A Case–Control Study
Ana Rubio, Alice Lee Vestner, John M. Stewart, Nicholas T. Forbes,
Yeates Conwell, and Christopher Cox
Background: The single most important risk factor for
Alzheimer’s pathology is age. Elderly individuals are also at increased risk for suicide, but comprehensive studies of the association between Alzheimer’s pathology and sui-cide are lacking. We designed the current study to deter-mine if Alzheimer’s disease changes are overrepresented in elderly people committing suicide.
Methods: The design is a case– control study. Cases (n5 28) were subjects older than 60 years of age who com-pleted suicide. For each case, two age- and gender-matched individuals who died naturally were selected as control subjects (n556). Neuropathologic examination of hippocampal sections was performed blindly and included a modified Braak scoring system and semiquantitative assessment of neurofibrillary tangles, amyloid deposition, Lewy bodies, and Lewy-associated neurites. Data were analyzed by conditional logistic regression.
Results: The brains of individuals who committed suicide
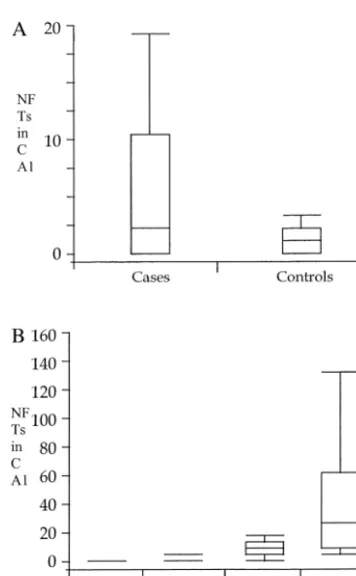
had higher modified Braak scores than those of matching control subjects (p5 .0028). The number of neurofibril-lary tangles in CA1 was not an independent predictor of suicide status in the statistical analysis (p5.16), although the distribution was more highly skewed among the cases (75th percentile of 10.5 for cases, vs. 2 for control subjects).
Conclusions: Severe Alzheimer’s disease pathology is
overrepresented in elderly patients who complete suicide. Biol Psychiatry 2001;49:137–145 © 2001 Society of Bio-logical Psychiatry
Key Words: Suicide, elderly, Alzheimer’s disease, neu-rofibrillary pathology
Introduction
T
he level of risk for suicide in patients with Alzhei-mer’s disease (AD) and other dementing illnesses, although controversial, is generally considered low (Chiu et al 1996; Draper et al 1998; Frierson 1991; Harris and Barraclough 1997; Hepple and Quinton 1997; Lyness et al 1992). Depression (Wragg and Jeste 1989), aggressive behavior (Aarsland et al 1996; Eastley and Wilcock 1997), and occasional awareness of limitations, common in AD (Kim and Hershey 1988), may be risk factors for suicide (Blumenthal 1988; Margo and Finkel 1990; Willis 1987). On the other hand, greater levels of supervision and deficits in cognitive function may protect from suicide (Conwell 1995). In our routine neuropathologic examina-tion of brains from elderly individuals who committed suicide, we found occasionally moderate to severe AD pathology, but no data are available regarding the relative incidence and severity of AD changes in the elderly suicide population compared with gender- and age-matched control subjects. A review of the literature revealed only a few case reports of clinically demented subjects who committed suicide and had neuropathologi-cally confirmed AD changes (Lecso 1989; Rodhe et al 1995), but comprehensive studies of elderly suicides do not commonly include neuropathologic evaluation (Con-well et al 1990; Ferris et al 1999; Margo and Finkel 1990). Alzheimer’s-related neuropathologic changes are com-monly found in the brains of older people. A recently published multicenter study (Braak and Braak 1997) with neuropathologic evaluation of 2661 subjects demonstrated the presence of AD pathology in the majority of elderly brains examined (more than 92% and 99% of subjects older than 70 and 80 years, respectively, had some neurofibrillary pathology). The severity and, most impor-tantly, the topography of these changes follow a stereo-typed pattern of progression with clearly defined stages (Braak and Braak 1991; Fewster et al 1991; The National Institute on Aging and Reagan Institute Working Group on Diagnostic Criteria for the Neuropathological Assessment of Alzheimer’s Disease 1997). Although the 1997 study did not include clinical information on individualsas-From the Departments of Pathology and Laboratory Medicine (AR, ALV, JMS), Psychiatry (YC), and Biostatistics (CC), University of Rochester School of Medicine, and Office of the Monroe County Medical Examiner (NTF), Rochester, New York.
Address reprint requests to Ana Rubio, M.D., Ph.D., University of Rochester Medical Center, Dept. of Pathology (Neuropathology Unit), 601 Elmwood Avenue, P.O. Box 626, Rochester NY 14642.
Received February 2, 2000; revised May 25, 2000; accepted May 31, 2000.
© 2001 Society of Biological Psychiatry 0006-3223/01/$20.00
sessed neuropathologically, other studies (Bancher et al 1996; Braak and Braak 1991; Braak et al 1993; Dickson 1997; Geddes et al 1996, 1997; Gertz et al 1996; Jellinger and Bancher 1997; Masliah 1995; Newell et al 1999; Xuereb et al 1995) have shown mixed correlation between the pathologic and the clinical findings. The greatest variation in cognitive status is among subjects with inter-mediate stages of AD pathology (limbic or Braak stages III–IV), where the deficits range from not noticeable to severe.
When the clinico-pathologic correlation is done by grouping individuals according to their clinical status, the results are equally heterogeneous. Of importance is the wide range of AD neuropathology among individuals with intact cognition (Davis et al 1999). Because the brunt of the burden in the intermediate stages of AD pathology localizes to the limbic system, alterations in affective regulation and emotional behavior are expected to be manifestations of these pathologic stages. It is thus plau-sible that mood changes or other emotional symptoms may precede detectable cognitive decline in the clinical pro-gression of the disease and may make subjects more vulnerable to suicidal behavior.
We undertook this pilot project with the purpose of determining if AD pathology was overrepresented in elderly patients committing suicide.
Methods and Materials
Selection of Cases and Control Subjects
Cases were individuals over the age of 60 who completed
suicide. Subjects were identified from the files of the Monroe County Medical Examiner’s Office. Other criteria for inclusion were the performance of a complete autopsy and the availability of hippocampal tissue sections.
Control subjects were chosen to match each case (two control
subjects per case) in age (within 3 years) and gender, and included individuals who died naturally, had a routine autopsy performed at the University of Rochester Medical Center, and had hippocampal tissue available for neuropathologic examina-tion. We selected the control subjects by reviewing the individual front-sheets of the autopsy files to detect control subjects matching each case for age and gender. Other factors, including social, environmental, and medical, were not considered. The presence of clinically diagnosed dementia and other chronic illnesses, including neurologic disease, was not an exclusion criterion for the selection of cases or control subjects.
Autopsy and clinical records were reviewed and the presence of cancer as well as severe cardiac, respiratory, or other illnesses was tabulated.
Psychologic Autopsies
As part of an ongoing study (“Suicide in later life: A psycho-logical autopsy study”), psychopsycho-logical autopsy evaluations were
available for 22 cases. Psychiatric diagnoses were determined by administration of the Structured Clinical Interview for DSM-III-R (Spitzer et al 1986) to knowledgeable informants and review of all available medical and psychiatric records. This methodology is described in detail elsewhere (Conwell et al 1996). The reports were reviewed for Axis I diagnoses of dementia, depression with and without psychosis, and substance abuse.
Neuropathologic Examination
Seven-micron sections obtained at the caudal end of the anterior hippocampus (generally at the level of the lateral geniculate body) were obtained and stained with hematoxylin– eosin and the modified Gallyas silver technique (Iqbal et al 1993). Tissue was also immunostained with antibodies for phosphorylated tau (paired helical filament [PHF] antibody, 1:20 dilution; kindly donated by Dr. S.H. Yen), ubiquitin (Chemicon, Temecula, CA; 1:5000 dilution), andb-amyloid (Dako, Carpinteria, CA; 1:100 dilution following pretreatment of the tissue with formic acid).
Neuropathologic evaluation was performed by a single neuro-pathologist, blind to the case or control status and to all clinical information. Neuropathologic staging was done by simplifying the Braak and Braak grading system (Braak and Braak 1991) to accommodate to hippocampus-only evaluation. A similar method including the hippocampus and inferior temporal cortex has been recently described and validated (Harding et al 2000). In our study the majority of the sections included the hippocam-pus proper and the entorhinal and perirhinal cortices (beyond the collateral sulcus). Individuals were characterized as stage 0 when no neurofibrillary pathology was observed and stages I–II when the neurofibrillary pathology was limited to the transentorhinal region. Brains with mild entorhinal pathology and/or one to few CA1 tangles at the junction of CA1 with the subiculum were also included in this stage. Stages III–IV included cases with moder-ate involvement of CA1 and those with few neurofibrillary tangles (NFTs) affecting CA4 of the hippocampus. In stages V–VI the AD pathology was most extensive and intense, affecting fascia dentata or CA2–3 of the hippocampus. The total number of PHF-positive NFTs in CA1 of the hippocampus was recorded for quantitative assessment of the intensity of Alzhei-mer’s-related pathology. Amyloid deposition in the cortex was semiquantitatively evaluated (from 0, when there were no depos-its, to 3, when the deposition was extensive) and the presence of amyloid (congophilic) angiopathy recorded. The presence of Lewy bodies and Lewy-associated neurites was determined by the presence of ubiquitpositive/tau-negative cytoplasmic in-clusions in the deep parahippocampal gyrus and granular linear deposits in the neuropil of the hippocampal CA3–2 area and superficial layers of the parahippocampal cortex.
We limited the neuropathologic examination to hippocampal sections because other brain areas were not uniformly available for the cases.
Statistical Analysis
matched control subjects, using conditional logistic regression models (McCullagh and Nelder 1989). The dependent variable in each regression analysis was case/control status. Logistic regres-sion models were used to examine associations with three predictor variables: Braak stage (treated as a score with a range of 0 –3 corresponding to Braak and Braak stages 0, I–II, III–IV, and V–VI), number of NFTs in CA1, and amyloid score (0 –3). The three predictor variables were examined separately and combined in a single analysis.
Results
General Characteristics of the Subjects
Twenty-eight cases and 56 control subjects were included in the study. Age, gender, presence of cognitive deficits, and the incidence of pathologically confirmed chronic cardiac, respiratory, or neoplastic illnesses are summa-rized in Table 1. Of note is the higher percentage of severe cardiac pathology (atherosclerotic disease) among the control subjects. The prevalence of cancer and severe respiratory illnesses was comparable in the two groups. Three (10.7%) cases and seven (12.5%) control subjects had clinical dementia.
Demographics of Suicide
Twelve of the 28 cases (43%) wrote a note of intent. Three other individuals (11%) had a conversation with a relative or friend that retrospectively could be interpreted as manifesting suicidal intent. The specific suicide method is shown in Table 2. The two most common methods were firearms, utilized by eight individuals (28.6%), seven of whom were men, and drug overdose, employed by eight subjects as well, seven of whom were women.
Twenty-two cases had a psychologic autopsy per-formed. Analysis of the Axis I evaluation is shown in Table 3. Sixteen cases (73%) had a diagnosable affective illness; five of the 16 had psychotic features. Eleven (50%) were in their first episode of major unipolar depression,
three had a recurrent major depressive episode, and two had dysthymia. Five subjects had an alcohol or drug use disorder, and four had other diagnosable conditions (schizophrenia [n 5 1], hypochondriasis [n 5 1], and undifferentiated somatiform disorder [n5 2]). A clinical diagnosis of dementia was made in three subjects.
Brain Weight
Brain weight did not differ between cases and control subjects, but the range was broader among the latter (Figure 1A). Possible explanations for this broader range are the higher incidence of brain edema among subjects dying of chronic illnesses (responsible for the upper brain weight value). Looking at the lower range, none of the cases but three of the control subjects had brains weighing less than 1000 g. The three were women with clinical dementia. The neuropathologic substrate of the dementia was AD in one case, Huntington’s disease (and mild AD pathology) in another, and multiple brain infarcts (and minimal AD pathology) in the third. As would be expected and can be seen in Figure 1B, brain weight declined with age (p 5 .035), with a gender difference (p , .0001). Brain weight decreased as modified Braak stage score increased (Figure 1C). The lack of apparent brain weight decline among subjects within the most severe modified Braak stage is most likely due to the increased represen-tation of men (with their inherent heavier brains) in that group (eight of 10 subjects were male).
Microscopic Studies
Figure 2 shows the distribution of cases and control subjects among the four modified Braak score groups. Although a similar proportion of both cases and control subjects fell within the mild AD pathology (stages I–II), 29% of the cases, as compared with 4% of the control subjects, were in the highest severity stages (V–VI). Concomitantly, there were fewer cases than control sub-jects (4% vs. 20%) with no neurofibrillary pathology (stage 0). The number of NFTs in CA1 was higher for cases than for control subjects (Figure 3A), and the values
Table 1. Demographic and Clinical Characteristics
Cases Control subjects
Number 28 56
Age
Mean6SD 75.768.7 75.668.2
Range 63–92 61–93
Gender
Men 17 (60.7%) 34 (60.7%) Women 11 (39.3%) 22 (39.3%) Dementia 3 (10.7%) 7 (12.5%) Other illnesses
Cancer 6 (21.4%) 18 (32.1%) Cardiac 7 (25%) 47 (83.9%) Respiratory 11 (39%) 21 (37.5%)
Table 2. Suicide Method with Gender Distribution
Method Number (%)a
Drug overdoseb 8 (29%), 1:7
Gunshot 8 (29%), 7:1
CO intoxication 6 (20%), 5:1 Strangulation (hanging) 3 (10%), 2:1 Asphyxia (plastic bag)b 2 (6%), 1:1
Drowning (bathtub) 1 (3%), 0:1 Trauma (jumping) 1 (3%), 1:0
aRatios are male:female.
increase proportionally according to the modified Braak stage (Figure 3B). The stage of amyloid deposition in the cortex was comparable for the two groups (Table 4). Five cases and 12 control subjects displayed amyloid angiopa-thy. One case and two control subjects had Lewy bodies in the parahippocampal gyrus, and ubiquitin-positive granu-lar neuropil staining in the superficial layers of the parahippocampal gyrus was identified in 10 (36%) cases and 20 (36%) control subjects.
Regression Analysis
The results of both the separate and combined logistic regression analyses were completely consistent. A higher Braak score predicted case status either alone (p5.0028) or combined with the other two predictor variables (p5
.014). This difference can be seen by comparing the frequency distributions for the Braak scores in the two groups (Figure 2). The cases clearly have relatively more scores of 3 (Braak stages V–VI) and relatively fewer scores of both 2 (Braak stages III–IV) and 0 (no neurofi-brillary pathology). There was no evidence for an associ-ation between amyloid score and case/control status either alone (p5.71) or combined with the other two variables (p 5 .75). This is consistent with the similarity of the distribution of scores in the two groups. The evidence for an association with NFTs was marginal (p 5 .16). The median numbers of NFTs were 2 for cases and 1 for control subjects; however, the 75th percentile was 10.5 for cases and 2 for control subjects, indicating a much more skewed distribution among the cases. This kind of differ-ence suggests that the assumption of a linear relationship on the logit scale that is required by the logistic regression model may be questionable for this variable.
Discussion
Our results show increased AD pathology in a population of elderly people committing suicide compared with a group of age- and gender-matched control subjects who died of natural causes. The prevalence of AD neuropathol-ogy in the control group is comparable to that seen in other population studies when stratified by age (Braak and Braak 1997). Due to the nature of the dependent variable being tested, suicide, the study has some intrinsic limita-tions, which we tried to minimize in our design. Specifi-cally we addressed the issue of selection and ascertain-ment biases as follows. The selection of cases was based on age, history of successful suicide, and availability of brain tissue. The clinical or neurologic status did not play a role in the performance of the autopsy, acquisition of brain tissue, or selection for the study. Because all suicides receive a forensic autopsy, we do not believe that social, educational, or other factors could have biased the selec-tion. Choosing appropriate control subjects is probably the key factor for any case– control study. Our purpose was to select a representative sample of the general population, comparable in age and gender to that of the cases. To increase statistical power, we chose two control subjects to match each case. We believe that in our community subjects receiving an autopsy at the university hospital are more representative of the general population as a whole than those having necropsies at the medical examiner’s office (violent deaths, unexpected and unexplained deaths, or deaths of individuals lacking physician attendance). It is probably true that individuals dying at the university hospital are sicker than those committing suicide (see also Table 2), given that they all die of natural causes. We do not believe that this factor biased the results. In fact, one of the genetic risk factors for cardiovascular disease (the
Table 3. Axis I Diagnosis (DSM-III-R) in 22 Cases with Psychological Autopsy
Case
1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22
Dementia f ea f
Major depression, single episode
f f f f f f f f f f f
Major depression, recurrent f f f
Dysthymia f f
Accompanying psychosis f f f f f
Alcohol dependence f f f
Alcohol abuse f
Drug abuse f f
Schizophrenia f
Hypochondriasis f
Undifferentiated somatiform disorder
f f
presence of one or more apolipoprotein E allele 4) is a risk factor for AD as well. If our control subjects had a higher prevalence of ApoE4, we could expect a higher degree of AD pathology in the control group, which was not the case. Because the neuropathologic grading was done blindly to the case status and to all clinical information, a biased assessment cannot explain the difference in AD pathology between cases and control subjects. The neuro-pathologic examination was limited because of the lack of
extensive brain tissue available for examination among the cases and the need to use the same methodology for the control subjects. Although we would prefer to have more brain areas available for testing, we believe that our modified scoring system is representative of the degree of AD pathology in the entire brain. This belief is substanti-ated by a recent study (Harding et al 2000) where the authors evaluated 72 brains in different stages of AD pathology and concluded that semiquantitative evaluation of NFTs in the hippocampus and inferior temporal cortices resulted in AD scores comparable to those obtained by the full original Braak method (kstatistic of .97).
Another factor limiting the scope of this study is the lack of homogeneity of clinical data for cases and control subjects. Although medical records were reviewed in both groups, cases had additional information resulting from the psychologic autopsy. As is common in case– control studies like this one, where death is the key factor for subject inclusion, the level of neuropsychologic evaluation
Figure 1. Brain weight by case/control status (A), age and gender (B), and modified Braak stage (C). Data in A and C are represented by box plots. The upper and lower limits of a box represent the 75th and 25th percentiles, respectively, and the line within the box indicates median values. Whiskers are represen-tative of the range.
Figure 2. Distribution of cases (top) and control subjects
was quite variable among individuals. Because of this, we did not include clinical status as a dependent variable and limited our end points to objective neuropathologic data obtained in a blind fashion. Thus, our results do not attempt to link suicide with AD (in the clinico-pathologic sense), but with Alzheimer’s neuropathology.
The cases included in our study shared the common experience of planning and carrying out an emotionally and intellectually challenging act. Postmortem psycho-logic evaluation was done in 22 of the 28 cases included in this study. Three individuals were identified as having dementia. A 79-year-old man had dementia of Parkinson disease. His neuropathologic evaluation revealed Demen-tia with Lewy bodies and mild AD changes. Secondly, a
75-year-old man with chronic schizophrenia was also diagnosed as having dementia. His neuropathologic exam-ination revealed mild AD pathology (Braak stages I–II), hardly justifying this patient’s cognitive decline. Lastly, the brain of a minimally demented 92-year-old female nursing home resident had severe AD pathology (Braak stages V–VI). Lack of clinical dementia in individuals with severe AD pathology has been reported (Davis et al 1999). Alzheimer’s disease is a clinico-pathologic entity (Berg and Morris 1994), and the currently recommended algorithm for the pathologic confirmation of AD requires the presence of clinical dementia (The National Institute on Aging and Reagan Institute Working Group on Diag-nostic Criteria for the Neuropathological Assessment of Alzheimer’s Disease 1997). We interpret this apparent lack of clinico-pathologic correlation as follows. Incipient Alzheimer’s pathology is the rule rather than the exception in the brains of elderly individuals. There is consensus among experts that these pathologic changes are progres-sive, and that the progression advances in a stereotyped manner according to brain topography. The tempo of the progression is slow, with the disease taking years to decades to advance from one Braak stage to another. The tempo would vary among different subjects, depending on known and unknown risk factors, both genetic (Arendt et al 1997; Li et al 1997; Nitsch et al 1996) and environmen-tal (Butler et al 1996; Itzhaki et al 1997). On the other hand, individual susceptibility (brain reserve) plays a role in the personal threshold for developing clinical dementia. Because dementia is the key for the clinical diagnosis of AD, we frequently observe neuropathologically compara-ble individuals with regard to Alzheimer’s pathology in very different cognitive stages. Thus, although the major-ity of humans would probably develop in time enough Alzheimer’s pathology to manifest clinical dementia, for those in whom neuropathology starts late in life and progresses slowly, or who have a larger “brain reserve,” AD, in the clinico-pathologic sense, will not be manifest within a normal life span. On the other hand, although dementia is a cardinal feature of AD, it is not the only one, and surely not the first to occur. Emotional-affective symptoms most likely precede dementia, and because cognitive decline is necessary for the clinical diagnosis of AD, emotional symptoms due to Alzheimer’s pathology would not be recognized as AD. In a recently published study (Smith et al 2000) of individuals carrying the Huntington disease mutation (so that the disease is genet-ically determined, provided patients live long enough), the authors were able to detect subtle movement abnormalities predicting disease progression. Similarly, in the limited fashion allowed by the case– control method, we suggest that suicide may be a manifestation of AD pathology. Indeed, the population of patients at risk for suicide
Figure 3. The number of neurofibrillary tangles (NFTs) in CA1 of the hippocampus plotted by case/control status (A) and by modified Braak stage (B). Data are represented by box plots. The upper and lower limits of a box represent the 75th and 25th percentiles, respectively, and the line within the box indicates median values. Whiskers are representative of the range.
Table 4. Amyloid Deposition in the Parahippocampal Cortex
Cases [n (%)]
Control subjects [n (%)]
because of such emotional changes may be larger than the population who are ever diagnosed with AD.
In contrast to the low prevalence of clinical dementia among the cases, postmortem psychological autopsy con-ferences demonstrated that the majority of individuals studied (79%) had some type of depressive disorder (Table 3), most commonly single-episode major depression (50% of the subjects with postmortem psychologic evaluation). Neuropathologic examination for the presence of Alzhei-mer’s-related changes in those subjects with a single episode of major depression revealed that nine of 11 individuals had early (Braak stages I–II) Alzheimer’s pathology. Depressive symptoms in these patients with early Alzheimer’s changes could either be a reaction to the subjects’ awareness of their cognitive decline or a direct manifestation of the entorhinal/limbic degeneration of incipient (preclinical) AD. Alternatively, it could be ar-gued that neurochemical imbalances usually seen in de-pressed patients committing suicide (for a review, see Mann 1998) may stress neuronal systems, inducing neu-rodegeneration by decreasing plasticity and synaptic repair (Brezun and Daszuta 1999). In fact, reduced activity of 5-hydroxytryptamine (5-HT) has been detected in AD patients (Meltzer et al 1998; Terry et al 1991) and has been linked to emotional imbalances, depression, and psychosis (Zubenko et al 1991; Zweig et al 1988). These neurochem-ical imbalances may predispose patients to aggressive or impulsive acts such as suicide, just as altered indices of central serotonin function have been associated with sui-cide (Ågren 1980, 1983; Bachus et al 1997; Banki et al 1984; Brown et al 1982; Coccaro et al 1990; Mann and Malone 1997; Mann et al 1996; Montgomery 1987; Ninan et al 1984; Roy et al 1986; Stockmeier and Meltzer 1995; Tra¨skman et al 1981; van Praag 1983; Virkkunen et al 1989) and other violent, aggressive behaviors (Virkkunen et al 1987, 1995).
The majority of serotonin-containing neurons are lo-cated in the brain stem, predominantly in the dorsal and ventral raphe (Frazer and Hensler 1999). The low seroto-nergic status seen in suicide victims does not seem to be due to fewer neurons in the raphe nuclei (Underwood et al 1999). On the other hand, medial raphe neurons, including serotonergic neurons, have NFTs in AD (Halliday et al 1992). Axons from the median raphe neurons project to the hippocampus and cerebral cortex, whereas from the dorsal raphe they reach the striatum and cortex. Numerous pre- and postsynaptic serotonin receptors have been iden-tified and their genes mapped. Polymorphisms for 5-HT2A
and 5-HT2Cmay be responsible not only for susceptibility
to psychosis (Gutierrez et al 1996; Williams et al 1997), but also for the psychotic symptomatology in patients with AD (Holmes et al 1998). These findings may be gender dependent, as indicated in animal models by the influence
of aromatase conversion of androgens to estrogens in the expression of 5-HT2A and serotonin transporters in the
brain (Fink et al 1999).
A third possible explanation of the association that we have observed is that both suicidal behavior and Alzhei-mer’s pathology may be independently linked to a genetic or environmental factor that affects both (Li et al 1997; Oliveira et al 1998).
Our conclusion that people with moderate to severe AD pathology are overrepresented in a sample of elderly patients who completed suicide should be validated in a larger, prospective study with psychologic autopsy data for control subjects as well as for cases. The psychologic examination should include cognitive, emotional, and behavioral assessment. We hypothesize that the emotion-al-affective state of cognitively intact or minimally im-paired individuals who later show AD symptoms may put them at risk for suicide before they reach clinically diagnosable AD.
Supported in part by grants AG-08665 and RO1 MH 54682.
The authors thank Ms. Frances Vito and Mr. Matt Frank for the histochemical and immunohistochemical preparations.
Presented at the “Unique Features of Neuropsychiatric Disease in the Elderly” panel, annual meeting of the American College of Neuropsy-chopharmacology, December 12–16, 1999, Acapulco, Mexico.
References
Aarsland D, Cummings JL, Yenner G, Miller B (1996): Rela-tionship of aggressive behavior to other neuropsychiatric symptoms in patients with Alzheimer’s disease. Am J
Psy-chiatry 153:243–247.
Ågren H (1980): Symptom patterns in unipolar and bipolar depression correlating with monoamine metabolites in the cerebrospinal fluid: I. General patterns. Psychiatry Res 3:211–223.
Ågren H (1983): Life at risk: Markers of suicidality in depres-sion. Psychiatry Dev 1:87–103.
Arendt T, Schindler C, Bruckner MK, Eschrich K, Bigl V, Zedlick D, Marcova L (1997): Plastic neuronal remodeling is impaired in patients with Alzheimer’s disease carrying apo-lipoprotein epsilon 4 allele. J Neurosci 17:516 –529. Bachus SE, Hyde TM, Akil M, Shannon Weikert C, Vawter MP,
Kleinman JE (1997): Neuropathology of suicide: A review and an approach. Ann N Y Acad Sci 836:201–219.
Bancher C, Jellinger K, Lassmann H, Fischer P, Leblhuber F (1996): Correlations between mental state and quantitative neuropathology in the Vienna Longitudinal Study of Demen-tia. Eur J Psychiatry Clin Neurosci 246:137–146.
Banki CM, Arato M, Papp Z, Kurcz M (1984): Biochemical markers in suicidal patients. Investigations with cerebrospinal fluid amine metabolites and neuroendocrine tests. J Affect
Disord 6:341–350.
Bick KL, editors. Alzheimer’s Disease. New York: Raven, 9 –25.
Blumenthal SJ (1988): Suicide: A guide to risk factors, assess-ment and treatassess-ment of suicidal patients. Med Clin North Am 72:937–971.
Braak H, Braak E (1991): Neuropathological staging of Alzhe-imer-related changes. Acta Neuropathol 82:239 –259. Braak H, Braak E (1997): Frequency of stages of
Alzheimer-related lesions in different age categories. Neurobiol Aging 18:351–357.
Braak H, Duyckaerts C, Braak E, Piette F (1993): Neuropatho-logical staging of Alzheimer-related changes correlates with psychometrically assessed intellectual status. In: Corain B, Iqbal K, Nicolini M, Wiblad B, Wisniewski H, Zatta P, editors. Alzheimer’s Disease: Advances in Clinical and Basic
Research. Chichester, UK: Wiley, 131–137.
Brezun JM, Daszuta A (1999): Depletion in serotonin decreases neurogenesis in the dentate gyrus and the subventricular zone of adult rats. Neuroscience 89:999 –1002.
Brown GL, Ebert MH, Goyer PF, Jimersom DC, Klein WJ, Bunney WE, et al (1982): Aggression, suicide and serotonin: Relationship to CSF amine metabolites. Am J Psychiatry 139:741–746.
Butler SM, Ashford JW, Snowdon DA (1996): Age, education, and changes in the Mini-Mental State Exam scores of older women: Findings from the Nun Study. J Am Geriatr Soc 44:675– 681.
Chiu HF, Lam LC, Pang AH, Leung CM, Wong CK (1996): Attempted suicide by Chinese elderly in Hong Kong. Gen
Hosp Psychiatry 18:444 – 447.
Coccaro EF, Silver LJ, Owen K, Davis KL (1990): Serotonin in mood and personality disorders. In: Coccaro EF, Murphy DL, editors. Serotonin in Major Psychiatric Disorders. Washing-ton, DC: APA Press, 71–97.
Conwell Y (1995): Dementia. Crisis 16:5– 6.
Conwell Y, Duberstein PR, Cox C, Herrmann JH, Forbes NT, Caine ED (1996): Relationships of age and axis I diagnoses in victims of completed suicide: A psychological autopsy study.
Am J Psychiatry 153:1001–1008.
Conwell Y, Rotenberg M, Caine ED (1990): Completed suicide at age 50 and over. J Am Geriatr Soc 38:640 – 644. Davis DG, Schnitt FA, Wekstein DR, Markesbery WR (1999):
Alzheimer neuropathologic alterations in aged cognitively normal subjects. J Neuropathol Exp Neurol 58:376 –388. Dickson DW (1997): The value of cross sectional
neuroanatomi-cal studies as a conceptual framework for prospective clini-copathological studies. Neurobiol Aging 18:382–386. Draper B, Moore CM, Brodaty H (1998): Suicidal ideation and
the “wish to die” in dementia patients: The role of depression.
Age Ageing 27:503–507.
Eastley R, Wilcock GK (1997): Prevalence and correlates of aggressive behaviours occurring in patients with Alzheimer’s disease. Int J Geriatr Psychiatry 12:484 – 487.
Ferris SH, Hofeldt GT, Carbone G, Masciandaro P, Troetel WM, Imbimbo BP (1999): Suicide in two patients with a diagnosis of probable Alzheimer disease. Alzheimer Dis Assoc Disord 13:88 –90.
Fewster PH, Griffin-Brooks S, MacGregor J, Ojalvo-Rose E,
Ball MJ (1991): A topographical pathway by which his-topathological lesions disseminate through the brain of pa-tients with Alzheimer’s disease. Dementia 2:121–132. Fink G, Sumner B, Rosie R, Wilson H, McQueen J (1999):
Androgen actions on central serotonin neurotransmission: relevance for mood, mental state and memory. Behav Brain
Res 105:53– 68.
Frazer A, Hensler JG (1999): Serotonin. In: Siegel GJ, editor.
Basic Neurochemistry. Molecular, Cellular and Medical Aspects, 6th ed. Philadelphia: Lippincott, Williams and
Wilkins, 263–292.
Frierson RL (1991): Suicide attempts by the old and the very old.
Arch Intern Med 151:141–144.
Geddes JW, Snowdon DA, Soultanian NS, Tekirian TL, Riley KP, Ashford JW, et al (1996): Braak stages III-IV of Alzheimer-related neuropathology are associated with mild memory loss, stages V-VI are associated with dementia: Findings from the Nun study. J Neuropathol Exp Neurol 55:617.
Geddes JW, Tekirian TL, Soultanian NS, Ashford JW, Davis DG, Markesbery WR (1997): Comparison of neuropathologic criteria for the diagnosis of Alzheimer’s disease. Neurobiol
Aging 18(suppl 4):S99 –S105.
Gertz HJ, Xuereb JH, Huppert FA, Brayne C, Kruger H, McGee MA, et al (1996): The relationship between clinical dementia and neuropathological staging (Braak) in a very elderly community sample. Eur Arch Psychiatry Clin Neurosci 246: 132–136.
Gutierrez B, Fananas L, Arranz MJ, Valles V, Guillamat R, van Os J, Collier D (1996): Allelic association analysis of the 5-HT2C receptor gene in bipolar disorder. Neurosci Lett 212:65– 67.
Halliday GM, McCann HL, Pamphlett R, Brooks WS, Creasey H, McCusker E, et al (1992): Brain stem serotonin-synthe-sizing neurons in Alzheimer’s disease: A clinicopathological correlation. Acta Neuropathol 84:638 – 650.
Harding AJ, Kril JJ, Halliday GM (2000): Practical measures to simplify the Braak tangle staging method for routine patho-logical screening. Acta Neuropathol 99:199 –208.
Harris EC, Barraclough B (1997): Suicide as an outcome for mental disorders: A meta-analysis. Br J Psychiatry 170:205– 228.
Hepple J, Quinton C (1997): One hundred cases of attempted suicide in the elderly. Br J Psychiatry 171:42– 46.
Holmes C, Arranz MJ, Powell JF, Collier DA, Lovestone S (1998): 5-HT2A and 5-HT2C receptor polymorphisms and psychopathology in late onset Alzheimer’s disease. Hum Mol
Genet 7:1507–1509.
Iqbal K, Braak H, Braak E, Grundke-Iqbal I (1993): Silver labeling of Alzheimer neurofibrillary changes and brain b
amyloid. J Histotechnol 16:335–342.
Itzhaki RF, Lin WR, Shang D, Wilcock GK, Faragher B, Jamieson GA (1997): Herpes simplex virus type 1 in brain and risk of Alzheimer’s disease. Lancet 349:241–244. Jellinger KA, Bancher C (1997): Proposals for re-evaluation of
current autopsy criteria for the diagnosis of Alzheimer’s disease. Neurobiol Aging 18(suppl 4):S55–S65.
Lecso PA (1989): Murder-suicide in Alzheimer’s disease. J Am
Geriatr Soc 37:167–168.
Li T, Homes C, Sham PC, Vallada H, Birkett J, Kirov G, et al (1997): Allelic functional variation of serotonin transporter expression is a susceoptibility factor for late onset Alzhei-mer’s disease. Neuroreport 8:683– 686.
Lyness JM, Conwell Y, Nelson JC (1992): Suicide attempts in elderly psychiatric inpatients. J Am Geriatr Soc 40:320 –324. Mann JJ (1998): The neurobiology of suicide. Nat Med 1998;4:
25–30.
Mann JJ, Malone KM (1997): Cerebrospinal fluid amines and higher lethality suicide attempts in depressed inpatients. Biol
Psychiatry 41:162–171.
Mann JJ, Underwood MD, Arango V (1996): Postmortem studies of suicide victims. In: Watson SJ, editor. Biology of
Schizo-phrenia and Affective Disease, 1st ed. Washington, DC: APP,
197–220.
Margo GM, Finkel JA (1990): Early dementia as a risk factor for suicide. Hosp Community Psychiatry 41:676 – 678.
Masliah E (1995): Natural evolution of the neurodegenerative alterations in Alzheimer’s disease. Neurobiol Aging 16:280 – 282.
McCullagh P, Nelder JA (1989): Generalized Linear Models, 2nd ed. London: Chapman and Hall.
Meltzer CC, Smith G, DeKosky ST, Pollock BG, Mathis CA, Moore RY, et al (1998): Serotonin in aging, late-life depres-sion, and Alzheimer’s disease: The emerging role of func-tional imaging. Neuropsychopharmacology 18:407– 430. Montgomery SA (1987): The psychopharmacology of borderline
personality disorders. Acta Psychiatr Belg 87:260 –266. The National Institute on Aging, Reagan Institute Working
Group on Diagnostic Criteria for the Neuropathological Assessment of Alzheimer’s Disease (1997): Consensus rec-ommendation for the postmortem diagnosis of Alzheimer’s disease. Neurobiol Aging 18(suppl 1):S1–S2.
Newell KL, Hyman BT, Growdon JH, Hedley-Whyte ET (1999): Application of the National Institute on Aging (NIA)-Reagan Institute criteria for the neuropathologic diagnosis of Alzhei-mer disease. J Neuropathol Exp Neurol 58:1147–1155. Ninan PT, van Kammen DP, Scheinin M, Linnoila M, Bunney
WE, Goodwin FK (1984): CSF 5-hydroxyindoleacetic acid levels in suicidal schizophrenic patients. Am J Psychiatry 141:566 –569.
Nitsch RM, Deng M, Growdon JH, Wurtman RJ (1996): Sero-tonin 5-HT2a and 5-HT2c receptors stimulate amyloid pre-cursor protein ectodomain secretion. J Biol Chem 271:4188 – 4194.
Oliveira JR, Gallindo RM, Maia LG, Brito-Marques PR, Otto PA, Passos-Bueno MR, et al (1998): The short variant of the polymorphism within the promoter region of the serotonin transporter gene is a risk factor for late onset Alzheimer’s disease. Mol Psychiatry 3:438 – 451.
Rodhe K, Peskind ER, Raskind MA (1995): Suicide in two patients with Alzheimer’s disease. J Am Geriatr Soc 43:187– 189.
Roy A, Ågren H, Pickar D, Linnoila M, Doran A, Cutler N, et al
(1986): Reduced CSF concentrations of homovanilic acid and homovanilic acid to 5-hydroxyindoleacetic acid ratios in depressed patients: relationship to suicidal behavior and dexamethasone nonsuppression. Am J Psychiatry 143:1539 – 1545.
Smith MA, Brandt J, Shadmehr R (2000): Motor disorder in Huntington’s disease begins as a dysfunction in error feed-back control. Nature 403:544 –549.
Spitzer RL, Williams JBW, Gibbon M (1986): Structured
Clin-ical Interview for DSM-IIIR (SCID). New York: New York
State Psychiatric Institute, Biometrics Research.
Stockmeier CA, Meltzer HY (1995): Biologic studies of suicide in schizophrenia. Clin Neuropharmacol 18(3):S9 –S17. Terry RD, Masliah E, Salmon DP, Butters N, DeTeresa R, Hill
R, et al (1991): Physical basis of cognitive alterations in Alzheimer’s disease: Synapse loss is the major correlate of cognitive impairment. Ann Neurol 30:572–580.
Tra¨skman L, Åsberg M, Bertilsson L, Sjo¨strand L (1981): Monoamine metabolites in CSF and suicidal behavior. Arch
Gen Psychiatry 38:631– 636.
Underwood MD, Khaibulina AA, Ellis SP, Moran A, Rice PM, Mann JJ, Arango V (1999): Morphometry of the dorsal raphe nucleus serotonergic neurons in suicide victims. Biol
Psychi-atry 46:473– 483.
van Praag HM (1983): CSF 5-HIAA and suicide in non-depressed schizophrenics. Lancet ii:977–978.
Virkkunen M, De Jong J, Bartko J, Linnoila M (1989): Psycho-biological concomitants of history of suicide attempts among violent offenders and impulsive fire setters. Arch Gen
Psy-chiatry 46:604 – 606.
Virkkunen M, Goldman D, Nielsen D, Linnoila M (1995): Low brain serotonin turnover rate (low CSF 5-HIAA) and impul-sive violence. J Psychiatr Neurosci 20:271–275.
Virkkunen M, Nuutila A, Goodwin FK, Linnoila M (1987): Cerebrospinal fluid monoamine metabolite levels in male arsonists. Arch Gen Psychiatry 44:241–247.
Williams J, McGuffin P, Nothen MM, Owen MJ, EMASS Consortium (1997): Meta-analysis of association between the 5-HT2a receptor T102C polymorphism and schizophrenia.
Lancet 349:1221.
Willis RH (1987): Suicide risk in elderly persons: Diagnosis and management. Mt Sinai J Med 54:14 –17.
Wragg RE, Jeste DV (1989): Overview of depression and psychosis in Alzheimer’s disease. Am J Psychiatry 146:577– 587.
Xuereb JH, Gertz HJ, Huppert F, Brayne C, Wischik CM, Mukaetoba-Ladinska E (1995): The application of Braak’s staging model of Alzheimer-type pathology to neuropatho-logical diagnosis of dementia. Neuropathol Appl Neurobiol 21:44.
Zubenko GS, Moosy J, Martinez AJ, Rao G, Claassen D, Rosen J, Kopp U (1991): Neuropathologic and neurochemical cor-relates of psychosis in primary dementia. Arch Neurol 48: 619 – 624.