The cortico-basal ganglia-thalamocortical circuit with
synaptic plasticity. I. Modification rules for excitatory and
inhibitory synapses in the striatum
Isabella Silkis *
Neurophysiology of Learning Laboratory,
Institute of Higher Ner6ous Acti6ity and Neurophysiology of the Russian Academy of Sciences,Butlero6a5a str.,
117865Moscow,Russia
Received 25 July 2000; accepted 28 August 2000
Abstract
It is pointed out that Ca2+-dependent modification rules for NMDA-dependent (NMDA-independent) synaptic
plasticity in the striatum are similar to those in the neocortex and hippocampus (cerebellum). A unitary postsynaptic mechanism of synaptic modification is proposed. It is based on the assumption that, in diverse central nervous system structures, long-term potentiation/depression (LTP/LTD) of excitatory transmission (depression/potentiation of inhibitory transmission, LTDi/LTPi) is the result of an increasing/decreasing the number of phosphorylated AMPA and NMDA (GABAA) receptors. According to the suggested mechanism, Ca2+/calmodulin-dependent protein kinase
II and protein kinase C, whose activity is positively correlated with Ca2+ enlargement, together with
cAMP-depen-dent protein kinase A (cGMP-depencAMP-depen-dent protein kinase G, whose activity is negatively correlated with Ca2+ rise)
mainly phosphorylate ionotropic striatal receptors, if NMDA channels are opened (closed). Therefore, the positive/
negative post-tetanic Ca2+ shift in relation to a previous Ca2+ rise must cause NMDA-dependent LTP+LTDi/
LTD+LTPi or NMDA-independent LTD+LTPi/LTP+LTDi. Dopamine D1/D2 or adenosine A2A/A1 receptor
activation must facilitate LTP+LTDi/LTD+LTPi due to an augmenting/lowering PKA activity. Activation of muscarinic M1/M4receptors must enhance LTP+LTDi/LTD+LTPi as a consequence of an increase/decrease in the
activity of protein kinase C/A. The proposed mechanism is in agreement with known experimental data. © 2000 Elsevier Science Ireland Ltd. All rights reserved.
Keywords:LTP; LTD; Striatum; Dopamine; Adenosine; Acetylcholine
www.elsevier.com/locate/biosystems
1. Introduction
It is widely believed that an activity-dependent modification in the efficacy of synaptic transmis-sion provides an important key to understanding the cellular mechanisms of learning and memory.
* Tel.: +7-95-3344345; fax:+7-95-3388500.
E-mail address:[email protected] (I. Silkis).
Such forms of plasticity as long-term potentiation (LTP) and long-term depression (LTD) in the efficacy of excitatory transmission, and inhibitory transmission (LTPi, LTDi), have been obtained in the neocortex, hippocampus, cerebellum, basal ganglia and other structures of the central
ner-vous system (CNS). It has been found that Ca2+
-dependent changes in the activity of protein kinases (PKs) and protein phosphatase 1 (PP1) are necessary for synaptic modification in these structures (Calabresi et al., 1992, 1994, 1999b; Bear and Malenka, 1994; Pisani et al., 1997; Daniel et al., 1998). The relatively high/low post-synaptic Ca2+
elevation usually causes
neocorti-cal or hippocampal LTP/LTD and cerebellar
LTD/LTP (Bear and Malenka, 1994; Hartell,
1994). In the neocortex or hippocampus (cerebel-lum), LTDi induction requires an additional
Ca2+ lowering (elevation) compared with the
Ca2+ level that causes LTPi (for a review, see
Silkis, 1998). Therefore, Ca2+-dependent
modifi-cation rules for excitatory and inhibitory synapses
are opposite. We have explained the diverse Ca2+
-dependent modification rules for the neocortical/
hippocampal and cerebellar cells by expression of different cyclic nucleotides, cAMP and cGMP, respectively (Silkis, 2000a,b).
In the striatum, the input structure of the basal
ganglia, experimentally observed Ca2+
depen-dence of LTP and LTD looks contradictory. On the one hand, it has been demonstrated that the
high/low Ca2+ elevation is required for LTD/
LTP (Calabresi et al., 1992, 1994). On the other, LTP induction had been facilitated by diverse
protocols that led to additional Ca2+ rise
(Cal-abresi et al., 1997; Pisani et al., 1997). The
mecha-nism explaining these controversial results
remains unknown. In addition, the sign of synap-tic modification in the striatum essentially de-pends on the activation of different types of receptors sensitive to dopamine, adenosine and acetylcholine (Calabresi et al., 1994, 1997, 1999a; Hernandes-Lopez et al., 1997). However, the mechanism of participation of these modulatory neurotransmitters in striatal plasticity is not clearly understood.
The aim of this work has been to analyze the possible mechanisms underlying experimentally
found features of striatal LTP/LTD. We analyzed
the role of NMDA receptor activation in the
Ca2+ dependence on the sign of synaptic
modifi-cation and modulatory role of dopamine D1/D2,
adenosine A1/A2A and acetylcholine muscarinic
M1/M4 receptor activation in synaptic plasticity. An earlier suggested unitary postsynaptic mecha-nism of plasticity (Silkis, 1998, 2000a,b) provided the basis for this analysis.
2. The proposed mechanism for synaptic plasticity in striatal spiny cells
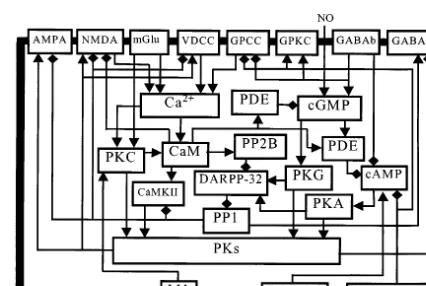
Different PKs phosphorylate ionotropic AMPA
(responsive to
a-amino-3-hydroxy-5-methyl-4-isoxazolepropionate) and NMDA (sensitive to
N-methyl-D-aspartate) receptors, increasing their
sensitivity to glutamate (Fig. 1). In the striatal spiny cells, AMPA and NMDA receptors can be phosphorylated by cAMP-dependent protein ki-nase A (PKA), cGMP-dependent protein kiki-nase
G (PKG), Ca2+/calmodulin-dependent protein
kinase II (CaMKII) and protein kinase C (PKC), and can be dephosphorylated by PP1 (Calabresi
et al., 1994, 1999b; Snyder et al., 1998; Oh et al., 1999; Yan et al., 1999). Since the same protein kinases (PKA, PKC, CaMKII, PKG) also
phos-phorylate GABAA receptors, decreasing
in-hibitory transmission (McDonald and Moss, 1997), the sign of modification of the inhibitory synapse must be opposite to that of the adjacent excitatory synapse.
Synaptically induced changes in the Ca2+
con-centration, and activity of PKs and PP1 in striatal spiny cells are provided by activation of different receptors (Fig. 1). The opening NMDA channels
strongly increase the postsynaptic Ca2+
concen-tration. The activation of metabotropic glutamate
(mGlu) receptors leads to Ca2+ elevation and
activation of PKC, CaMKII and Ca2+
/ calmod-ulin-dependent protein phosphatase 2B (PP2B).
An activation of GABAB receptors results in a
lowering the Ca2+ and cAMP level (Hashimoto
and Kuriyama, 1997). In the striatal spiny cells, wherein adenylate cyclase is Ca2+/calmodulin
in-dependent (Polli and Kincaid, 1994), an activation
of dopamine-sensitive D1/D2 receptors or
adenosine-sensitive A2A/A1 receptors causes an
increase/decrease in cAMP concentration (Collis
and Hourani, 1993; Snyder et al. 1998). In
addi-tion, D2 receptor activation causes a decrease of
Ca2+ influx (Strange, 1993). Activation of
acetyl-choline muscarinic M1 or M3 receptors causes
Ca2+ efflux from intracellular stores and an
in-crease in PKC activity (de la Vega et al., 1997), while an activation of muscarinic M4or M2 recep-tors leads to a decrease in cAMP concentration (Olianas et al., 1996). Conjunctive action on
mGlu and A2Areceptors increases cAMP
accumu-lation, while activation of mGlu and D1receptors
decreases cAMP level (Wang and Johnson, 1995). The elevating cGMP concentration in striatal spiny cells is believed to be the result of nitric oxide (NO) action on soluble guanylate cyclase (Calabresi et al., 1999b). However, NO synthase is expressed in the axon terminals of striatal in-terneurons (Calabresi et al., 1999b), the number of which is very small, while cGMP concentration is high (Surmeier et al., 1995). We hypothesized that cGMP elevation could be also caused by activation of membrane-bound guanylate cyclase
through GABABreceptors (Silkis, 2000a,b). Such
effect we have obtained in the cerebellar cortex. The activity of PP1 in the striatum is controlled by its inhibitor DARPP-32, which is phosphory-lated by PKA and PKG and dephosphoryphosphory-lated by PP2B (Snyder et al., 1998; Greengard et al., 1999). The supposed sequence of interconnected bio-chemical processes in the dendritic spine of a striatal neuron underlying postsynaptic mecha-nisms of excitatory and inhibitory synaptic plas-ticity (Fig. 1), to a large extent, is similar to those we earlier suggested for the neocortical/
hippocam-pal pyramidal cells and Purkinje/deep cerebellar
nuclei cells (Silkis, 2000a,b). Using a computa-tional model of post-tetanic biochemical processes in the dendritic spine of a pyramidal cell, we have found that the efficacy of the excitatory synaptic transmission, which is proportional to the number of phosphorylated AMPA and NMDA receptors,
depends on the ratio PKs/PP1 (Silkis, 1998,
2000a). This ratio is strongly affected by
post-tetanic Ca2+ elevation and completely defined by
parameters of stimulation. The necessary condi-tion for synaptic modificacondi-tion is a post-tetanic shift in the ratio PKs/PP1 in relation to the value produced by previous stimulation (Silkis, 1998, 2000a).
According to the current view, phosphorylation of AMPA receptors by PKC and PKG may un-derlie striatal and cerebellar LTD (Calabresi et al., 1994, 1999b; Nakazawa et al., 1995). We have pointed out (Silkis, 2000a,b) that such mechanism of LTD implies that properties of AMPA recep-tors on striatal or cerebellar cells are distinctive from those on hippocampal or neocortical cells,
wherein AMPA receptor phosphorylation/
dephosphorylation underlies LTP/LTD (Bear and
Malenka, 1994). We have postulated that sensitiv-ity of the same type of receptors in different CNS structures identically depends on their phosphory-lation (Silkis, 2000a). Therefore, the striatal LTP/
LTD (LTDi/LTPi) must be the consequence of an
increase/decrease in the number of highly (low)
sensitive phosphorylated AMPA and NMDA
(GABAA) receptors.
The suggested postulate for striatal plasticity is
supported by data that LTP/LTD in this structure
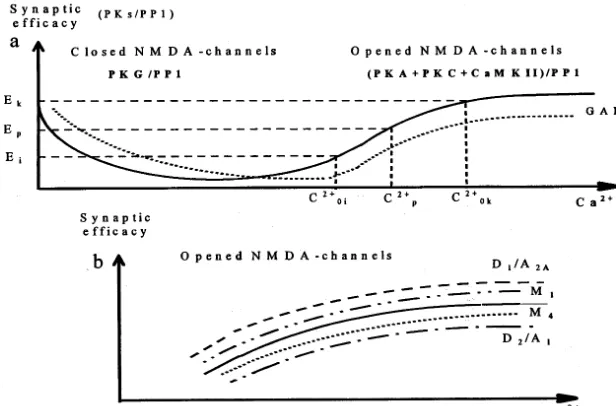
Fig. 2. The influence of neuromodulators on the efficacy of excitatory synaptic transmission. (a) Influence of inhibition on the efficacy of excitatory synaptic input; solid line, the proposed dependence of the ratio PKs/PP1 (that determine synaptic efficacy) on postsynaptic Ca2+elevation for activation of excitatory input alone; dotted line, additional activation of GABA
Breceptors; Ca 2+ 0i or Ca2+
0k and Ca 2+ P , a Ca
2+ rise produced by diverse prior activation and current stimulation, respectively. (b) Influence of dopamine, adenosine and acetylcholine on NMDA-dependent modification of excitatory synaptic input; dashed line, activation of D1or A2Areceptors; dash – dotted line, activation of D2or A1receptors, dash – double dotted line, activation of M1or M3receptors; dotted line, activation of M4or M2receptors.
of PP1 (Stefani et al., 1995; Hernandes-Lopez et al., 1997; Pisani et al., 1997; Snyder et al., 1998; Martin et al., 1999; Oh et al., 1999). LTP was obtained instead of LTD after increasing the concentration of phosphodiesterase (PDE) inhibitor (Calabresi et al., 1999b). This protocol causes an increase in the ratio PKs/PP1 due to a rising the cyclic nucleotide concentration (see Fig. 1). Phosphorylation also resulted in an augmentation of the efficacy of
striatal voltage-dependent Ca2+ channels
(VD-CCs), while an inhibition of PKG reduced the
Ca2+ current through VDCCs (Trautwein and
Hescheier, 1990; Surmeier et al., 1995). On the contrary, it was found that LTDi/LTPi in the basal
ganglia correlates with phosphorylation/
dephos-phorylation of GABAA receptors by (PKA and
PKC)/PP1. A decrease of the current through
GABAA receptors due to PKA activation and/or
PP1 inhibition has been found not only in striatal spiny cells, but also in cholinergic interneurons and dopamine neurons of the ventral tegmental area (Bonci et al., 1997; Yan and Surmeier, 1997; Flores-Hernandez et al., 2000).
3. The proposed role of NMDA receptor activation in the Ca2+
dependence of striatal modification rules
The activity of PKC and CaMKII increases
with Ca2+ enlargement, while cGMP
concentra-tion and PKG activity are downregulated by
Ca2+/calmodulin (Baltrons et al., 1997). So,
Ca2+ entry through NMDA channels must
influ-ence the ratio PKs/PP1 in the dendritic spine of
the spiny striatal neuron as well as the efficacy of excitatory synaptic transmission (Silkis, 2000c). If
NMDA channels are closed, Ca2+ rise and
activ-ity of Ca2+-dependent PKC and CaMKII can not
be large, while PKG activity in the striatum is initially high (Surmeier et al., 1995). The role of PKA is possibly low, since cAMP can be hy-drolyzed by highly effective cGMP-dependent
PDE. The ratio PKG/PP1 must strongly decrease
with Ca2+ rise (Fig. 2a, left part of curve), since
a lowering PKG activity is followed by enlarge-ment of PP1 activity (see Fig. 1). If NMDA
achieved and the activity of PKG becomes negli-gible. In this case, PKC and CaMKII must play the significant role in the ratio PKs/PP1 (Fig. 2a, right part of curve). Actually, NMDA-dependent LTP has been induced without PKG activation (Calabresi et al. 1999b). It is obvious from the curve in Fig. 2a that the positive/negative postsy-naptic Ca2+ shift must cause increase/decrease in
synaptic efficacy if NMDA channels are opened
(NMDA-dependent LTP/LTD), and decrease/
in-crease in synaptic efficacy if NMDA channels are
closed (NMDA-independent LTP/LTD). Thus,
modification rules for NMDA-dependent and NMDA-independent synaptic plasticity in the striatum are opposite (Table 1). In terms of the proposed mechanism, this effect is the result of
involving PKG and PKA+PKC+CaMKII in
NMDA-independent and NMDA-dependent ef-fects, respectively. Thus, non-unique striatal
mod-ification rules that look like
neocortical/hippocampal (cerebellar) modification rules, if NMDA channels are opened (closed), could be the sequence of involving cAMP (cGMP) in the postsynaptic cascades.
The diverse modification rules in the striatum manifest itself in experiments performed in vitro and in vivo. In normal striatal slices, EPSP usu-ally consists of the AMPA component only, while
both AMPA and NMDA components are
recorded in vivo (Calabresi et al., 1994). In agree-ment with suggested modification rules, an artifi-cial elevating/lowering of the intracellular Ca2+
concentration in striatal slices resulted in
NMDA-independent LTD/LTP (Calabresi et al., 1992,
1994; Dos-Santos-Villar and Walsh, 1999). The
Ca2+ influx through NMDA channels reversed
Ca2+ dependence of the sign of synaptic
modifi-cation in the striatum (Calabresi et al., 1992, 1997). In experiments in vivo, any one protocol
that had augmented Ca2+ concentration and/or
protein kinase activity facilitated NMDA-depen-dent LTP in spiny striatal cells (Charpier and Deniau, 1997; Pisani et al., 1997). It must be noted that the same stimulation protocol, which
leads to the same post-tetanic Ca2+ elevation,
may result in LTP (LTD), if this concentration
exceeds (is less than) a Ca2+ rise produced by
prior stimulation, Ca2+
0i (Ca20k+) (Fig. 2a).
There-fore, both LTP and LTD could be obtained in adjacent cells after tetanization. Such an effect has been observed in spiny striatal neurons (Dos-San-tos-Villar and Walsh, 1999). The firing rate of corticostriatal neurons can exceed 100 Hz (Cal-abresi et al., 1992). Thus, the usually used
parameters of rhythmic stimulation are
physiological.
4. Influence of GABA, dopamine, adenosine and acetylcholine on the sign and magnitude of synaptic modification
It follows from the suggested mechanism of
plasticity that a decrease in Ca2+ and cAMP
concentration due to GABAB receptor activation
must lead to reducing PKA activity and lowering the number of phosphorylated AMPA and NMDA receptors (Fig. 2a, dashed line, right part of curve). This effect leads to NMDA-dependent LTD. If NMDA channels are closed, the
addi-tional activation of GABABreceptors must
facili-tate NMDA-independent LTP due to the
enlargement of PKG activity and rising the ratio PKG/PP1 (Fig. 2a, dashed line, left part of curve). An activation of D1/D2 receptors or A2A/A1
re-ceptors can also result in rising/decreasing PKA
activity and subsequent elevating/lowering of the
ratio PKs/PP1 in striatal spiny cells (Fig. 2b,
dashed/dash – dotted line). For this reason, an
ad-ditional activation of D1 or A2A receptors must
increase the magnitude of NMDA-dependent
LTP and LTDi, while an activation of D2 or A1
receptors can decrease these effects (Table 1) or even reverse NMDA-dependent LTP into LTD and LTDi into LTPi. On the contrary, a blockade
of D2 or A1 receptors must promote
NMDA-de-pendent LTP together with LTDi that can appear in a rising cell activity. Indeed, in experiments in
vivo, the administration of a D2receptor
antago-nist resulted in the increase in bursting activity of synaptically excited striatal cells (Finch, 1999).
Activation of D1 receptors resulted in LTP of
I
.
Silkis
/
BioSystems
57
(2000)
187
–
196
Table 1
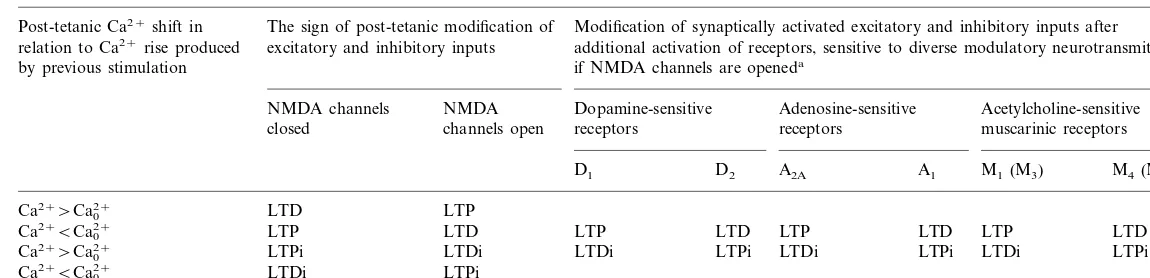
Modification rules for cortico-striatal synaptic plasticity and influence of neuromodulators on NMDA-dependent effects
The sign of post-tetanic modification of Modification of synaptically activated excitatory and inhibitory inputs after Post-tetanic Ca2+shift in
additional activation of receptors, sensitive to diverse modulatory neurotransmitters, relation to Ca2+rise produced excitatory and inhibitory inputs
by previous stimulation if NMDA channels are openeda
NMDA channels NMDA Dopamine-sensitive Adenosine-sensitive Acetylcholine-sensitive
muscarinic receptors
closed channels open receptors receptors
A1 M1(M3) M4(M2)
D1 D2 A2A
LTP Ca2+\Ca2+
0 LTD
LTP LTD LTP LTP LTD LTP LTD
Ca2+BCa2+
0 LTD
Ca2+\Ca2+
0 LTPi LTDi LTDi LTPi LTDi LTPi LTDi LTPi
LTPi Ca2+BCa2+
0 LTDi
aThe sign of modification could be reversed after the blockade of receptors, sensitive to diverse modulatory neurotransmitters. LTP/LTD, Long-term
LTP into LTD, while D2 receptor inactivation resulted in the enhancement of LTP (Calabresi et
al., 1997). On the other hand, activation of D2
receptors can facilitate NMDA-independent LTP as well as NMDA-independent LTDi due to
de-creasing Ca2+ concentration and subsequent
ris-ing PKG activity. Actually, in the presence of AMPA and NMDA receptor blockators, an inhi-bition of GABAergic synaptic response in striatal
cells has been obtained after D2 receptor
activa-tion (Delgado et al., 1999). A2A receptor
activa-tion (inactivaactiva-tion) that leads to a rise (decrease) in PKA activity also resulted in striatal LTDi (LTPi) (Mori et al., 1996). A depressive (facilitating)
action of A2A receptor agonist (antagonist) on
inhibition of striatal cell firing mediated by D2
receptor agonist has been shown previously (Stromberg et al., 1998).
Experimental data supporting the proposed role of adenosine in synaptic plasticity have been mostly obtained in the hippocampus. A blockade
of A2 receptors resulted in the preventing LTP
and augmentation of depotentiation or LTD (Kessey et al., 1997; Fujii et al., 1999). A depoten-tiation produced by adenosine has been shown to be the consequence of a decrease in PKA activity (Huang et al., 1999). This result is in accordance with our conclusion that the same mechanism (dephosphorylation) underlies both LTD and de-potentiation (Silkis, 1998). On the contrary, a
blockade of A1 receptors caused a facilitating
LTP and decreasing depotentiation (Fujii et al.,
1999). However, an activation of A1 receptors
augmented depotentiation and prevented LTP (Hogan et al., 1998).
In terms of the suggested mechanism, an
activa-tion of M1 or M3 receptor and a subsequent
increase in Ca2+ concentration and PKC activity
(Fig. 2b, dash – double dotted line) must promote NMDA-dependent LTP and LTDi. Oppositely,
an activation of M2 or M4 receptors that causes
lowering cAMP concentration and PKA activity (Fig. 2b, dotted line) must facilitate NMDA-de-pendent LTD and LTPi. Indeed, an activation of
M1 receptors as well as a blockade of M2
recep-tors on striatal cells enhanced NMDA-dependent LTP (Calabresi et al., 1998a, 1999a). In addition,
an activation of M1 and M3 receptors decreased
the amplitude of inhibitory current in the spiny cells (Szabo et al., 1998), while an enhancement of GABA-activated current has been obtained after
activation of M4 receptors (Hu et al., 1999). The
blockade of muscarinic receptors must cause the opposite effects. One can expect that acetylcholine
also influences NMDA-independent synaptic
modification, since neuronal NO-synthase activity
and cGMP formation could be stimulated by M1
receptor activation (Wotta et al., 1998). However, in experimental conditions, no changes of AMPA
response has been found after M1receptor
activa-tion (Calabresi et al., 1998b). Possibly, the amount of cGMP, produced through NO-stimu-lated cascade, is insufficient. The suggested scheme of postsynaptic striatal processes (Fig. 1) can be supplemented by those triggered by activa-tion of other receptor types that cause changes in
the ratio PKs/PP1. For example, it follows from
the suggested mechanism that an activation of
opiate m and d receptors, and subsequent
inhibi-tion of cAMP formainhibi-tion (Greengard et al., 1999), may prevent LTP and promote LTD.
5. Conclusion
The earlier suggested principles of unitary post-synaptic mechanism of excitatory and inhibitory plasticity, and known features of postsynaptic processes in striatal spiny cells made it possible to explain the experimentally obtained properties of
LTP/LTD in the striatum. According to the
pro-posed mechanism of striatal plasticity, LTP/LTD
(LTDi/LTPi) is the result of an increasing
/decreasing the number of highly (low) sensitive
phosphorylated AMPA and NMDA (GABAA)
receptors. We assume that Ca2+/
calmodulin-dependent protein kinase II and protein kinase C,
the activity of which increases with Ca2+ rise,
together with cAMP-dependent protein kinase A (cGMP-dependent protein kinase G, the activity of which is initially high and decreases with Ca2+
enlargement) mainly phosphorylate ionotropic re-ceptors on striatal cells if NMDA channels are opened (closed). Therefore, the modification rules for NMDA-dependent and NMDA-independent
Ca2+ shift in relation to Ca2+ concentration
produced by prior stimulation must cause
NMDA-dependent LTP/LTD together with
LTDi/LTPi (NMDA-independent LTD/LTP
to-gether with LTPi/LTDi). Modification rules for
NMDA-dependent and NMDA-independent ef-fects in the striatum are similar to those in the
neocortex/hippocampus and cerebellum,
respec-tively. Dopamine D1/D2and/or adenosine A2A/A1
receptor activation must facilitate LTP/LTD and
LTDi/LTPi in consequence of augmenting/
lower-ing cAMP concentration and protein kinase A activity. Activation of muscarinic M1 (M3)/M4
(M2) receptors must enhance LTP/LTD together
with LTDi/LTPi due to an increase/decrease in
the activity of protein kinase C/A. The suggested mechanism for striatal synaptic plasticity is in accordance with known experimental data.
Induc-tion of LTP/LTD in corticostriatal synapses can
underlie the well known ‘excitatory’ (via D1 recep-tors) and ‘inhibitory’ (via D2 receptors) influence of dopamine on striatal cells (De Long, 1990). The proposed modification rules can be used for the complication of diverse models of the neu-ronal networks, which include the basal ganglia.
Acknowledgements
This work was partly supported by Russian Foundation of Fundamental Research, grant 98-04-48368.
References
Baltrons, M.A., Saadoun, S., Agullo, L., Garcia, A., 1997. Regulation by calcium of the nitric oxide/cyclic GMP system in cerebellar granule cells and astroglia in culture. J. Neurosci. Res. 49, 333 – 341.
Bear, M.F., Malenka, R.C., 1994. Synaptic plasticity: LTP and LTD. Curr. Opin. Neurobiol. 4, 389 – 399.
Bonci, F., Grillner, P., Sinesculchi, A., Mercuri, N.B., Bernardi, G., 1997. Glutamate metabotropic receptor ago-nists depress excitatory and inhibitory transmission on rat mesencephalic principal neurons. Eur. J. Neurosci. 9, 2359 – 2369.
Calabresi, P., Maj, R., Pisani, A., Mercuri, N.B., Bernardi, G., 1992. Long-term synaptic depression in the striatum: phys-iological and pharmacological characterization. J. Neu-rosci. 12, 4224 – 4233.
Calabresi, P., Pisani, A., Mercuri, N.B., Bernardi, G., 1994. Post-receptor mechanisms underlying striatal long-term de-pression. J. Neurosci. 14, 4871 – 4881.
Calabresi, P., Saiardi, A., Pisani, A., Baik, J.H., Centonze, D., Mercuri, N.B., Bernardi, G., Borrelli, E., 1997. Abnormal synaptic plasticity in the striatum of mice lacking do-pamine D2receptors. J. Neurosci. 17, 4536 – 4544. Calabresi, P., Centonze, D., Gubellini, P., Pisani, A., Bernardi,
G., 1998a. Blockade of M2-like muscarinic receptors en-hances long-term potentiation at corticostriatal synapses. Eur. J. Neurosci. 10, 3020 – 3023.
Calabresi, P., Centonze, D., Gubellini, P., Pisani, A., Bernardi, G., 1998b. Endogenous ACh enhances striatal NMDA-re-sponses via M1-like muscarinic receptors and PKC activa-tion. Eur. J. Neurosci. 10, 2887 – 2895.
Calabresi, P., Centonze, D., Gubellini, P., Bernardi, G., 1999a. Activation of M1-like muscarinic receptors is required for the induction of corticostriatal LTP. Neuropharmacology 38, 323 – 326.
Calabresi, P., Gubellini, P., Centonze, D., Sancesario, G., Morello, M., Giorgi, M., Pisani, A., Bernardi, G., 1999b. A critical role of the nitric oxide/cGMP pathway in corti-costriatal long-term depresion. J. Neurosci. 19, 2489 – 2499. Charpier, S., Deniau, J.M., 1997. In vivo activity-dependent plasticity at cortico-striatal connections: evidence for phys-iological long-term potentiation. Proc. Natl. Acad. Sci. USA 94, 7036 – 7040.
Collis, M.G., Hourani, S.O., 1993. Adenosine receptor syb-types. Trends Neurosci. 14, 360 – 366.
Daniel, H., Levenes, C., Crepel, F., 1998. Cellular mechanisms of cerebellar LTD. Trends Neurosci. 21, 401 – 407. de la Vega, M.T., Nunez, A., Arias-Montano, J.A., 1997.
Muscarinic M1and M3receptors in rat striatum: a binding study. Arch. Med. Res. 28, 493 – 497.
Delgado, A., Sierra, A., Querejeta, E., Valdiosera, R.F., Aceves, J., 1999. Inhibitory control of the GABAergic transmission in the rat neostriatum by Dinf 2 dopamine receptors. Neuroscience 95, 1043 – 1048.
Dos-Santos-Villar, F., Walsh, J.P., 1999. Modulation of long-term synaptic plasticity at excitatory striatal synapses. Neuroscience 90, 1031 – 1041.
Finch, D.M., 1999. Plasticity of responses to synaptic inputs in rat ventral striatal neurons after repeated administration of the dopamine D2antagonist raclopride. Synapse 31, 297 – 301.
Flores-Hernandez, J., Hernandez, S., Snyder, G.L., Yan, Z., Fienberg, A.A., Moss, S.J., Greengard, P., Surmeier, D.J., 2000. D1 dopamine receptor activation reduces GABAa receptor currents in neostriatal neurons through a PKA/
DARPP-32/PP1 signaling cascade. J. Neurophysiol. 83, 2996 – 3004.
Greengard, P., Allen, P.B., Nairn, A.C., 1999. Beyond the dopamine receptor: the DARPP-32/protein phosphatase-1 cascade. Neuron 23, 435 – 447.
Hartell, N.A., 1994. Induction of cerebellar long-term depres-sion requires activation of glutamate metabotropic recep-tors. Neuroreport 5, 913 – 916.
Hashimoto, T., Kuriyama, K., 1997. In vivo evidence that GABA(B) receptors are negatively coupled to adenylate cyclase in the rat striatum. J. Neurochem. 69, 365 – 370. Hernandes-Lopez, S., Bargas, J., Surmeier, D.J., Reyes, A.,
Gallabarraga, E., 1997. D1 receptor activation enhances evoked discharge in neostriatal medium spiny neurons by modulating an L-type Ca2+conductance. J. Neurophysiol. 17, 3334 – 3342.
Hogan, Y.H., Hawkins, R., Alkadhi, K.A., 1998. Adenosine A1receptor activation inhibits LTP in sympathetic ganglia. Brain Res. 807, 19 – 28.
Hu, H.Z., Shao, M., Li, Z.W., 1999. Enhancement of GABA-activated current by muscarine in rat dorsal root ganglion neurons. Neuroscience 89, 883 – 890.
Huang, G.-C., Liang, Y.-C., Hsu, K.-S., 1999. A role for extracellular adenosine in time-dependent reversal of long-term potentiation by low frequency stimulation at hippocampal CA1 synapses. J. Neurosci. 19, 9728 – 9738. Kessey, K., Trommer, B.L., Overstreet, L.S., Ji, T., Mogul,
D.J., 1997. A role for adenosine A2 receptors in the induction of long-term potentiation in the CA1 region of rat hippocampus. Brain Res. 756, 184 – 190.
Martin, G., Ahmed, S.H., Blank, K.T., Spiess, J., Koob, G.F., Siggins, G.R., 1999. Chronic morphine treatment alters NMDA receptor mediated synaptic transmission in the nucleus accumbens. J. Neurosci. 19, 9081 – 9089.
McDonald, B.J., Moss, S.J., 1997. Conserved phosphorylation of intracellular domains of -aminobutyric acid type A receptor subunits by cAMP-dependent protein kinase, protein kinase C, calcium/calmodulin type 2-dependent protein kinase and cGMP-dependent protein kinase. Neu-ropharmacology 36, 1377 – 1385.
Mori, A., Shindou, T., Ichimura, M., Nonaka, H., Kase, H., 1996. The role of adenosine A2A receptors in regulating GABAergic synaptic transmission in striatal medium spiny neurons. J. Neurosci. 16, 605 – 611.
Nakazawa, K., Mikawa, S., Hashikawa, T., Ito, M., 1995. Transient and persistent phosphorylation of AMPA-type glutamate receptor subunits in cerebellar Purkinje cells. Neuron 15, 697 – 709.
Oh, J., Vaughan, C.L., Chase, T.N., 1999. Effect of dopamine denervation and dopamine agonist administration on ser-ine phosphorylation of striatal NMDA receptor subunits. Brain Res. 821, 433 – 442.
Olianas, M.C., Adem, A., Karlsson, E., Onali, P., 1996. Rat striatal muscarinic receptors coupled to the inhibition of adenylyl cyclase activity: potent block by the selective m4 ligand muscarinic toxin 3 (MT3). Br. J. Pharmacol. 118, 283 – 288.
Pisani, A., Calabresi, P., Centonze, D., Bernardi, G., 1997. Enhancement of NMDA responses by group I
metabo-tropic glutamate receptor activation in striatal neurones. Br. J. Pharmacol. 120, 1007 – 1014.
Polli, J.W., Kincaid, R.L., 1994. Expression of calmodulin-de-pendent phosphodiesterase isoform (PDE1B1) correlates with brain regions having extensive dopaminergic innerva-tion. J. Neurosci. 14, 1251 – 1261.
Price, C.J., Kim, P., Raymond, L.A., 1999. D1 dopamine receptor-induced cyclic AMP-dependent protein kinase phosphorylation and potentiation of striatal glutamate re-ceptors. J. Neurochem. 73, 2441 – 2446.
Silkis, I.G., 1998. The unitary modification rules for neural networks with excitatory and inhibitory synaptic plasticity. Biosystems 48, 205 – 213.
Silkis, I.G., 2000a. Unitary postsynaptic mechanisms of LTP and LTD in the neocortex, hippocampus and cerebellum. In: Miller, R., Ivanitsky, A.M., Balaban, P.M. (Eds.), Complex Brain Functions: Conceptual Advances in Rus-sian Neuroscience. Harwood, pp. 21 – 51.
Silkis, I., 2000b. Interrelated modification of excitatory and inhibitory synapses in three layer olivary-cerebellar neural network. Biosystems 54, 141 – 149.
Silkis, I.G., 2000c. The unitary postsynaptic mechanism of plasticity in the striatum, neocortex, hippocampus and cerebellum. Russ. J. Physiol. 86, 519 – 531 (in Russian). Snyder, G.L., Fienberg, A.A., Huganir, R.L., Greengard, P.,
1998. A dopamine/D1 receptor/protein kinase A/ Do-pamine- and cAMP-regulated phosphoprotein (Mr 32 kDa)/protein phosphatase-1 pathway regulates dephospho-rylation of the NMDA receptor. J. Neurosci. 18, 10297 – 10303.
Stefani, A., Pisani, A., Bernardi, G., Bonci, A., Mercuri, N.B., Stratta, F., Calabresi, P., 1995. The modulation of do-pamine receptors in rat striatum. J. Neural. Transm. Suppl. 45, 61 – 66.
Strange, P., 1993. New insights into dopamine receptors in the central nervous system. Neurochem. Int. 22, 223 – 226. Stromberg, I., Ferre, F., Fuxe, K., 1998. Neurophysiological
evidence for striatal adenosine A2A and dopamine D2 receptor – receptor interactions. Soc. Neurosci. Abstr. 24, 824.12.
Surmeier, D.J., Bargas, J., Hemmings, H.C., Nairn, A.C., Greengard, P., 1995. Modulation of calcium currents by a D1dopaminergic protein kinase/phosphatase cascade in rat neostriatal neurons. Neuron 14, 385 – 397.
Szabo, B., Dorner, L., Pfreundtner, C., Norenberg, W., Starke, K., 1998. Inhibition of GABAergic inhibitory post-synaptic currents by cannabinoids in rat corpus striatum. Neuroscience 85, 395 – 403.
Trautwein, W., Hescheier, J., 1990. Regulation of cardiac L-type calcium current by phosphorylation and G proteins. Ann. Rev. Physiol. 52, 257 – 274.
Wang, J., Johnson, K.M., 1995. Regulation of striatal cyclic-3%,5%-adenosine monophosphate accumulation and GABA release by glutamate metabotropic and dopamine D1 re-ceptors. J. Pharmacol. Exp. Ther. 275, 877 – 884. Wotta, D.R., Parsons, A.M., Hu, J., Grande, A.W.,
and prolonged phases of neuronal nitric oxide synthase activity: involvement of different calcium pools. J Neu-rochem. 71, 487 – 497.
Yan, Z., Surmeier, D.J., 1997. D5 dopamine receptors enhance Zn2+-sensitive GABAa currents in striatal cholinergic in-terneurons through a PKA/PP1 cascade. Neuron 19,
1115 – 1126.
Yan, Z., Hsieh-Wilson, L., Feng, J., Tomizawa, K., Allen, P.B., Fienberg, A.A., Nairn, A.C., Greengard, P., 1999. Protein phosphatase 1 modulation of neostriatal AMPA channels: regulation by DARPP-32 and spinophilin. Nat. Neurosci. 2, 13 – 17.
.