Classical and biochemical endpoints in the evaluation of
phytotoxic effects caused by the herbicide trichloroacetate
Claudemir Marcos Radetski *, Sylvie Cotelle, Jean-Franc¸ois Fe´rard
Centre des Sciences de l’En6ironnement,Uni6ersite´ de Metz,1,rue des Re´collets,57000Metz,France
Received 31 March 2000; received in revised form 7 July 2000; accepted 8 July 2000
Abstract
Three terrestrial plant species, oat (A6ena sati6a), Chinese cabbage (Brassica campestriscv. chinensis) and lettuce
(Lactuca sati6a), were exposed to different concentrations of herbicide TCA (sodium trichloroacetate) in a growth test
according to guideline OECD c 208. Classical (i.e. germination and biomass) and biochemical (i.e., antioxydant enzyme activities) endpoints were investigated. Germination rate decreased significantly at 3.9 mg TCA kg dry soil−1
(for oat and lettuce) and 62.5 mg TCA kg dry soil−1(for Chinese cabbage). Biomass decreased significantly only at
1.9 mg TCA kg dry soil−1(for oat and lettuce) and 15.6 mg TCA kg dry soil−1(for Chinese cabbage). The activities
of superoxide dismutase (EC 1.15.1.1), catalase (EC 1.11.1.6), peroxidase (EC 1.11.1.7) and glutathione reductase (EC 1.6.4.2) increased significantly at the lowest concentration of TCA tested, i.e. 0.03 mg TCA kg dry soil−1(for oat and
lettuce) and 0.48 mg TCA kg dry soil−1(for Chinese cabbage). Our results showed a ranking of sensitivity among
the different endpoints for the three plant species: enzyme activities\biomass\germination rate. The increase in antioxidant enzyme activities observed in this study ensured the detoxification of increased levels of active oxygen species, and presumably prevented the plants from undergoing oxidative stress damage. Thus, the use of enzyme activities will permit the detection of early injury in plant growth testing. © 2000 Elsevier Science B.V. All rights reserved.
Keywords:A6ena sati6a; Biomarkers;Brassica campestris;Lactuca sati6a; Oxidative stress; Phytotoxicity
www.elsevier.com/locate/envexpbot
1. Introduction
The two chlorinated aliphatic compounds 2,2-dichloropropionic acid (dalapon) and trichloroacetic acid (TCA) were widely used as
herbicides (Foy, 1969; Ashton and Crafts, 1981). Both compounds are particularly effective against grasses, but they also control certain broad leaf weeds. The most important application of trichloroacetic acid (sodium salt) herbicide is the use for the management of aquatic plants in drainage and in supply canals (Bowmer, 1987). TCA is still used for various purposes in industry (e.g. plastic, galvanization and textile), and maybe the least harmful of the C2halogenated acids that
* Correspoding author. Present adress: Departamento de Quı´mica, Universidade Federal de Santa Catarina, 88040-900 Floriano´polis SC, Brazil.
E-mail address:[email protected] (C.M. Radetski).
are formed in the atmosphere by photochemical processes (Mu¨ller et al., 1996; Hashimoto et al., 1998). It is readily absorbed by roots and leaves and primarily translocated via the transpiration stream system. However small amounts are trans-ported via the symplast system (Blanchard, 1954). The initial step of plant injury caused by TCA is regarded as a modification of protein structure and such changes could alter enzyme activity and membrane permeability (Ashton and Crafts, 1981).
On the other hand, when plants are subjected to stress of many kinds (physical, chemical, bio-logical), bursts of active oxygen occur within min-utes after exposure (Foyer et al., 1994). Although the formation of toxic oxygen species is generally considered to be detrimental to cellular function, these molecules are formed in normal cell metabolism and their production and destruction is a regulated phenomenon (Asada, 1993). An imbalance between oxidants and antioxidants in favor of the oxidants, potentially leading to dam-age, is termed oxidative stress (Sies, 1997). Up to date, there is no report of active oxygen species associated with TCA toxicity.
Plants are well adapted for minimizing damage that could occur from toxic oxygen species. The natural antioxidative defence system (Elstner, 1982; Winston, 1990; Smirnoff, 1993; Sies, 1997) includes three general classes: (a) lipid soluble, membrane-associated antioxidants (e.g. alpha-to-copherol and beta-carotene); (b) water soluble reductants (e.g. tripeptide glutathione and ascor-bate); and (c) enzymatic antioxidants including superoxide dismutase, catalase, peroxidase and the enzymes involved in the synthesis and regener-ation of the reduced forms of the antioxidants (e.g. enzymatic pool of glutathione). This system is present in both intra- and extra-cellular com-partments. Since oxidative stress comprises a complex set of phenomena, it is highly unlikely that a single response will provide a general marker for it, thus simultaneous increase in sev-eral components of the antioxidative defence sys-tem would be necessary in order to obtain a substantial increase in stress tolerance (Foyer et al., 1994). Plant antioxidant enzyme activities were considered as relevant endpoints in different
stress situations, they have been investigated mainly with air pollutants and metals (Keller, 1974; Van Assche et al., 1988) and reviews were published giving some theoretical rationale for using biomarkers (Ernst and Peterson, 1994; Vangronsveld et al., 1997).
In the present investigation, three terrestrial plants, oat (A6ena sati6a), Chinese cabbage
(Brassica campestris cv. chinensis) and lettuce (Lactuca sati6a), were exposed to different
con-centrations of herbicide TCA in a growth test according to guideline of Organization for Eco-nomic Cooperation and Development (1984). The phytotoxic effects of soil contaminated with TCA were measured by classical endpoints (i.e. germi-nation rate and biomass) and by biochemical endpoints (antioxidant enzyme activities). The purposes of this work were (i) to investigate more sensitive endpoints for evaluating TCA contami-nated soils; (ii) to examine the ratios of sensitivity between classical and biochemical endpoints car-ried out simultaneously according to the same phytotoxicity protocol. As a matter of fact, the utilization of guidelines offers the advantage of minimizing differences in the generation of results, and thus comparison of phytotoxicity results can be more realistic and feasible.
It is proposed that changes in enzyme activities might be used as plant biomarkers in the evalua-tion of the phytotoxicity of soils contaminated by the chlorinated aliphatic herbicides.
2. Materials and methods
2.1. Chemical, plant material, soil, and growth conditions
The chemical used in the tests was TCA (as NaTCA; Aloricu, lot c 19078-0). Plant species were oat (A6ena sati6a L.), Chinese cabbage
(Brassica campestris L. cv. chinensis) and lettuce (Lactuca sati6a L.), and the seeds have been
26%, silt 52%, and sand 22%) and samples were taken from the 0 – 25 cm surface layer. Some characteristics of the soil include: carbon 1.5%, organic matter 3.0%, pH=6.6, and C/N 8.2. The soil was dried (110 – 130°C; 30 h) before use. Test concentrations were drop-pippeted into the soil surface at 66% of the maximum water-holding capacity. In this study, maximum water-holding capacity was 37 ml water per 100 g soil; thus, water dropped to controls was 25 ml and the different toxic test concentrations were dropped at the same volume (25 ml) in corresponding pot treatments.
Three tests were performed to each plant and for each test three pots (disposable plastic, diame-ter 7 cm, height 5 cm) were used for each TCA treatment (or controls). Sixteen seeds for oats and 40 seeds for Chinese cabbage and lettuce were sown in each pot containing 100 g of soil. Water evaporation was determined by daily weighing of pots and compensated by addition of distilled water. Plants were grown under controlled condi-tions at a temperature regimen of 2592°C (day) and of 1792°C (night), with irradiation flux of 72 mE m−2 s−1 from fluorescent tubes under a
16/8 light/dark photoperiod. Ten days after sow-ing, the plants were cut at soil level and the wet weight of the plant material was immediately determined using an analytical balance. For each pot, the total weight of the germinated plants (expressed in g wet weight) was divided by the number of germinated plants (plants that devel-oped roots at least 20 mm long were considered germinated). The pH of the soil was measured at the beginning and end of each ten-day experiment.
2.2. Assay of enzyme acti6ities
Fresh weight biomass (oat=0.125 g ml−1
buffer, Chinese cabbage=0.137 g ml−1
buffer, lettuce=0.100 g ml−1 buffer) for each replicate
was homogenized using a mortar and pestle under ice-cold conditions in 4 ml of 50 mM potassium phosphate buffer (pH=7.6). The samples were sonicated on ice three times for 20 s (to avoid sample overheating), centrifuged (10 000×g; 10 min; 4°C), and supernatants were stored at −
20°C until subsequent measurements were carried out at 25°C using a Uvikon Spectrometer 930 (Kontron Instruments).
Superoxide dismutase (EC 1.15.1.1) activity was measured by an indirect method (Paoletti and Mocali, 1990) following the oxidation of reduced nicotinamide adenine dinucleotide (NADH) at 340 nm. Prior to the assay, each sample was poured into a Pharmacia column containing Sep-hadex G-25 equilibrated with 100 mM tri-ethanolamine-diethanolamine (TDB)-HCl buffer (pH=7.4). In a 2.13 ml reaction volume contain-ing 75 mM TDB, 0.28 mM NADH, 2.30/1.15 mM ethylenediaminetetraacetic acid (EDTA)/MnCl2
and 0.1 ml sample, 0.93 mM of beta-mercap-toethanol was added to start the reaction. Super-oxide dismutase (SOD) activity was expressed in units according to Asada method (Asada et al., 1974), where 1 unit was defined as the amount of enzyme required to inhibit the rate of NADH oxidation of the control by 50%. The results were reported as specific activities (Asada Units).
Peroxidase (EC 1.11.1.7) activity was measured using a slightly (see below) modified version of published method (Byl et al., 1994). Just prior to the assay, 0.7 ml of solution A (810 mg phenol and 25 mg 4-aminoantipyrene in 50 ml distilled water) and 0.2 ml of sample were added to a 3 ml glass cuvette. A 0.75 ml aliquot of solution B (0.01% of hydrogen peroxide in 100 mM 4-(2-hy-droxyethyl)-1-piperazineethane sulfonic acid (HEPES) buffer (pH=7.1) was mixed in order to start the reaction and the change in absorbance was monitored at 510 nm.
Catalase (EC 1.11.1.6) activity was determined by following the consumption of hydrogen perox-ide at 240 nm (Beers and Sizer, 1951). This proce-dure was slightly modified as follows: 1 ml of 0.15% buffered hydrogen peroxide (in potassium phosphate buffer, pH=7.6) was added to 1 ml of 50 mM potassium phosphate buffer (pH=7.6) and 0.1 ml of diluted sample.
Glutathione reductase (EC 1.6.4.2) activity was assayed following NADPH oxidation at 340 nm (Bergmeyer et al., 1983). The final reaction vol-ume contained 66.6 mM hydroxymethyl aminomethane (TRIS) buffer (pH=8.0), 0.2 mM Na2EDTA, 12 mM oxidized glutathione (GSSG),
Activities of all enzymes (except SOD) were expressed in enzyme units per mg of protein, where 1 enzyme unit is defined as a change of 0.01 absorbance min−1 caused by the enzyme sample.
Protein sample content was determined using bovine serum albumin as a standard (Bradford, 1976).
2.3. Statistics
Three tests were performed for each plant, and data for enzyme activity, biomass and germina-tion rate were generated in triplicate for each treatment (or control), thus n=9. No observed effect concentration (NOEC) and lowest observed effect concentration (LOEC) values were deter-mined using Williams’ test (P50.05) (Williams, 1971) after checking homogeneity of variances with the Hartley test. The software TOXSTAT 3.0 (University of Wyoming, Laramie, WY, USA) was used for these different calculations. The coefficient of variation is representative of
re-peatability (triplicates) and of reproducibility (tests realized at three different times).
3. Results
The pH values of the soils were practically unaltered (from 7.0 to 6.7) during the test period. Tables 1, 2 and 3 show biological and biochemical data for oat (A6ena sati6a), Chinese cabbage
(Brassica campestris cv. chinensis) and lettuce (Lactuca sati6a) exposed to TCA herbicide.
3.1. Effects of TCA on germination rate and plant growth
Although no visible symptoms such as wilting or leaf coloring were caused by the lower TCA concentrations, weak leaf chlorosis was observed in some plants of lettuce and Chinese cabbage species at the highest TCA concentration. The biomass (fresh weight) and germination rate
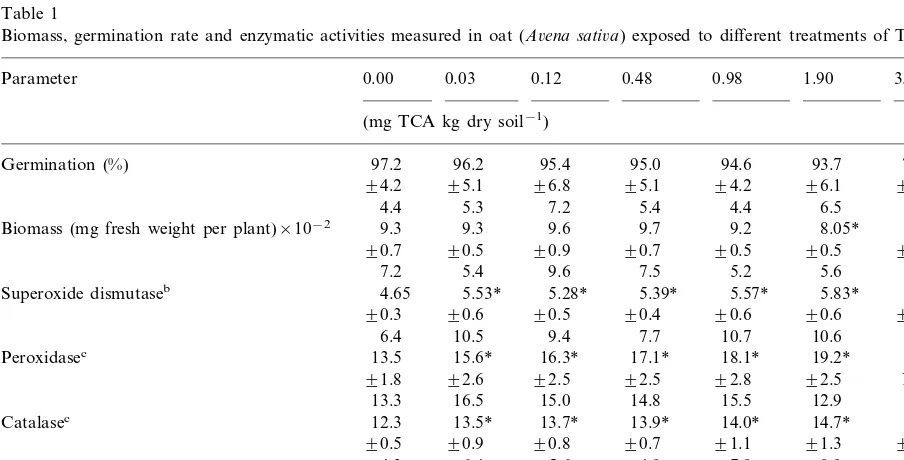
Table 1
Biomass, germination rate and enzymatic activities measured in oat (A6ena sati6a) exposed to different treatments of TCAa
Parameter 0.00 0.03 0.12 0.48 0.98 1.90 3.90
(mg TCA kg dry soil−1)
95.0 94.6 93.7
Germination (%) 97.2 96.2 95.4 73.3*
96.8
94.2 95.1 95.1 94.2 96.1 96.4 7.2
4.4 5.3 5.4 4.4 6.5 8.8
6.1* 8.05*
9.2 9.7
Biomass (mg fresh weight per plant)×10−2 9.3 9.3 9.6
90.7 90.5 90.5 90.3 90.7 90.5 90.9
7.2 5.4 9.6 7.5 5.2 5.6 4.4
Superoxide dismutaseb 4.65 5.53* 5.28* 5.39* 5.57* 5.83* 5.78* 90.6
90.6
90.4 90.8 90.5
90.6 90.3
6.4 10.5 9.4 7.7 10.7 10.6 14.5
17.1* 18.1* 19.2*
Peroxidasec 13.5 15.6* 16.3*
91.8 92.6 92.5 92.5 92.8 92.5 NR
13.3 16.5 15.0 14.8 15.5 12.9
Catalasec 12.3 13.5* 13.7* 13.9* 14.0* 14.7* 15.9*
90.5 90.9 90.8 90.7 91.1 91.3 91.7 4.9
6.4
4.3 5.6 7.9 8.9 10.5
Glutathione reductasec 16.9 19.8* 21.5* 22.1* 22.1* 23.6* 94.6
92.4 92.8
90.7 90.6 94.4 NR 10.7
13.2
2.9 20.6
4.0 18.4
aData (n=9) are listed in the following order: mean, standard deviation and coefficient of variation; NR, not realized. b(Asada units mg protein−1)×10−1.
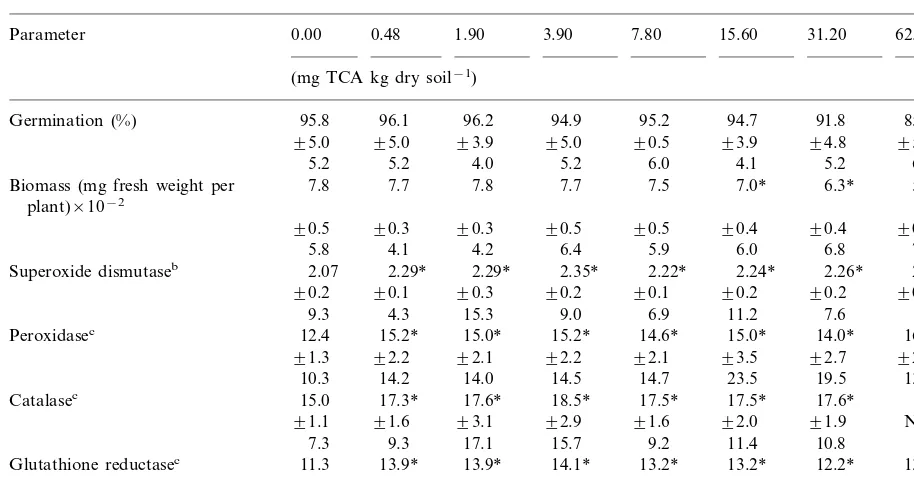
Table 2
Biomass, germination rate and enzymatic activities measured in Chinese cabbage (Brassica campestris cv. chinensis) exposed to different treatments of TCAa
0.48 1.90 3.90 7.80
0.00 15.60
Parameter 31.20 62.50
(mg TCA kg dry soil−1)
96.1 96.2 94.9 95.2 94.7 91.8 85.7
Germination (%) 95.8
95.0 93.9 95.0 90.5
95.0 93.9 94.8 95.8
5.2 4.0 5.2 6.0 4.1 5.2
5.2 6.8
7.8 7.7 7.8 7.7 7.5
Biomass (mg fresh weight per 7.0* 6.3* 5.6*
plant)×10−2
90.3 90.3 90.5 90.5
90.5 90.4 90.4 90.4
4.1 4.2 6.4 5.9
5.8 6.0 6.8 7.4
Superoxide dismutaseb 2.07 2.29* 2.29* 2.35* 2.22* 2.24* 2.26* 2.30* 90.1 90.3 90.2 90.1
90.2 90.2 90.2 90.3
9.3 4.3 15.3 9.0 6.9 11.2 7.6 1.9
15.2*
Peroxidasec 12.4 15.0* 15.2* 14.6* 15.0* 14.0* 16.4*
92.2 92.1 92.2 92.1
91.3 93.5 92.7 92.2
14.2 14.0 14.5 14.7 23.5
10.3 19.5 13.1
17.3* 17.6* 18.5* 17.5*
15.0 17.5*
Catalasec 17.6*
91.1 91.6 93.1 92.9 91.6 92.0 91.9 NR
9.3 17.1 15.7 9.2
7.3 11.4 10.8
11.3
Glutathione reductasec 13.9* 13.9* 14.1* 13.2* 13.2* 12.2* 13.1* 91.5
90.7 91.9 91.6 91.6 92.8 91.6 91.8
10.9 13.9 11.4 12.0 21.2 12.8
6.3 13.7
aData (n=9) are listed in the following order: mean, standard deviation and coefficient of variation; NR, not realized. b(Asada units mg protein−1)×10−1.
c(Enzymatic units mg protein−1)×10−2. * Statistically significant differences (P50.05).
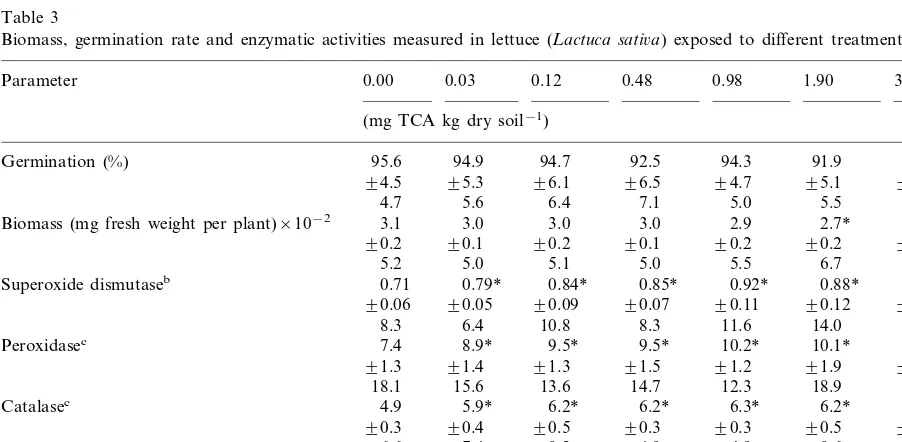
clearly decreased with increasing TCA concentra-tions for the three plant species. Germination rates decreased significantly with LOEC values of 3.9 mg TCA kg dry soil−1for oat and lettuce and
62.5 mg TCA kg dry soil−1 for Chinese cabbage.
The reproducibility of germination endpoint was good, with coefficients of variation between 4.0 and 8.8%.
Concerning biomass in species response, lettuce and oat showed the same sensitivity for TCA with an LOEC value of 1.9 mg TCA kg dry soil−1,
both species being more sensitive than Chinese cabbage which showed an LOEC value of 15.6 mg TCA kg dry soil−1. For biomass endpoint, the
reproducibility was similar to the germination endpoint, with coefficients of variation between 4.1 and 9.6%.
3.2. Effects of TCA on enzyme acti6ities
Activities of all enzymes were significantly dif-ferent from plant controls at the lowest concentra-tion of TCA tested, i.e. 0.03 mg TCA kg dry soil−1 for oat and lettuce and 0.48 mg TCA kg
dry soil−1 for Chinese cabbage. As all test
superoxide dismutase (30% for lettuce, 25% for oat, and 14% for Chinese cabbage).
The peroxidase showed a higher coefficient of variation, ranging from 10.3 to 23.5% (mean CV=15.2%), while catalase showed the lower coefficient of variation (mean CV=8.3%).
At highest concentrations of TCA, severe biomass and germination rate diminution were observed, as well strong reduction on enzyme activities (results not shown).
4. Discussion
Toxicity assessment of compounds on plants includes methods that evaluate critical develop-mental stages in plant life history, such as germi-nation and shooting phases. Regarding sensitivity, our results showed that germination rate endpoint was less sensitive than biomass and than biochem-ical endpoints. This trend agrees with other stud-ies (USEPA, 1982; Gorsuch et al., 1990). The lowest sensitivity of seed germination endpoint is
mainly due to the fact that the nutritional require-ment comes from the seed storage materials and not from the environment (Wang, 1985). In our study, the LOEC values for germination rate were 3.9 mg TCA kg dry soil−1for oat and lettuce and
62.5 mg TCA kg dry soil−1for Chinese cabbage,
while LOEC values for biomass endpoint were 1.9 mg TCA kg dry soil−1 for oat and lettuce and
15.6 mg TCA kg dry soil−1for Chinese cabbage.
Therefore the LOEC ratios between germination and biomass endpoints are relatively close from one plant species to another: for oat and lettuce, this ratio was 2, while for Chinese cabbage it was 4.
The different sensitivity between plant species can be attributed to genetic and physiological differences (Gerakis et al., 1980; Fletcher et al., 1990). Lettuce is generally considered as a sensi-tive plant species in phytotoxicity tests (Ratsch, 1983; Adema and Henzen, 1989). The same sensi-tivity in biomass response between oat (mono-cotyledon) and lettuce (di(mono-cotyledon) observed in this study may be explained by the fact that TCA
Table 3
Biomass, germination rate and enzymatic activities measured in lettuce (Lactuca sati6a) exposed to different treatments of TCAa
0.00 0.03 0.12
Parameter 0.48 0.98 1.90 3.90
(mg TCA kg dry soil−1)
95.6
Germination (%) 94.9 94.7 92.5 94.3 91.9 81.4*
95.3 96.1 96.5 94.7
Superoxide dismutaseb 0.85* 0.92* 0.88* 0.87*
90.10
Peroxidasec 7.4 8.9* 9.5* 9.5* 10.2* 10.1* 10.1*
91.3 91.4 91.3 91.5 91.2 91.9 91.5
18.1 15.6 13.6 14.7 12.3 18.9 15.2
4.9 5.9* 6.2*
Glutathione reductasec 6.7* 6.4* 6.5* 6.1* 6.0*
90.6
aData (n=9) are listed in the following order: mean, standard deviation and coefficient of variation; NR, not realized. b(Asada units mg protein−1)×10−1.
is particularly effective against monocotyledons. The same observation was made by other authors (Adema and Henzen, 1989) who found LOEC values of 3.2 and 1.0 mg TCA kg dry soil−1 for
lettuce and oat respectively in a phytotoxicity test according to OECD guideline c 208.
The activities of superoxide dismutase, catalase, peroxidase and glutathione reductase increased significantly at the lowest tested concentration of TCA i.e. 0.03 mg TCA kg dry soil−1(for oat and
lettuce) and 0.48 mg TCA kg dry soil−1
(for Chinese cabbage). These increases in enzyme ac-tivities at lower TCA concentrations are not sur-prising, since molecular alterations are usually the first detectable responses to exposure and effects of compounds at toxic concentrations (Stegeman et al., 1992). Concerning plant enzyme increase even after ten days of exposure, it was reported (Foy, 1969) that TCA can persists in the plant for weeks, but in soil it is degraded rapidly by mi-croorganisms (Tortensson, 1976). A low microbial population (:5×102 CFU g soil−1) present in
the dried soil used in our experiments could also explain the longer phytoavailability of TCA .
The higher coefficients of variation presented by the peroxidase enzyme (between 10.3 and 23.5%) could be due to their extreme sensitivity to several experimental or natural factors (Endress et al., 1980; Gaspar et al., 1982).
Globally, the increase in enzyme activities showed that the presence of TCA activated the protection system to increase its capacity to scav-enge active oxygen species, but we observed dif-ferent concentration – response relationships: a plateau (e.g. SOD in Table 2); constant increase (e.g. peroxidase in Table 1); increase and decrease (e.g. GR in Table 3). The moderate increase in enzymatic response might be better understood if we consider that developmentally controlled mechanisms determining basal antioxidant en-zyme activities, and not inductive responses, can appear to be the critical factors mediating short-term oxidative stress resistance (Donahue et al., 1997). It is pertinent to note here that increase of antioxidant enzyme activities is not TCA-specific, as such responses as those described can be mea-sured after exposure to a wide variety of chemical contaminants (Stegeman et al., 1992). Thus,
Kraus and Fletcher (1994) reported a moderate increase in antioxidant enzyme activities in wheat seedlings exposed to stress conditions (heat and paraquat), and they supported the hypothesis that paclobutrazol-induced protection in these seedlings was in part mediated by enhanced detoxification of active oxygen. This triazole com-pound (paclobutrazol) stimulated an increase in the activities of superoxide dismutase (16%), ascorbate peroxidase (32%), glutathione reductase (21%), and within the cytoplasm an increase in activities of catalase (45%) and guaiacol perox-idase (29%) on a fresh weight basis.
The responses of plants to herbicides are varied, highly complex and by no means a plethora of theories have been advanced to explain toxic ac-tions. A herbicide can show different effects in the cell. Although TCA has been reported to alter carbohydrate (Rebstock et al., 1953), lipid (Dewey et al., 1962), and nitrogen metabolism (Mashtakov and Moshchuk, 1967), most of these responses are probably of a secondary nature and reflect a more basic primary mechanism of action common to all of these biomolecules. Our results showed that, in vivo, the oxygen derived species may be implicated in the toxic action of plants exposed to herbicide TCA, since there was a significant increase in the activities of most impor-tant antioxidant enzymes. Our present investiga-tion did not include measurements of biomarkers of oxidative damage to biological macromolecules (e.g., malonaldehyde — MDA, or 8-hydroxy de-oxyguanosine — 8-OHdG), but it was reported (Navari-Izzo and Izzo, 1994) that SO2-fumigated
leaves of wheat, which had neither visible injury nor lipid peroxidation, greatly increased activities of most important antioxidant enzymes and non-protein thiol GSH.
Germination and growth responses have the ad-vantage of high ecological significance, but rela-tively low sensitivity to stress. Conversely, biochemical markers are very sensitive and have the advantage of a rapid response time: Van Assche and Clijsters (1990) reported the transient detection of cationic peroxidases after 2 h of exposure. Moreover, they offer the possibility to give some explanations on the mechanism(s) of action and they reveal that plant are (perhaps) ‘suffering’ without any perceptible biomass mod-ifications (Vangronsveld et al., 1997).
In conclusion, our results showed a ranking of sensitivity among the different endpoints for the three plant species: enzyme activities\biomass\ germination rate. The increase in antioxidant en-zyme activities observed in this study ensured the detoxification of increased levels of active oxygen species, and presumably prevented the plants from undergoing oxidative stress damage. Thus, the use of enzyme activities will permit the detec-tion of early injury in plant growth testing. Never-theless, a better knowledge of ecological relevance of these biomarkers is needed in the future.
Acknowledgements
Claudemir Marcos Radetski greatly acknowl-edges the fellowship support of CNPq (Brazilian Conselho Nacional de Desenvolvimento Cientı´fico e Tecnolo´gico).
References
Adema, D.M.M., Henzen, L., 1989. A comparison of plant toxicities of some industrial chemicals in soil culture and soil-less culture. Ecotox. Environ. Saf. 18, 219 – 229. Asada, K., 1993. Production and action of active oxygen
species in photosynthetic tissues. In: Foyer, C.H., Mullineaux, P.M. (Eds.), Causes of Photo-Oxidative Stress and Amelioration of Defense Systems in Plants. CRC Press, Boca Raton, FL, pp. 77 – 104.
Asada, K., Takahashi, M.A., Nagate, M., 1974. Assay and inhibitors of spinach superoxide dismutase. Agric. Biol. Chem. 38, 471 – 473.
Ashton, F.M., Crafts, A.S., 1981. Mode of Action of Herbi-cides. Wiley Publishers, New York.
Beers, Jr., R.F., Sizer, I.W., 1951. A spectrophotometric method for measuring the breakdown of hydrogen perox-ide by catalase. J. Biol. Chem. 195, 133 – 140.
Bergmeyer, H.U., Grabßl, M., Walter, H.E., 1983. Biochemi-cal reagents for general use: enzymes. In: Bergmeyer, H.U., Bergmeyer, J., Grabßl, M. (Eds.), Methods of Enzymatic Analysis, vol. II. Verlag Chemie, Weinheim, pp. 126 – 327. Blanchard, F.A., 1954. Uptake, distribution, and metabolism of carbon-14 labeled TCA in corn and pea plants. Weeds 3, 274 – 278.
Bowmer, K.H., 1987. Residues of Dalapon and TCA in sedi-ments and irrigation water. Pestic. Sci. 18, 1 – 13. Bradford, M.M., 1976. A rapid and sensitive method for the
quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248 – 254.
Byl, T.D., Sutton, H.D., Klaine, S.J., 1994. Evaluation of peroxidase as a biochemical indicator of toxic chemical exposure in the aquatic plant Hydrilla6erticillataRoyle. Environ. Toxicol. Chem. 13, 509 – 515.
Dewey, O.R., Hartley, G.S., Mackaughlan, J.W.G., 1962. External leaf waxes and their modification by heat-treat-ment of plants with trichloroacetate. Proc. Roy. Soc. 155, 432 – 450.
Donahue, J.L., Okpodu, C.M., Cramer, C.L., Grabau, E.A., Alscher, R.G., 1997. Plant responses of antioxidants to paraquat in pea (Pisum sati6um) leaves. Plant Physiol. 113, 249 – 257.
Elstner, E.F., 1982. Oxygen activation and oxygen toxicity. Ann. Ver. Plant Physiol. 33, 73 – 96.
Endress, A.G., Suarez, S.J., Taylor, O.C., 1980. Peroxidase activity in plant leaves exposed to gaseous HCl or ozone. Environ. Pollut. 22, 47 – 58.
Ernst, W.H.O., Peterson, P.J., 1994. The role of biomarkers in environmental assessment (4). Terrestrial plants. Ecotoxi-cology 3, 189 – 192.
Fletcher, J.S., Johnson, F.L., McFarlane, J.C., 1990. Influence of greenhouse versus field testing and taxonomic differ-ences on plant sensitivity to chemical treatment. Environ. Toxicol. Chem. 9, 769 – 776.
Foy, C.L., 1969. The chlorinated aliphatic acids. In: Kearney, P.C., Kaufman, D.D. (Eds.), Degradation of Pesticides. Marcel Dekker, New York, pp. 207 – 253.
Foyer, C.H., Descourvie`res, P., Kunert, K.J., 1994. Protection against oxygen radicals: an important defense mechanism studied in transgenic plants. Plant Cell Environ. 17, 507 – 523.
Gaspar, T., Penel, C., Thorpe, T., Greppin, H., 1982. Peroxi-dases 1970 – 1980. A survey of their biochemical and physi-ological roles in higher plants. Centre de Botanique, Universite´ de Geneve, Geneve.
Gerakis, P.A., Veresglou, D.S., Sakellariadis, S.D., 1980. Dif-ferential response of sugar beetBeta6ulgarisL. cultivars to lead. Environ. Pollut. 21, 77 – 83.
(Eds.), Plants for Toxicity Assessment, ASTM STP 1091. American Society for Testing and Materials, Philadelphia, PH, pp. 49 – 58.
Hashimoto, S., Azuma, T., Otsuki, A., 1998. Distribution, sources, and stability of haloacetic acids in Tokyo bay, Japan. Environ. Toxicol. Chem. 17, 798 – 805.
Keller, T., 1974. The use of peroxidase activity for monitoring and mapping air pollution areas. Eur. J. Forest Pathol. 4, 11 – 19.
Kraus, T.E., Fletcher, R.A., 1994. Paclobutrazol protects wheat seedlings from heat and paraquat injury. Is detoxifi-cation of active oxygen involved? Plant Cell Physiol. 35, 45 – 52.
Mashtakov, S.M., Moshchuk, P.A., 1967. The effect of sodium trichloroacetate on the content of nitrogenous substances in varities of lupin resistant and sensitive to herbicides. Agrokhimiya 9, 80 – 89.
Mu¨ller, S.R., Zweifel, H.-R., Kinnison, D.J., Jacobsen, J.A´ ., Meier, M.A., Ulrich, M., Schwarzenbach, R.P., 1996. Oc-curence, sources, and fate of trichloroacetic acid in Swiss waters. Environ. Toxicol. Chem. 15, 1470 – 1478. Navari-Izzo, F., Izzo, R., 1994. Induction of enzyme activities
and antioxidant production in barley plants as a result of SO2fumigation. Plant Sci. 96, 31 – 40.
Organization for Economic Cooperation and Development (OECD), 1984. Terrestrial plants, growth test. Guidelines for Testing of Chemicals, OECD, Nc 208, Paris. Paoletti, F., Mocali, A., 1990. Determination of superoxide
dismutase activity by purely chemical system based on NAD(P)H oxidation. In: Packer, L., Glazer, N.A. (Eds.), Methods in Enzymology, vol. 186. Academic Press, New York, pp. 209 – 219.
Ratsch, H.C., 1983. Interlaboratory root elongation testing of toxic substances on selected plant species. EPA 600/ S3-83-051. United States Environmental Protection Agency, En-vironmental Research Laboratory, Corvallis, OR. Rebstock, T.L., Hamner, C.L., Lueeke, R.W., Sell, H.M.,
1953. The effect of sodium trichloroacetate upon the metabolism of wheat seedlings (Triticum6ulgareL.). Plant Physiol. 28, 437 – 442.
Sies, H., 1997. Oxidative stress: oxidants and antioxidants. Exp. Physiol. 82, 291 – 295.
Smirnoff, N., 1993. The role of active oxygen in the response of plants to water deficit and desiccation. New Phytol. 125, 27 – 58.
Stegeman, J.J., Brouwer, M., Di Giulio, R.T., Fo¨rlin, L., Fowler, B.A., Sanders, B.M., Van Veld, P.A., 1992. Molec-ular responses to environmental contamination: enzyme and protein systems as indicators of chemical exposure and effect. In: Huggett, R.J., Kimerle, R.A., Mehrle, P.M., Jr, Bergman, H.L. (Eds.), Biomarkers. Lewis Publishers, Boca Raton, FL, pp. 235 – 335.
Tortensson, L., 1976. Effects of some factors on persistence of TCA in arable soil. Swed. Weed Conf. 17, 8 – 13. USEPA, 1982. Environmental Effects Test Guidelines. EPA
560/6-82-002, Office of Toxic Substances, Washington, DC, USA.
Van Assche, F., Clijsters, H., 1990. Effects of metals on enzyme activity in plants (review). Plant Cell Environ. 13, 195 – 206.
Van Assche, F., Cardinaels, C., Clijsters, H., 1988. Induction of enzyme capacity in plants as a result of heavy-metal toxicity: dose-response relations in Phaseolus6ulgaris L. treated with zinc and cadmium. Environ. Pollut. 52, 103 – 115.
Vangronsveld, J., Mocquot, B., Mench, M., Clijsters, H., 1997. Biomarqueurs du stress oxydant chez les ve´ge´taux. In: Lagadic, L., Caquet, T., Amiard, J.C., Ramade, F. (Eds.), Biomarqueurs en Ecotoxicologie — Aspects Fondamen-taux. Masson, Paris, pp. 165 – 184.
Wang, W., 1985. The use of plant seeds in toxicity tests of phenolic compounds. Environ. Int. 11, 49 – 55.
Williams, D.A., 1971. A test for differences between treatment means when several dose levels are compared with a zero dose control. Biometrics 27, 103 – 117.
Winston, G.W., 1990. Physiochemical basis for free radical formation in cells: production and defenses. In: Alscher, R.G., Cumming, J.R. (Eds.), Stress Responses in Plants: Adaptation and Acclimation Mechanisms. Wiley-Liss, New York, pp. 57 – 86.