www.elsevier.com / locate / bres
Research report
Androgen receptor-immunoreactivity in the forebrain of the Eastern
Fence lizard (Sceloporus undulatus)
a ,
*
b a bM.M. Moga
, B.M. Geib , D. Zhou , G.S. Prins
a
Terre Haute Center for Medical Education, Indiana University School of Medicine, Terre Haute, IN 47809, USA b
Department of Urology, University of Illinois at Chicago, Chicago, IL 60612, USA
Accepted 25 July 2000
Abstract
Androgen receptor (AR) distribution in the lizard forebrain and optic tectum was examined using PG21 immunohistochemistry. In the male Eastern Fence lizard, AR-immunoreactive (-ir) nuclei were observed in the medial preoptic area, ventromedial and arcuate hypothalamic nuclei, periventricular hypothalamus, premammillary nucleus, bed nucleus of the stria terminalis, and ventral posterior amygdala. Punctate immunostaining of neuronal processes (axons and / or dendrites) was concentrated in the cortex, hypothalamus, and optic tectum. AR-ir nuclei in the female brain were confined to the ventral posterior amygdala and ventromedial hypothalamic nucleus. The AR distribution in the lizard brain is similar to that reported for other vertebrate classes. Sex differences in AR-immunoreactivity may contribute to sex-specific behaviors in the Eastern Fence lizard. 2000 Elsevier Science B.V. All rights reserved.
Theme: Other systems of the CNS
Topic: Comparative neuroanatomy
Keywords: Steroid receptor; Hypothalamus; Amygdala; Cortex; Reptile
1. Introduction species-specific AR-ir cell clusters, such as the song control nuclei of songbirds [1,2], and have increased our Male reproductive behavior is activated by circulating understanding of the neural basis of reproductive behaviors sex steroids, particularly the androgens testosterone and [20].
5a-dihydrotestosterone (DHT). Androgens elicit sex-spe- To date, there has been no immunocytochemical study cific behaviors by acting directly on androgen receptors in of AR in the reptilian brain. An early study by Morrell et
3
the brain or through indirect activation of brain estrogen al. [26] employed H-testosterone autoradiography to receptors via androgen aromatization [32]. In whiptail and identify androgen-concentrating cells in the lizard brain. anole lizards, androgens are more effective than estrogenic Some of the androgen-concentrating cells which they metabolites in eliciting male sexual behavior [14,38,42]. identified may actually have been estrogen-concentrating The distribution of androgen receptor (AR) protein and cells, as circulating testosterone is aromatized to estrogen mRNA in the brain shows a similar pattern among the in certain areas of the brain. Recently, Young et al. [46] different vertebrate classes, including bird [2,36], fish [19], analyzed the distribution of AR mRNA in the brain of the reptile [46], and mammal [21,35]. In each of these classes, whiptail lizard using in situ hybridization. Although highly AR-positive cells are concentrated in the hypothalamus sensitive, this method cannot distinguish between cyto-and amygdala, specifically in brain nuclei that have been plasmic and nuclear staining, or identify AR-positive implicated in male reproductive and aggressive behavior. dendrites and axons. In the present study, we map the Comparative studies have proven useful in delineating distribution of AR in the forebrain of the Eastern Fence lizard (Sceloporus undulatus) using immunocytochemistry. The AR antibody employed in the present experiments
*Corresponding author. Tel.: 11-812-237-3420; fax: 1
1-812-237-(PG21) is directed against amino acids 1–21 of the rat AR
7646.
E-mail address: [email protected] (M.M. Moga). [30]. The lizard AR shows a high degree of sequence
homology with ARs in other species [47]. The DNA- (Elite Standard ABC kit; Vector) for 1–2 h. After several binding and C-terminal ligand-binding domains of AR are rinses in PBS, the sections were reacted in a solution of the most highly conserved [37]. However, positions 1–35 0.05% 3,39-diaminobenzidine tetrahydrochloride (DAB; and 230–268 in the AR are far less variant than the rest of Sigma)-0.025% nickel chloride-0.01% H O in 0.1 M Tris2 2 the N-terminal domain [37]. A low degree of variance in buffer, pH 7.4, for 10 min. The sections were mounted on amino acids 1–21 of the AR may explain the ability of the gel-coated slides, dehydrated through alcohols and xylene, PG21 antibody to label ARs in diverse tissue types in a and coverslipped with Permount (Fisher).
variety of vertebrates, including mammal [11,13,18,21, The specificity of the PG21 antibody was tested by, (1) 44,45,49], fish [15], bird [2,36], and amphibian [16]. preabsorption of the primary antibody with its respective peptide (AR21; amino acids 1–21 of the rat AR), (2) preabsorption of the primary antibody with a peptide 2. Methods derived from a different portion of the rat AR (AR462; amino acids 462–478), and (3) omission of the primary Male (n56) and female (n55) lizards (Sceloporus antibody. In the two preabsorption controls, sections were undulatus) were purchased from Charles D. Sullivan Co., incubated in 1 ml of diluted antibody (1:5000) preincu-Inc. (Nashville, TN) during the spring / summer breeding bated with 370 ng of peptide (AR21 or AR462). No season (May through September). Nine of the lizards (5 immunocytochemical staining was observed in controls males, 4 females) were classified as S. undulatus consob- where, (1) the primary antibody was omitted, or (2) the rinus (Southern Prairie subspecies); the other two in- primary antibody was preincubated with its respective dividuals (experiments L8 and L18) were identified as S. peptide (AR21). Staining was intact in control experiments undulatus garmani (Northern Prairie subspecies). Animals involving preabsorption with the distant, unrelated peptide
were maintained in captivity for 2–8 weeks in glass (AR462).
aquaria (2 to 3 animals per aquaria) with peat moss as The distribution of AR-immunoreactivity in representa-substrate, and sticks and stones for climbing. Photoperiod tive sections was plotted on a microscope (BH-2, was maintained at 12 h light: 12 h dark. Room temperature Olympus) with a digital stage readout head attached to a was 21–238C, with additional heat (up to 388C) provided computer with Neurolucida software (MicroBrightField, by a heat lamp situated at one end of the terrarium. Water Inc.). A camera lucida was used to add cytoarchitectural was available at all times. Animals were fed crickets 2 to 3 details obtained from adjacent Nissl-stained sections. times per week, supplemented with reptile vitamins.
At the time of sacrifice, animals were given an overdose
of sodium pentobarbital followed by decapitation. The 3. Results dorsal skullcase was removed, and the brain was
im-mersion-fixed in situ in 4% paraformaldehyde 0.1 M AR-immunoreactivity was observed in select brain areas phosphate buffer, pH 7.4, for 5–12 days. Following in both male and female S. undulatus. The distribution of fixation, the brains were removed from the skull and AR-immunoreactivity in the male Eastern Fence Lizard embedded in 10% gelatin. The gelatin-brain blocks were brain was examined in six experiments. One male lizard fixed in 4% paraformaldehyde for an additional 4–6 days. (experiment L2) was obtained and sacrificed in July, 1998; Next, the brains were cut into 20 mm sections on a two males (experiments L8 and L24) were obtained in Vibratome. One series of sections (one-of-three) was September, 1998 and sacrificed in November, 1998; and processed for AR-immunocytochemistry, and a second three males (experiments L21, L22 and L25) were obtained series was mounted on gel-coated slides and Nissl-stained and sacrificed in June, 1999. The pattern of AR-ir labeling
with thionin. was identical in these six experiments, but the number of
The immunocytochemical method was as follows. Sec- labeled cells and the intensity of the immunolabeling tions were treated with 0.3% hydrogen peroxide in 0.01 M varied by experiment. The following description specifical-phosphate-buffered saline (PBS), pH 7.4, for 10 min, ly refers to representative experiment L8, which exhibited rinsed in PBS, treated with 0.5% sodium borohydride in the most robust and widespread labeling.
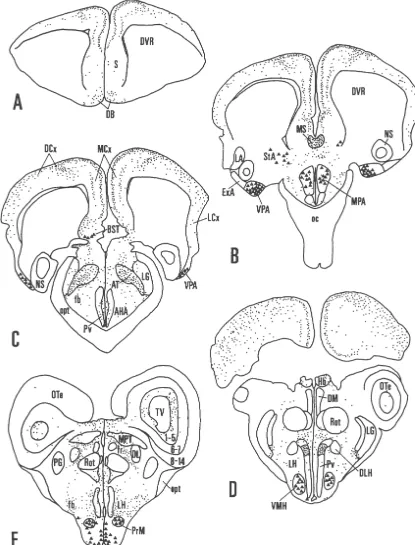
PBS for 5–10 min, and preincubated for 1 h in a PBS At rostral levels in experiment L8, AR-immunoreactivi-solution containing 2% normal donkey serum (NDS) and ty was concentrated in the medial and dorsal cortices (Figs. 0.3% Triton X-100 (TX). Sections were then incubated 1A–D; 2A–B). AR-ir fibers in the medial cortex were with the AR antibody (PG21), diluted 1:1,000–1:5,000 organized in a bilaminar pattern with both layers juxtap-(0.2–1.0 mg / ml, final concentration) in PBS-NDS-TX, for osed to the principal neuron layer (Fig. 2A). In the dorsal 3–6 days at 48C. Next, the sections were rinsed in PBS, cortex, AR-ir fibers displayed numerous varicosities (Fig. incubated with a secondary antibody (biotin–SP-conju- 2). At more caudal levels, AR-ir fibers were also found in gated donkey anti-rabbit IgG; Jackson ImmunoResearch; the lateral cortex (Fig. 1B, C). Cytoplasmic staining was
diluted 1:200 in PBS-NDS-TX) for 1–2 h at room observed in a small number of neurons scattered in the
dense concentration of labeled fibers in the medial septal experiment L8 (November) than in representative
experi-nucleus (Fig. 1B). ments L2 (July) and L21 (June). Conversely, more labeled
Several cell groups in the amygdala contained AR-ir cells were observed in the bed nucleus of the stria nuclei. In many of the labeled cell nuclei, a darkly stained terminalis and striatoamygdalar area in experiment L2 than AR-ir nucleolus was evident. One amygdalar cell group in L8 or L21. The intensity of the immunolabeling (both with AR-ir nuclei formed a band of labeling ventral to the AR-ir nuclei and fibers) was greatest in L8, moderate in striatum (Fig. 1B); this cell group corresponded at rostral L2, and least in L21.
levels to the striatoamygdalar area, and at caudal levels, to AR-immunoreactivity in the female Eastern Fence lizard the medial / interstitial amygdala [7]. Labeled neurons in brain was examined in five experiments. Two female this cell group (striatoamygdalar area, medial / interstitial lizards (experiments L17 and L18) were obtained in amygdala) were large in size and widely spaced. A few, September, 1998 and sacrificed in November, 1998; and small, lightly stained AR-ir nuclei were observed in the three females (experiments L19, L20 and L23) were adjacent bed nucleus of the stria terminalis (Fig. 1B, C). A obtained and sacrificed in May, 1999. The distribution of dense cluster of darkly stained, AR-ir nuclei was found in AR-immunoreactivity was identical for each case. the ventral posterior nucleus of the amygdala (Figs. 1B, C, In representative experiment L23, AR-ir nuclei were 3A) [7,25]. This cell group, composed of closely packed, confined to the ventral posterior amygdala (Fig. 4A) and small neurons, formed a wedged-shaped protuberance at the ventromedial hypothalamic nucleus. Labeled nuclei in rostral levels (Fig. 1B); it extended caudally as a thin band these areas were more lightly stained and fewer in number of cells ventrolateral to the nucleus sphericus (Fig. 1C). than in the male brain. No nuclear labeling was observed In the hypothalamus, some lightly stained AR-ir nuclei in the medial preoptic area, the arcuate nucleus, the bed were observed in the medial preoptic area, particularly in nucleus of the stria terminalis, or the striatoamygdalar area the caudal dorsal portion (Fig. 1B). Scattered AR-ir fibers, in any experiment involving female lizards (Fig. 4B). punctate in appearance, were present throughout the preop- The pattern of AR-ir fiber labeling in representative tic and anterior hypothalamic areas (Fig. 2C). Immediately experiment L23 (female) closely resembled that in L8 caudal to the optic chiasm, we observed a concentration of (male). In both male and female, AR-ir fibers were AR-ir fibers in a dorsal section of the periventricular concentrated in the medial cortex, dorsal cortex, medial hypothalamus (Fig. 1D). In Nissl-stained sections, this septum, area triangularis, dorsal portion of the periven-hypothalamic area showed a distinct cytoarchitecture with tricular hypothalamus, habenular nuclei, optic tectum, many small, dark-staining neurons as well as larger, lateral forebrain bundle, and the white matter surrounding medium-staining neurons. Adjacent areas in the periven- the third ventricle.
tricular hypothalamus contained only large, medium-stain-ing neurons. A cluster of lightly labeled AR-ir nuclei was
observed the ventromedial nucleus (Fig. 1D). Many darkly 4. Discussion stained, AR-ir nuclei with prominent, stained nucleoli were
present in the premammillary and arcuate nuclei (Figs. 1E; Our results show that AR-immunoreactivity is concen-3B, C). Scattered AR-ir fibers were found throughout the trated in limbic areas of the lizard forebrain, particularly
hypothalamus. the medial cortex, amygdala, and hypothalamus, and that
AR-immunoreactivity was observed in a few other areas this immunoreactivity is sexually dimorphic, with a wider of the diencephalon. AR-ir fibers were concentrated in a distribution of AR-ir nuclei present in the male brain as cell-dense area located medial to the lateral geniculate compared to the female.
nucleus and dorsal to the forebrain bundles (Fig. 1C); this In the male fence lizard, we observed AR-ir cell nuclei region corresponded to the area triangularis [9]. Axonal in the medial preoptic area, the ventromedial hypothalamic labeling was present throughout the lateral forebrain nucleus, the arcuate nucleus, the caudal ventral part of the bundle (Fig. 1B–E). Many AR-ir fibers coursed dorsally periventricular hypothalamus, the premammillary nucleus, through the white matter adjacent to the third ventricle, but the bed nucleus of the stria terminalis, the striatoamygdalar did not extend into the gray matter of the thalamus (Fig. area, and the ventral posterior amygdala. This distribution 1E). A band of AR-ir labeling was observed in the is similar to the AR mRNA distribution reported for the fasciculus retroflexus, ventral to the medial pretectal whiptail lizards, Cnemidophorus uniparens and C. inor-nucleus [28] and dorsolateral to the caudal portion of the natus [46]. In the whiptail lizard, AR is expressed in the nucleus rotundus (Fig. 1E). In the optic tectum, labeled external amygdalar nucleus, the ventromedial hypo-fibers were concentrated in the deep, ventricular layers 1–5 thalamic nucleus, the medial preoptic area, the
premammil-(Figs. 1E; 3D). lary nucleus, and the periventricular hypothalamus. The
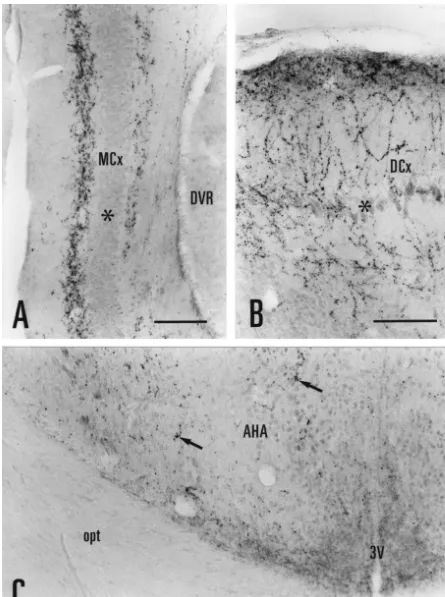
Fig. 3. Photomicrographs of AR-ir nuclei and fibers in the male lizard brain (experiments L2 and L8). (A) A prominent cluster of AR-nuclei was observed in the ventral posterior nucleus of the amygdala. (B) Darkly stained AR-ir nuclei and fibers were present in the premammillary nucleus. (C) AR-ir nuclei were abundant in the arcuate nucleus and periventricular hypothalamus. (D) Punctate labeling was concentrated in the deep layers of the optic tectum surrounding the tectal ventricle. Arc, arcuate nucleus; OTe, optic tectum; Pv, periventricular hypothalamus; PrM, premammillary nucleus; TV, tectal ventricle, VPA, ventral posterior nucleus of the amygdala; 3V, third ventricle. Scale bar5100mm.
in [46]). Young et al. [46] observed several additional tional AR cell populations detected in their study. Alter-AR-positive cell clusters in the whiptail lizard that we did natively, there may be species differences in AR expres-not detect in the Eastern Fence lizard, such as the dorsal sion between whiptail and fence lizards.
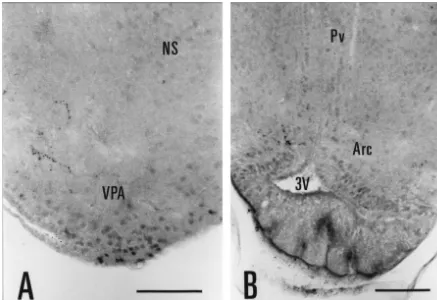
Fig. 4. Photomicrographs of AR-ir nuclei and fibers in the female lizard brain (experiment L23). (A) In the ventral posterior amygdala, most of the AR-ir nuclei were lightly stained. (B) A few AR-ir fibers, but no AR-ir nuclei, were observed in the arcuate nucleus and periventricular hypothalamus. Arc, arcuate nucleus; NS, nucleus sphericus; Pv, periventricular hypothalamus; VPA, ventral posterior nucleus of the amygdala; 3V, third ventricle. Scale bar5100mm.
medial preoptic area; the arcuate, ventromedial and neostriatum [1], the supraoptic nucleus [13], the optic paraventricular hypothalamic nuclei; the ventral premam- tectum [19] and the nucleus of the lateral olfactory tract millary nucleus; the medial and cortical amygdalar nuclei; [21], may be involved in more specialized aspects of the CA1 hippocampus; and the cerebral cortex reproductive behavior (e.g., song production, intruder-chas-[10,12,21,34,43,45,49]. A similar pattern is observed in the ing) or in other adaptive behaviors (e.g., water homeosta-bird brain, with AR-ir nuclei concentrated in the medial sis).
long fixation times (9–18 days) involved in post mortem Acknowledgements immersion fixation and gelatin blocking.
AR cytoplasmic labeling was observed with the PG21 This work was supported by the Terre Haute Center for antibody in the Eastern Fence lizard brain. The punctate Medical Education, Indiana University School of Medi-staining pattern of the AR-ir fibers was consistent with a cine. We are grateful to Dr Diana Hews for introducing us synaptic localization on either axon terminals and / or to Sceloporus lizards.
dendrites. Glial processes may also possess steroid re-ceptors [22], but the length and diameter of the AR-ir fibers in the lizard brain makes a glial origin unlikely. We
References believe this cytoplasmic immunostaining to be specific for
AR for the following reasons. (1) In control experiments,
[1] J. Balthazart, A. Foidart, E.M. Wilson, G.F. Ball,
Immuno-AR-immunoreactivity in both cell nuclei and neuronal
cytochemical localization of androgen receptors in the male songbird
processes was completely abolished when the PG21 anti- and quail brain, J. Comp. Neurol. 317 (1992) 407–420.
body was preabsorbed with its immunogenic peptide [2] J. Balthazart, A. Foidart, M. Houbart, G.S. Prins, G.F. Ball,
(AR21) or when the primary antibody was omitted. (2) Distribution of androgen receptor-immunoreactive cells in the quail forebrain and their relationship with aromatase immunoreactivity, J.
Cytoplasmic staining of steroid receptors is well
docu-Neurobiol. 35 (1998) 323–340.
mented and has been reported for the estrogen [4,5,29],
[3] J.D. Blaustein, J.C. King, D.O. Toft, J. Turcotte,
Immunocytochemi-androgen [18,44], progestin [3,31], and glucocorticoid [33] cal localization of progestin receptor-immunoreactivity in guinea pig receptors. In the guinea-pig hypothalamus of ovarectom- hypothalamus and preoptic area, Brain Res. 474 (1988) 1–15.
ized females, estrogen receptor-immunoreactivity is pres- [4] J.D. Blaustein, J.C. Turcotte, Estrogen receptor-immunostaining of neuronal cytoplasmic processes as well as cell nuclei in guinea pig
ent in dendrites and axon terminals, as well as in the cell
brain, Brain Res. 495 (1989) 75–82.
soma [5]. A punctate staining pattern, similar to that
[5] J.D. Blaustein, M.N. Lehman, J.C. Turcotte, G. Greene, Estrogen
observed in the present study, has been demonstrated for receptors in dendrites and axon terminals in the guinea pig hypo-membrane glucocorticoid and estrogen receptors thalamus, Endocrinology 131 (1992) 281–290.
[29,33,40]. Future studies will examine the ultrastructure [6] G. Boivin, P. Mesguich, J.W. Pike, R. Bouillon, P.J. Meunier, M.R. Haussler, P.M. Dubois, G. Morel, Ultrastructural
immunocytochemi-and possible neuronal origin of the AR-ir fibers in the
cal localization of endogenous 1,25-dihydroxyvitamin D3 and its
fence lizard brain.
receptors in osteoblasts and osteocytes from neonatal mouse and rat
A sex difference in AR-immunoreactivity was detected calvaria, Bone and Mineral 3 (1987) 125–136.
in the hypothalamus and amygdala. AR-ir nuclei were [7] L.L. Bruce, T.J. Neary, The limbic system of tetrapods: a compara-abundant in the hypothalamus of the male fence lizard but tive analysis of cortical and amygdalar populations, Brain Behav.
Evol. 46 (1995) 224–234.
were restricted to the ventromedial nucleus in the female.
[8] A.B. Butler, W. Hodos, Comparative Vertebrate Neuroanatomy:
AR-immunostaining in the amygdala of the female lizard
Evolution and Adaptation, Wiley–Liss, New York, 1996.
was limited to the most ventral portion of the ventral
[9] A.B. Butler, R.G. Northcutt, Architectonic studies of the
dien-posterior nucleus; staining in the male was more extensive cephalon of Iguana iguana, J. Comp. Neurol. 149 (1973) 439–462. and spanned the ventral posterior nucleus, the [10] J.V.A. Choate, O.D. Slayden, J.A. Resko, Immunocytochemical
striatoamygdalar area and the bed nucleus of the stria localization of androgen receptors in brains of developing and adult male rhesus monkeys, Endocrine 8 (1998) 51–60.
terminalis. Few studies have examined the brains of both
[11] P. Ciofi, J.E. Krause, G.S. Prins, M. Mazzuca, Presence of nuclear
sexes for possible sex differences in AR protein or mRNA
androgen receptor-like immunoreactivity in neurokinin B-containing
expression. Six-day-old male rats show robust AR im- neurons of the hypothalamic arcuate nucleus of the adult male rat, munostaining of the ventral premammillary nucleus; this Neurosci. Lett. 182 (1994) 193–196.
nucleus is unstained in female rats of the same age [45]. In [12] A.N. Clancy, R.W. Bonsall, R.P. Michael, Immunohistochemical labeling of androgen receptors in the brain of rat and monkey, Life
the fetal mouse (E16), an intense AR mRNA signal is
Sci. 50 (1992) 409–417.
present in the male embryo brain with only weak AR
[13] A.N. Clancy, C. Whitman, R.P. Michael, H.E. Albers, Distribution
signal detected in the female brain [48]. These and the of androgen receptor-like immunoreactivity in the brains of intact present results suggest that a sex comparison of AR- and castrated male hamsters, Brain Res. Bull. 33 (1994) 325–332.
immunoreactivity in the brain of an adult bird or mammal [14] D. Crews, V. Traina, F.T. Wetzel, C. Muller, Hormonal control of male reproductive behavior in the lizard, Anolis carolinensis: role of
would be worthwhile.
testosterone, dihydrotestosterone, and estradiol, Endocrinology 103
Sex differences in the brain are generally correlated with
(1978) 1814–1821.
sexually dimorphic behaviors [20]. Male and female S. [15] K.D. Dunlap, H.H. Zakon, Behavioral actions of androgens and undulatus show sex differences in size, skin coloring, and androgen receptor expression in the electrocommunication system of
aggressive behavior [39]. Males have blue belly patches an electric fish, Eigenmannia virescens, Horm. Behav. 34 (1998) 30–38.
which they display during frequent aggressive encounters.
[16] S.B. Emerson, A. Greig, L. Carroll, G.S. Prins, Androgen receptors
Females are physically larger than males, lack blue
colora-in two androgen-mediated, sexually dimorphic characters of frogs,
tion on their belly, and display little or no aggression. The Gen. Comp. Endocrinol. 114 (1999) 173–180.
marked sexual dimorphism in brain AR-immunoreactivity [17] O.M. Echeverrıa, A. Gonzalez Maciel, A.M. Traish, H.H. Wotiz, E.´ ´ ´
localiza-tion of estradiol receptor in cells of male and female reproductive [34] M. Sar, D.B. Lubahn, F.S. French, E.M. Wilson, Immunohistoch-and non-reproductive organs, Biol. Cell 81 (1994) 257–265. emical localization of the androgen receptor in rat and human [18] L.M. Freeman, B.A. Padgett, G.S. Prins, S.M. Breedlove, Dis- tissues, Endocrinology 127 (1990) 3180–3186.
tribution of androgen receptor immunoreactivity in the spinal cord [35] R.B. Simerly, C. Chang, M. Muramatsu, L.W. Swanson, Distribution of wild-type, androgen-insensitive and gonadectomized male rats, J. of androgen and estrogen receptor mRNA-containing cells in the rat Neurobiol. 27 (1995) 51–59. brain: an in situ hybridization study, J. Comp. Neurol. 294 (1990) [19] D. Gelinas, G.V. Callard, Immunolocalization of aromatase- and 76–95.
androgen receptor-positive neurons in the goldfish brain, Gen. [36] G.T. Smith, E.A. Brenowitz, G.S. Prins, Use of PG-21 immuno-Comp. Endocrinol. 106 (1997) 155–168. cytochemistry to detect androgen receptors in the songbird brain, J. [20] J. Godwin, D. Crews, Sex differences in the nervous system of Histochem. Cytochem. 44 (1996) 1075–1080.
reptiles, Cell. Molec. Neurobiol. 17 (1997) 649–669. [37] J.W. Thornton, D.B. Kelley, Evolution of the androgen receptor: [21] J. Iqbal, J.J. Swanson, G.S. Prins, C.D. Jacobson, Androgen structure–function implications, BioEssays 20 (1998) 860–869.
receptor-like immunoreactivity in the Brazilian opossum brain and [38] R.R. Tokarz, Hormonal regulation of male reproductive behavior in pituitary: distribution and effects of castration and testosterone the lizard Anolis sagrei: a test of the aromatization hypothesis, replacement in the adult male, Brain Res. 703 (1995) 1–18. Horm. Behav. 20 (1986) 364–377.
[22] C.L. Jordan, Glia as mediators of steroid hormone action on the [39] M.B. Vinegar, Comparative aggression in Sceloporus virgatus, S. nervous system: an overview, J. Neurobiol. 40 (1999) 434–445. undulatus consobrinus, and S. u. tristichus (Sauria: Iguanidae), [23] M.M. Kessels, B. Qualmann, H.H. Thole, W.D. Sierralta, Subcellular Anim. Behav. 23 (1975) 279–286.
localization of estradiol receptor in MCF7 cells studied with [40] C.S. Watson, B. Gametchu, Membrane-initiated steroid actions and nanogold-labelled antibody fragments, Eur. J. Histochem. 42 (1998) the proteins that mediate them, Proc. Soc. Exp. Biol. Med. 220
259–270. (1999) 9–19.
[24] P.A. Kingston, D. Crews, Effects of hypothalamic lesions on [41] J.M. Wheeler, D. Crews, The role of the anterior hypothalamus– courtship and copulatory behavior in sexual and unisexual whiptail preoptic area in the regulation of male reproductive behavior in the lizards, Brain Res. 643 (1994) 349–351. lizard, Anolis carolinensis: lesion studies, Horm. Behav. 11 (1978)
´ ´ ´
[25] E. Lanuza, C. Font, A. Martınez-Marcos, F. Martınez-Garcıa, 42–60.
Amygdalo-hypothalamic projections in the lizard Podarcis his- [42] S.M. Winkler, J. Wade, Aromatase activity and regulation of sexual
panica: a combined anterograde and retrograde tracing study, J. behaviors in the green anole lizard, Physiol. Behav. 64 (1998)
Comp. Neurol. 384 (1997) 537–555. 723–731.
[26] J.I. Morrell, D. Crews, A. Ballin, A. Morgenthaler, D.W. Pfaff, [43] R.I. Wood, R.K. Brabec, J.M. Swann, S.W. Newman, Androgen and
3 3 3
H-Estradiol, H-testosterone and H-dihydrotestosterone localiza- estrogen receptor-containing neurons in chemosensory pathways of tion in the brain of the lizard Anolis Carolinensis: an autoradiog- the male Syrian hamster brain, Brain Res. 596 (1992) 89–98. raphic study, J. Comp. Neurol. 188 (1979) 201–224. [44] R.I. Wood, S.W. Newman, Intracellular partitioning of androgen [27] C.B. Nemeroff, C.A. Lamartiniere, G.A. Mason, R.E. Squibb, J.S. receptor immunoreactivity in the brain of the male Syrian hamster: Hong, S.C. Bondy, Marked reduction in gonadal steroid hormone effects of castration and steroid replacement, J. Neurobiol. 24 levels in rats treated neonatally with monosodium L-glutamate: (1993) 925–938.
further evidence for disruption of hypothalamic–pituitary–gonadal [45] M. Yokosuka, G.S. Prins, S. Hayashi, Co-localization of androgen axis regulation, Neuroendocrinology 33 (1981) 265–267. receptor and nitric oxide synthase in the ventral premammillary [28] R.G. Northcutt, Forebrain and midbrain organization in lizards and nucleus of the newborn rat: an immunohistochemical study, Dev.
its phylogenetic significance, in: N. Greenberg, P.D. MacLean Brain Res. 99 (1997) 226–233.
(Eds.), Behavior and Neurology of Lizards, NIMH, Rockville, [46] L.J. Young, G.F. Lopreato, K. Horan, D. Crews, Cloning and in situ Maryland, 1978, pp. 11–64. hybridization analysis of estrogen receptor, progesterone receptor, [29] T.C. Pappas, B. Gametchu, C.S. Watson, Membrane estrogen and androgen receptor expression in the brain of whiptail lizards receptors identified by multiple antibody and impeded ligand (Cnemidophorus uniparens and C. inornatus), J. Comp. Neurol. 347 labeling, FASEB J. 9 (1995) 404–410. (1994) 288–300.
[30] G.S. Prins, L. Birch, G.L. Greene, Androgen receptor localization in [47] L.J. Young, J. Godwin, M. Grammar, M. Gahr, D. Crews, Reptilian different cell types of the adult rat prostate, Endocrinology 129 sex steroid receptors: amplification, sequence and expression analy-(1991) 3187–3199. sis, J. Steroid Biochem. Molec. Biol. 55 (1995) 261–269. [31] W.G. Rossmanith, S. Wolfahrt, A. Ecker, E. Eberhardt, The demon- [48] W.-J. Young, C. Chang, Ontogeny and autoregulation of androgen
stration of progesterone, but not of estrogen, receptors in the receptor mRNA expression in the nervous system, Endocrine 9 developing human placenta, Horm. Metab. Res. 29 (1997) 604–610. (1998) 79–88.
[32] B.D. Sachs, R.L. Meisel, The physiology of male sexual behavior, [49] L. Zhou, J.D. Blaustein, G. De Vries, Distribution of androgen in: E. Knobil, J. Neil (Eds.), The Physiology of Reproduction, receptor immunoreactivity in vasopressin- and oxytocin-immuno-Raven Press, New York, 1988, pp. 1393–1485. reactive neurons in the male rat brain, Endocrinology 134 (1994) [33] F.N.A. Sackey, C.S. Watson, B. Gametchu, Cell cycle regulation of 2622–2627.