anastomosis grouping and virulence ofRhizoctonia solaniKu¨hn isolates associated with bean plants grown in Samsun/Turkey. Pakistan J Biol Sci. 5(4):434–437.
Kronland WC, Stanghellini ME. 1988. Clean slide technique for the observation of anastomosis and nuclear condition ofRhizoctonia solani. Phytopathology. 78:820–822.
Archives of Phytopathology and Plant Protection 83
Leiner RH, Carling DE. 1994. Characterization of Waitea circinata (Rhizoctonia) isolated from agricultural soils in Alaska. Plant Dis. 78:385–388.
Martin SB. 1988. Identification, isolation frequency, and pathogenicity of anastomosis groups of binucleateRhizoctoniaspp. from strawberry roots. Phytopathology. 78:379–384. Muyolo NG, Lipps PE, Schmitthenner AF. 1993. Anastomosis grouping and variation in
virulence among isolates of Rhizoctonia solaniassociated with dry bean and soybean in Ohio and Zaire. Phytopathology. 83:438–444.
Nelson B, Helms T, Christianson T, Kural I. 1996. Characterization and pathogenicty of Rhizoctoniafrom soybean. Plant Dis. 80:74–80.
Ogoshi A. 1996. Introduction – the genusRhizoctonia. In: Sneh B, et al., editors.Rhizoctonia species: taxonomy, molecular biology, ecology, pathology and disease control. Nether-lands: Kluwer Academic Publishers. p. 1–9.
Ogoshi A, Cook RJ, Bassett EN. 1990.Rhizoctoniaspecies and anastomosis groups causing root-rot of wheat and barley in the Pasific Northwest. Phytopathology. 80:784–788. Philips AJL. 1991. Variation in virulence to dry beans, soybeans and maize among isolates of
Rhizoctonia solanifrom beans. Ann Appl Biol. 118:9–17.
Ploetz RC, Mitchell DJ, Gallaher RN. 1985. Characterization and pathogenicity of Rhizoctoniaspecies from a reduced-tillage experiment multicropped to rye and soybean in Florida. Phytopathology. 75:833–839.
Rothrock CS, Winters SA, Kinney PM. 1993. Occurrence of Rhizoctonia solani AG-7 in Arkansas. Plant Dis. 77:1262.
Sharon M, Kuninaga S, Hyakumachi M, Naito S, Sneh B. 2008. Classification ofRhizoctonia spp. using rDNA-ITS sequence analysis supports the genetic basis of the classical anastomosis grouping. Mycoscience. 49:93–114.
Sinclair JB, Backman PA, editors. 1989. Compendium of soybean diseases. 3rd ed. St. Paul, MN: American Phytopathological Society.
Sneh B, Burpee L, Ogoshi A. 1991. Identification ofRhizoctoniaspecies. St. Paul, MN: APS Press. p. 133.
Sumner DR. 1985. Virulence of anastomosis groups ofRhizoctonia solaniandRhizoctonia-like fungi on selected germ plasm of snap bean, lima bean, and cowpea. Plant Dis. 69:25–27. Tuncer G, Erdiller G. 1990. The identification of Rhizoctonia solani Ku¨hn anastomosis groups isolated from potato and some other crops in Central Anatolia. J Turk Phytopathol. 19(2):89–93.
Woodhall JW, Lees AK, Edwards SG, Jenkinson P. 2007. Characterization ofRhizoctonia solanifrom potato in Great Britain. Plant Pathology. 56:286–295.
Yuen GY, Craig ML, Giesler LJ. 1994. Biological control ofRhizoctonia solanion tall fescue using fungal antagonists. Plant Dis. 78:118–123.