Basal Ganglia: Response to Cocaine Administration
James D. Christensen, Marc J. Kaufman, Blaise deB. Frederick, Stephanie L. Rose,
Constance M. Moore, Scott E. Lukas, Jack H. Mendelson, Bruce M. Cohen, and
Perry F. Renshaw
Background: Proton magnetic resonance spectroscopy was used to determine the effects of intravenous cocaine or placebo administration on human basal ganglia water and metabolite resonances.
Methods: Long echo time, proton magnetic resonance spectra of water and intracellular metabolites were con-tinuously acquired from an 8-cm3voxel centered on the left caudate and putamen nuclei before, during, and after the intravenous administration of cocaine or a placebo in a double-blind manner.
Results:Cocaine, at both 0.2 and 0.4 mg/kg, did not alter the peak area for water. Cocaine at 0.2 mg/kg induced small and reversible increases in choline-containing com-pounds andN-acetylaspartate peak areas. Cocaine at 0.4 mg/kg induced larger and more sustained increases in choline-containing compounds and N-acetylaspartate peak areas. No changes in either water or metabolite resonances were noted following placebo administration. Conclusions:These increases in choline-containing com-pounds and N-acetylaspartate peak areas may reflect increases in metabolite T2 relaxation times secondary to osmotic stress and/or increased phospholipid signaling within the basal ganglia following cocaine administration. This is the first report of acute, drug-induced changes in the intensity of human brain proton magnetic resonance spectroscopy resonance areas. Biol Psychiatry 2000;48: 685– 692 ©2000 Society of Biological Psychiatry
Key Words:Cocaine, osmotic stress, basal ganglia, nu-clear magnetic resonance, phospholipid turnover, sub-stance abuse
Introduction
H
uman brain imaging studies have documented co-caine-induced alterations in cerebral metabolism and perfusion (Gollub et al 1998; Johnson et al 1998; Kaufman et al 1998a, 1998b; London et al 1990; Mathew et al 1996; Pearlson et al 1993; Wallace et al 1996); however, there remains a paucity of data on the biochemical responses of the human brain to cocaine administration. Proton mag-netic resonance spectroscopy (1H MRS) offers a means to characterize some of cocaine’s effects by allowing nonin-vasive detection of brain water and proton-containing metabolites present in millimolar concentrations.Metabolites that are detected in the human brain in vivo include cytosolic choline-containing compounds (Cho), creatine and phosphocreatine (Cre), andN-acetylaspartate (NAA). The1H MRS choline resonance arises primarily
from phosphocholine (PC) and glycerophosphocholine (GPC; Bluml et al 1999). Changes in the Cho peak may reflect receptor-mediated phospholipid turnover (Exton 1994) or alterations in regional glucose utilization (Duc et al 1997). The Cre peak, which contains contributions from both creatine and phosphocreatine (an important energy reservoir molecule), remains relatively stable across phys-iologic states (Petroff et al 1988, 1989).N-Acetylaspartate has been suggested to serve as a marker of neuronal density (Urenjak et al 1993). Its levels are reduced by the injection of kainic acid, an excitotoxin, in the rat brain (Guimaraes et al 1995) and in neurodegenerative pro-cesses (Tsai and Coyle 1995; Tzika et al 1993). Recent work suggests that NAA also participates as a neuronal osmolyte (Taylor et al 1995). These compounds can thus be used as markers of cerebral biochemical function.
The purpose of this study was to determine whether cocaine-induced biochemical changes in the basal ganglia are detectable as changes in the 1H MRS signals of proton-containing metabolites. Cocaine receptors associ-ated with the dopamine transporter are thought to contrib-ute to cocaine’s behavioral effects (Giros et al 1996). Consequently, the basal ganglia was selected as a region of interest, since it is enriched with dopamine terminals and From the Brain Imaging Center (JDC, MJK, BdBF, SLR, CMM, BMC, PFR) and
Alcohol & Drug Abuse Research Center (MJK, SEL, JHM), McLean Hospital, and the Consolidated Department of Psychiatry, Harvard Medical School (JDC, MJK, BdBF, SLR, CMM, SEL, JHM, BMC, PFR), Belmont, Massachusetts.
Address reprint requests to Perry F. Renshaw, M.D., Ph.D., McLean Hospital, Brain Imaging Center, 115 Mill Street, Belmont MA 02478-9106.
Received November 23, 1999; revised March 20, 2000; accepted April 7, 2000.
© 2000 Society of Biological Psychiatry 0006-3223/00/$20.00
transporter sites and, in animals, has been shown to undergo cocaine-induced biochemical changes. We hy-pothesized that cocaine would induce an increase in the Cho peak area, by elevating striatal dopamine and, thereby, increasing phospholipid turnover through second-messenger signaling pathways.
Methods and Materials
All study subjects were men aged 2865 years (mean6SD) and were interviewed by a psychiatrist (PFR) to rule out the presence of an Axis I disorder other than a past history of substance abuse. No study subject met criteria for substance dependence. Ongoing use of alcohol and tobacco averaged 666 drinks/week and 146
28 cigarettes/week; 19 of the 30 subjects did not smoke. All subjects reported occasional cocaine use, 13 6 14 lifetime exposures, with intranasal administration being the most com-mon route of exposure. We have recently reported that illicit cocaine use patterns are not altered in this population following participation in an imaging study during which cocaine is administered by slow intravenous infusion (Kaufman et al 2000). No subject was taking any psychoactive medications at the time of study.
All subjects underwent a complete physical examination and were judged to be in good health. All structural brain MRI examinations were normal. Immediately before the study all subjects tested negative for recent cocaine, amphetamine, opiate, phencyclidine, benzodiazepine, and barbiturate exposure by a urine test (Triage Test, Biosite Diagnostics, San Diego) and for alcohol exposure by a breathalyzer test (Alco Sensor III, In-toximeters, St. Louis). Subjects were implanted with an 18-gauge angiocath in the antecubital vein for intravenous drug adminis-tration. Subjects were fit with noninvasive, cardiovascular mon-itoring equipment (In Vivo Research, Orlando) for continuous vital sign measurements. This included a four-lead electrocardio-gram, infrared fingertip pulse oxymetry oxygen detection sys-tem, and automated blood pressure cuff. Vital signs were recorded at 5-min intervals throughout the study.
Magnetic resonance spectroscopy was performed using a 1.5-T clinical magnetic resonance scanner with a proton quadra-ture head coil (General Electric Medical Systems, Milwaukee). The point-resolved spectroscopy localization technique (Moonen et al 1989) was used to acquire proton (1H) signals from an
8-cm3 cubic voxel centered on the head of the left caudate
nucleus and putamen. Acquisition parameters were as follows: interpulse repetition time (TR)52 sec, echo time (TE)5135 msec, number of points51024, spectral width 52 kHz, and phase cycles52. Water suppression was achieved using a series of chemical shift–selective radiofrequency pulses and gradient dephasing. Signal pairs consisting of an unsuppressed water signal (16 averages) and a water-suppressed metabolite signal (128 averages) were obtained in a sequential manner over a 5-min period. The TR and TE values were the same for both the suppressed and unsuppressed spectra.
Three baseline signal pairs were obtained before drug chal-lenges. Subjects (n 5 30) then were administered a placebo intravenously (saline;n510) or cocaine at low (0.2 mg/kg;n5
10) or high (0.4 mg/kg; n510) doses as a slow push over 1 min in a double-blind manner. Seven additional signal pairs were acquired immediately following placebo/cocaine administration, yielding 10 pairs of water and water-suppressed spectra for each subject, with the exception of six subjects from whom only water-suppressed spectra were obtained.
Magnetic resonance spectroscopy data was processed in a fully automated manner requiring no subjective operator input (Webb et al 1994). The Cho, Cre, NAA, and water peaks were fit to Lorentzian line shapes using an interative Marquardt algo-rithm for peak areas. A singular value decomposition algoalgo-rithm was used to determine peak line widths (van den Boogaart et al 1994). For this analysis, fitting of the raw, unfiltered, and unapodized free induction decay was done in the time domain with no Fourier reconstruction, eliminating the artificial line width distortion that would arise from apodization.
Repeated-measures analysis of variance (ANOVA) was used to detect between-group differences in normalized water and metabolite peak areas. Because we found a moderate degree of between-subject variance in the time course of cocaine’s effects, we also performed an area under the curve (AUC) analysis. The AUC was calculated as the sum of the normalized metabolite resonance intensities for each subject. Before summation the normalized baseline value (1.00) was subtracted from each postdrug measurement. The AUC was defined as
S[(Postdrug Time 121.00), (Postdrug Time 221.00) . . . ,
(Postdrug Time 721.00)]
Missing data values (one postdrug water-suppressed metabolite spectrum in three subjects and a single unsuppressed water signal in one subject) were estimated by interpolation (Winer 1971).
Results
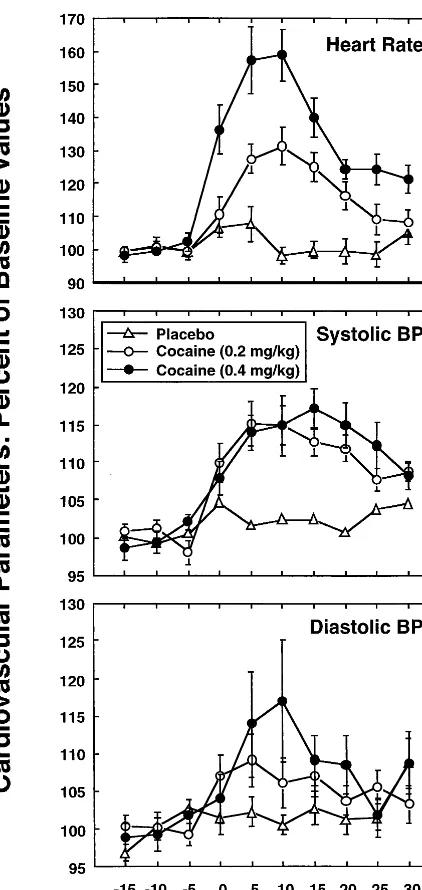
Baseline cardiovascular parameters were normal in all subjects and statistically equivalent across experimental groups, with heart rate (HR)5 606 8 beats per minute (mean6SD), systolic pressure (Sys)5118611 mm Hg, diastolic pressure 5 70 6 8 mm Hg, and mean arterial pressure 5 87 6 8 mm Hg. Cocaine induced transient, dose-dependent increases in HR and Sys, with peak values occurring about 15–20 min after drug administration (Figure 1).
Cocaine administration was not associated with statis-tically significant changes in water peak areas. The vari-ance of the water resonvari-ance area was, in each case, less than 2%, documenting the consistency and reproducibility of the technique.
cocaine dose (0.4 mg/kg) resulted in a progressive increase in the Cho peak, which reached a maximum of about 135% of basal level 20 min following drug administration and persisted to the end of the experiment (Figure 3, top). For the NAA peak, 0.2 mg/kg cocaine also induced a minimal metabolite increase, whereas 0.4 mg/kg cocaine induced a larger increase that peaked at about 120% of
basal levels 20 min after drug administration and remained elevated for the rest of the study (Figure 3, middle). A slight but nonsignificant increase in the Cre peak area was observed with high-dose cocaine (Figure 3, bottom).
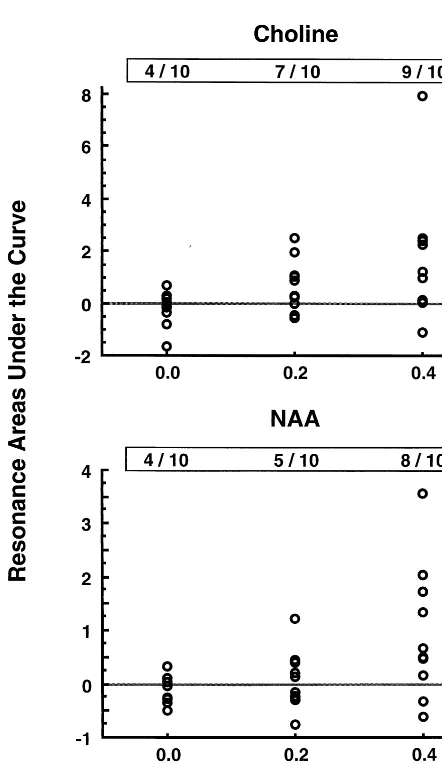
Figure 4 displays a scattergram of areas under the curve for the Cho and NAA resonance lines over time following cocaine administration. Note that for the Cho resonance (Figure 4, top) four of 10 subjects had an AUC . 0, compared with seven of 10 subjects receiving 0.2 mg/kg cocaine and nine of 10 subjects receiving 0.4 mg/kg. By x2
, the number of subjects with an increased AUC was larger in the cohort receiving cocaine (x25
4.8,p,.03). For the NAA resonance (Figure 4, bottom), four of 10 subjects had an AUC . 0, compared with five of 10 subjects receiving 0.2 mg/kg cocaine and eight of 10 subjects receiving 0.4 mg/kg. By x2
, there was a weak trend for the number of subjects with an increased AUC to be larger in the cohort receiving cocaine (x25
1.7,p, .20). In both instances, the number of subjects with an increased resonance intensity increased with dose. For the Cre resonance, no trends between resonance area and cocaine data were noted (data not shown).
Metabolite line widths were unchanged by cocaine administration; repeated-measures ANOVA analysis de-tected no dose or time effects of cocaine on the Cho (5.16 0.8 Hz), Cre (6.161.8 Hz), and NAA (5.161.3 Hz) line widths (mean 6SD). This suggests that any confound in the data resulting from subject motion is minimal.
Subject motion could also influence the observed MRS signal amplitudes, if the volume of interest shifted over time in such a manner as to include different amounts of the lateral ventricles. Movement would also likely influ-ence the shim and, therefore, the degree of water
suppres-Figure 1. Effects of cocaine/placebo administered at time 0 on cardiovascular parameters. Shown are means 6 SEs of 10 subjects per dose level. Cocaine induced transient dose-depen-dent increases in heart rate (F 5 21.8, p , .0001; top) and systolic blood pressure (F 5 13.0, p 5 .0001; middle). A significant time effect was detected for diastolic pressure (F5 4.3,p,.0001;bottom). Data are presented as mean6SEM and values have been normalized to 100% for baseline measures.
sion; however, these effects of subject motion would not account for the differential changes in signal intensity that were observed in the NAA, Cho, and Cre resonance areas. Although plasma cocaine levels were not measured in this study, plasma analysis with gas chromatography in
separate subject groups receiving cocaine via an identical administration protocol revealed that 0.2 (n5 6) and 0.4 (n 5 6) mg/kg cocaine produced peak plasma levels 10 min after administration averaging 110 and 230 ng/mL, respectively (Mendelson et al 1999).
Discussion
Our results demonstrate dose- and time-dependent effects of cocaine on basal ganglia metabolite peak areas. These effects temporally paralleled neuroendocrine and cardio-vascular changes and plasma cocaine concentrations in separate subject groups receiving cocaine via an identical administration protocol as previously described (Mendel-son et al 1999). In those studies, cocaine pharmacokinetics
Figure 3. Proton metabolite peak area changes induced by intravenous cocaine/placebo administration at time 0. Cocaine induced dose-dependent (F53.3,p5.05) and time-dependent (F52.5,p,.01) increases in the cytosolic choline-containing compounds (Cho) peak area (top). Cocaine induced dose-dependent increases in the N-acetylaspartate (NAA) peak area (F 5 5.1, p , .02;middle). Cocaine administration was not associated with statistically significant alterations in the creatine and phosphocreatine (Cre) resonance (bottom). Data are pre-sented as means6SDs.
following intravenous administration of 0.2 and 0.4 mg/kg to men were determined; Tmax(and T1/2) were 6.7 min (45
min) and 8.0 min (48 min), respectively (Mendelson et al 1999). A comparable time course for cocaine-induced striatal dopamine increases has been reproduced in both rodents and nonhuman primates. Microdialysis studies in rats (Bradberry et al 1993) and in primates (Iyer et al 1995) suggest that striatal dopamine levels peak 10 –20 minutes following the intravenous administration of co-caine and remain elevated for at least 100 min following injection.
Cocaine’s effects on the Cho and NAA peak areas may reflect changes in transverse relaxation times (T2*),
caused by osmotic stress and increased intracellular water content. Cocaine inhibits the Na1/K1 -adenosinetriphos-phatase (Na1/K1-ATPase; Lien et al 1994), probably through its action as an indirect agonist of dopamine D1
receptors (Bertorello et al 1990). Since volume regulation is dependent upon Na1/K1-ATPase activity (Olson et al 1986), inhibition of this enzyme could lead to ion imbal-ance, osmotic stress, and cell swelling, as both sodium and water move into the intracellular space (Hertz et al 1992). Metabolite relaxation times can be greatly affected by such changes in the cellular environment. In this regard, osmotic stress secondary to Na1/K1-ATPase inhibition and secondary energy failure has been associated with enhanced metabolite mobility and increased Cho and NAA relaxation times in some reports (Cady 1996; Cady et al 1994), but not all (Fujimori et al 1998). Such an effect on NAA relaxation has also been observed in brain edema (Kamada et al 1994). Importantly, NAA has recently been shown to serve as a neuronal osmolyte (Taylor et al 1995), and GPC, which contributes substantially to the1H MRS Cho peak (see below), has been shown to function as a renal osmolyte (Wolff and Balaban 1990).
Alternatively, cocaine may alter metabolite concentra-tions. For example, cocaine-stimulated phosphatidylcho-line (PtdCho; brain concentration;21 mmol/L) hydroly-sis might result in increased levels of PC (0.57 mmol/L) and/or GPC (0.94 mmol/L; Bluml et al 1999; Klein et al 1993). With these concentration estimates, a 35% increase in the 1H MRS Cho resonance would require the
hydro-lysis of 0.53 mmol/L, or approximately 2.5% of cellular PtdCho. Turnover rates for PtdCho are difficult to measure in vivo and may vary with regional cerebral activity; however, Purdon and Rapoport (1998) have noted that the turnover rates for fatty acids in phospholipids may be 100 times faster than previously thought. The relatively de-layed time frame for the cocaine-induced Cho increase is consistent with the general temporal sequence of phospho-lipid turnover, in which phosphatidylinositol turnover is often the initial response, followed by PtdCho turnover (Exton 1994).
We did not observe a statistically significant increase in the intensity of the Cre resonance; however, across a number of studies (for review, see Kreis 1997), T2*
relaxation times for the Cre resonance are substantially shorter than those of Cho and NAA at 1.5 T. Thus, increases in the intensity of the Cre resonance due to an increase in intracellular water would be smaller than those predicted for the Cho or NAA resonances. It may also be the case that the 10% increase observed in the Cre resonance following 0.4 mg/kg cocaine would have reached statistical significance had a larger number of subjects been studied.
To date, acute, drug-induced changes in the intensities of1H MRS resonance lines have not been reported in the
peer-reviewed literature; however, in general agreement with the present results, Li and colleagues (1998) have presented a preliminary report documenting a statistically significant, 15% increase in NAA/Cre and a nonsignifi-cant, 10% increase in Cho/Cre 12 min after the adminis-tration of a comparable dose of cocaine (40 mg/70 kg) to a cohort of cocaine-dependent subjects. These data were obtained from a 5-cm3 volume centered on the left
thalamus.
Since the present data were collected at a single TE, it is not possible to conclude whether changes in Cho and NAA peak areas resulted from changes in concentration, changes in relaxation times, or both effects. Kreis (1997) has reported that the mean T2* for NAA across multiple
brain regions is 376 msec. An increase in T2* to
approx-imately 500 msec would be needed to increase the NAA signal intensity by 10%. Of note, Cady et al (Cady 1996; Cady et al 1994) have reported increases of this magnitude in brain metabolite T2* values during secondary energy
failure; however, the fact that the intensities of two intracellular resonance lines increased, as well as the relatively large size of these increases, tend to argue in favor of a change in relaxation times. A 35% increase in the Cho resonance and an 20% increase in the NAA resonance would correspond to increases in these metab-olite pools of at least 500mmol/L and 1 mmol/L, respec-tively, which are not likely. To the extent that the increased Cho and NAA resonance intensities can be directly attributed to dopamine release within the basal ganglia, 1H MRS may provide a unique window into central nervous system dopaminergic neurotransmission.
not been associated with the long-term administration of dopaminergic agonists and efforts to detect basal ganglia pathology in human cocaine users have produced mixed results (e.g., Kish et al 1999; Little et al 1998; Staley et al 1997). An alternative experimental approach would be to use diffusion-weighted imaging (Le Bihan et al 1986) to detect increased intracellular water, which should lead to increased water diffusion.
Other factors may also contribute to the increased Cho and NAA resonance intensities. For example, cocaine administration to nonhuman primates has been associated with a 13–22% decrease in basal ganglia cerebral meta-bolic rate (Lyons et al 1996). Limited available data suggest that local cerebral metabolic rates, as determined by positron emission tomography, are inversely related to the intensity of the1H MRS choline resonance. Another
factor that may play a role is cocaine-induced vasocon-striction. The intravenous administration of cocaine has been reported to cause decreases in cerebral blood flow (Gollub et al 1998; Wallace et al 1996) and volume (Kaufman et al 1998a). Alterations in cerebral blood flow and blood volume can lead to vascular susceptibility changes (i.e., BOLD effects) that may increase (if the local concentration of deoxyhemoglobin is decreased) or de-crease (if the concentration of deoxyhemoglobin is in-creased) the signal intensity of both water and nearby tissue metabolites. In our study the signal intensity of water, which traverses the intravascular space, was not increased while the intensity of intracellular metabolites, NAA and Cho, was elevated. This observation tends to argue against a BOLD effect. Of concern, our data would be consistent with a decrease in blood flow to an extent that causes basal ganglia ischemia (Cady 1996; Cady et al 1994). Therefore, further work to clarify the mechanism by which intracellular metabolite resonance intensities are increased may be warranted.
With regard to the effects of chronic cocaine on basal ganglia 1H MRS metabolites, Li and coworkers (1999) have reported that there are no differences in 1H MRS metabolite ratios between cocaine users and comparison subjects. In contrast, a 17% reduction in NAA/Cr was noted in the left thalamus. Chang and colleagues (1997) have reported increased parietal white matter Cre and
myo-inositol concentrations in heavy cocaine users. In a follow-up study (Chang et al 1999), decreased NAA and increasedmyo-inositol were noted in frontal lobe gray and white matter in a cohort of abstinent subjects with a history of crack cocaine dependence. In our study the use of a 135-msec TE precluded the accurate assessment of the
myo-inositol resonance. Thus, it is uncertain how the acute, cocaine-induced increases in 1H MRS resonance
intensities are related to the neurochemical consequences of chronic heavy drug use.
In summary, intravenous cocaine administration tran-siently induced dose-dependent increases of basal ganglia Cho and NAA peak areas. This study’s results are sugges-tive of cocaine-induced osmotic stress, but are also con-sistent with an increase in phospholipid turnover within the basal ganglia. Further studies characterizing proton metabolite changes following cocaine administration are warranted to clarify this issue and to determine whether the observed changes are relevant to the physiologic and behavioral effects of cocaine.
This research was supported by National Institute on Drug Abuse Grants Nos. DA09448, DA04059, DA00064, DA00115, DA00329, and DA00343.
We gratefully acknowledge the excellent technical assistance provided by Ms. Kim Appelmans.
JDC’s current affiliation: Department of Psychiatry, University of Louisville, Louisville, Kentucky.
References
Bertorello AM, Hopfield JF, Aperia A, Greengard P (1990): Inhibition by dopamine of (Na1/K1)ATPase activity in neostriatal neurons through D1 and D2 dopamine receptor synergism.Nature347:386 –388.
Bluml S, Seymour KJ, Ross BD (1999): Developmental changes in choline- and ethanolamine-containing compounds mea-sured with proton-decoupled 31P MRS in in vivo human brain.Magn Reson Med42: 643– 654.
Bradberry CW, Nobiletti JB, Elsworth JD, Murphy B, Jatlow P, Roth RH (1993): Cocaine and cocaethylene: Microdialysis comparison of brain drug levels and effects on dopamine and serotonin.J Neurochem60:1429 –1435.
Cady EB (1996): Metabolite concentrations and relaxation in perinatal cerebral hypoxic-ischemic injury. Neurochem Res
21:1043–1052.
Cady EB, Lorek A, Penrice J, Wylezinska M, Cooper CE, Brown GC, et al (1994): Brain-metabolite transverse relaxation times in magnetic resonance spectroscopy increase as adenosine triphosphate depletes during secondary energy failure follow-ing acute hypoxia-ischaemia in the newborn piglet.Neurosci Lett182:201–204.
Chang L, Ernst T, Strickland TL, Mehringer M (1999): Gender effects on persistent cerebral metabolite changes in the frontal lobes of abstinent cocaine users.Am J Psychiatry156:716 – 722.
Chang L, Mehringer CM, Ernst T, Rosemarie M, Myers H, Forney D, Satz P (1997): Neurochemical alterations in asymptomatic abstinent cocaine users: A proton magnetic resonance spectroscopy study.Biol Psychiatry42:1105–1114. Duc CO, Weber AH, Trabesinger AH, Meier D, Boesiger P
(1997): Recycling the cholines. In: International Society of Magnetic Resonance in Medicine VI.Berkeley, CA: ISMRM, 1210.
Exton JH (1994): Phosphatidylcholine breakdown and signal transduction.Biochem Biophys Acta1212:26 – 42.
relaxation of cerebral metabolites during transient global ischemia in rat brain.Magn Reson Med39:647– 650. Giros B, Jaber M, Jones SR, Wightman RM, Caron MO (1996):
Hyperlocomotion and indifference to cocaine and amphet-amine in mice lacking the dopamphet-amine transporter. Nature
379:606 – 612.
Gollub RL, Breiter HC, Kantor H, Kennedy D, Gastfriend D, Mathew RT, et al (1998): Cocaine decreases cortical cerebral blood flow but does not obscure regional activation in functional magnetic resonance imaging in human subjects.
J Cereb Blood Flow Metab18:724 –734.
Guimaraes AR, Schwartz P, Prakash MR, Carr CA, Berger UV, Jenkins BG, et al (1995): Quantitative in vivo 1H nuclear magnetic resonance spectroscopic imaging of neuronal loss in rat brain.Neuroscience69:1095–1101.
Gur RE, Maany BA, Mozley PD, Swanson C, Bilker W, Gur RC (1998): Subcortical MRI volumes in neuroleptic-naive and treated patients with schizophrenia. Am J Psychiatry 155: 1711–1717.
Hertz L, Code WE, Sykova E (1992): Ions, water and energy in brain cells: A synopsis of interrelations. Can J Physiol Pharmacol70:S100 –S106.
Iyer RN, Nobiletti JB, Jatlow PI, Bradberry CW (1995): Cocaine and cocaethylene: effects on extracellular dopamine in the primate.Psychopharmacology120:150 –155.
Johnson B, Lamki L, Fang B, Barron B, Wagner L, Wells L, et al (1998): Demonstration of dose dependent global and regional cocaine-induced reductions in brain blood flow using a novel approach to quantitative single photon emission computerized tomography. Neuropsychopharmacology 18: 377–384.
Kamada K, Houkin K, Hida K, Matsuzawa H, Iwasaki Y, Abe H, Nakada T (1994): Localized proton spectroscopy of focal brain pathology in hurnans: Significant effects of edema on spin-spin relaxation time.Magn Reson Med31:537–540. Kaufman MJ, Levin JM, Kukes TJ, Villafuerte RA, Hennen J,
Lukas SE, et al (2000): Illicit cocaine use patterns in intra-venous-naive cocaine users following investigational intrave-nous cocaine administration.Drug Alcohol Depend58:35– 42. Kaufman MJ, Levin JM, Maas LC, Rose SL, Kukes TJ,
Men-delson JH, et al (1998a): Cocaine decreases relative cerebral blood volume in humans: A dynamic susceptibility contrast magnetic resonance imaging study. Psychopharmacology
138:76 – 81.
Kaufman MJ, Levin JM, Ross MH, Lange N, Rose SL, Kukes TJ, et al (1998b): Cocaine induced cerebral vasoconstriction detected in humans with magnetic resonance angiography.
JAMA279:376 –380.
Kish SJ, Kalasinsky KS, Furukawa Y, Guttman M, Ang L, Li L, et al (1999): Brain choline acetyltransferase activity in chronic, human users of cocaine, methamphetamine, and heroin.Mol Psychiatry4:26 –32.
Klein J, Gonzalez R, Koppen A, Loffelholz K (1993): Free choline and choline metabolites in rat brain and body fluid: Sensitive determination and implications for choline supply to the brain.Neurochem Int31:293–300.
Kreis R (1997): Quantitative localized 1H MR spectroscopy for clinical use.Prog Nucl Magn Reson Spectrosc11:155–195. Le Bihan D, Breton E, Lallemand D, Grenier P, Cabanis E,
Laval-Jeantet M (1986): MR imaging of intravoxel incoher-ent motions: Applications to diffusion and perfusion in neurologic disorders.Radiology161:401– 407.
Li S-J, Wang Y, Pankiewicz J, Stein EA (1999): Neurochemical adaptation to cocaine abuse: Reduction of N-acetyl aspartate in thalamus of human cocaine users. Biol Psychiatry 45: 1481–1487.
Li S-J, Wang Y, Risinger R, Rainey C, Harsch H, Pankiewicz J, et al (1998): Acute cocaine administration induces NAA increase detected by 1H MRS. In:International Society of Magnetic Resonance in Medicine VI.Berkeley, CA: ISMRM, 1714.
Lien R, Mishra OP, Graham E, Delivoria-Papadopoulos M, Anday EK (1994): Alteration of brain cell membrane function following cocaine exposure in the fetal guinea pig.Brain Res
637:249 –254.
Little KY, McLaughlin DP, Zhang L, McFinton PR, Dalack GW, Cook EH Jr, et al (1998): Brain dopamine transporter mes-senger RNA and binding sites in cocaine users: A postmortem study.Arch Gen Psychiatry550:793–799.
London ED, Cascella NG, Wong DF, Phillips RL, Dannals RF, Links JM, et al (1990): Cocaine-induced reduction of glucose utilization in human brain. A study using positron emission tomography and [fluorine 18]-fluorodeoxyglucose.Arch Gen Psychiatry47:567–574.
Lyons D, Friedman DP, Nader MA, Porrino LJ (1996): Cocaine alters cerebral metabolism within the ventral striatum and limbic cortex of monkeys.J Neurosci16:1230 –1238. Mathew RJ, Wilson WH, Lowe JV, Humphries D (1996): Acute
changes in cranial blood flow after cocaine hydrochloride.
Biol Psychiatry40:609 – 616.
Mendelson JH, Mello NK, Sholar MB, Siegel AJ, Kaufman MJ, Levin JM, et al (1999): Cocaine pharmacokinetics in men and in women during the follicular and luteal phases of the menstrual cycle.Neuropsychopharmacology21:294 –303. Moonen CT, von Kienlin M, van Zijl PC, Cohen J, Gillen J, Daly
P, Wolf G (1989): Comparison of single-shot localization methods (STEAM and PRESS) for in vivo proton NMR spectroscopy.NMR Biomed2:201–208.
Olson J, Sankar R, Holtzman D, James A, Fleischhacker D (1986): Energy-dependent volume regulation in primary cul-tured cerebral astrocytes.J Cell Physiol128:209 –215. Pearlson GD, Jeffery PJ, Harris GJ, Ross CA, Fischman MW,
Camargo EE (1993): Correlation of acute cocaine-induced changes in local cerebral blood flow with subjective effects.
Am J Psychiatry150:495– 497.
Petroff OAC, Ogino T, Alger JR (1988): High-resolution proton magnetic resonance spectroscopy of rabbit brain: Regional metabolite levels and postmortem change. J Neurochem
51:163–171.
Petroff OAC, Spencer DD, Alger JR, Prichard JW (1989): High-field proton magnetic resonance spectroscopy of human cerebrum during surgery for epilepsy.Neurology 39:1197– 1202.
Purdon AD, Rapoport SI (1998): Energy requirements for two aspects of phospholipid metabolism in mammalian brain.
Biochem J335:313–318.
MP, et al (1997): Radioligand binding and immunoautoradio-graphic evidence for a lack of toxicity to dopaminergic nerve terminals in human cocaine overdose victims. Brain Res
274:219 –229.
Taylor DL, Davies SEC, Obrenovitch TP, Doheny MH, Patsalos PN, Clark JB, Symon L (1995): Investigation into the role of N-acetylaspartate in cerebral osmoregulation. J Neurochem
65:275–281.
Tsai G, Coyle JT (1995): N-Acetylaspartate in neuropsychiatric disorders.Prog Neurobiol46:531–540.
Tzika AA, Ball WSL, Vigneron DB, Dunn RS, Kirks DR (1993): Clinical proton MR spectroscopy of neurodegenerative dis-ease in childhood.AJNR Am J Neuroradiol14:1267–1281. Urenjak J, Williams SR, Gadian DG, Noble M (1993): Proton
nuclear magnetic resonance spectroscopy unambiguously identifies different neural cell types.J Neurosci13:981–989.
van den Boogaart A, Ala-Korpela M, Jokisaari J, Griffiths JR (1994): Time and frequency domain analysis of NMR data compared: an application to 1D 1H spectra of lipoproteins.
Magn Reson Med31:347–358.
Wallace EA, Wisniewski G, Zubal G, van Dyck CH, Pfau SE, Smith EO, et al (1996): Acute cocaine effects on absolute cerebral blood flow.Psychopharmacology128:17–20. Webb PG, Sailasuta N, Kohler SJ, Raidy T, Moats RA, Hurd RE
(1994): Automated single-voxel proton MRS: Technical de-velopment and multisite verification. Magn Reson Med31: 365–373.
Winer BJ (1971):Statistical Principles in Experimental Design.
New York: McGraw-Hill.
Wolff SD, Balaban RS (1990): Regulation of the predominant renal medullary organic solutes in vivo. Annu Rev Physiol