Jurusan Teknologi Hasil Pertanian Fakultas Pertanian Universitas Lampung
elaborated under certain conditions by particular moulds that can grow on grains, seeds, and nuts (47). If present during processing, they can contaminate oils that are derived from these sources.
Regulations on maximum allowable levels for these contaminants have been legislated in various countries: heavy PAHs—5 mg/kg and for sum of heavy and light PAHs—25 mg/kg, German Society for Fat Science (45); dioxins—0.75 pg TEQWHO/g fat in the EU (50) where TEQWHOmeans Toxic Equivalence expressed as the sum individual toxicities for 17 toxic polychlorinated-p-dibenzo dioxins and furans as identified by the World Health Organization; aflatoxin B1—5mg/kg (51) in the EU. Regulatory limits for PAHs, PCBs, and dioxins have not yet been set in the United States; the Food and Drug Administration (FDA) has established an action level of 20 ppb for aflatoxins in human foods (52).
4.1. Effect of Lipid Constituents on Oil Quality; Determination of Oil Quality
Trace constituents in oils are removed (or destroyed) in varying degrees during refining, bleaching, and deodorization steps. Figure 7 summarizes the various steps in oil processing and the major constituents removed in each of them. The main objectives of oil processing are to enhance appearance, flavor, and oil stability and to ensure safety for human consumption.
Phospholipids, if not removed from oils before deodorization, can lead to dark-colored oils and serve as off-flavor precursors (53, 54). Chlorophyll (55), pheophytins and pyropheophytins (56), and metal ions (57, 58) are prooxidants that decrease oil stability. Iron and copper at levels as low as 0.01 and 0.1 ppm, respectively, are capable of lowering flavor and oxidative stability (59). Free fatty acids, besides representing a refining loss, have also been shown to act as prooxi-dants (60) and to lower smoke points (61) of oils during frying. Linolenic acid has
Figure 7. Major impurities removed by different processing steps.
TRACE CONSTITUENTS IN LIPID OILS AND FATS 299
O I L
P U R I F I C A T I O N
1
Jurusan Teknologi Hasil Pertanian ! Fakultas Pertanian Universitas Lampung
D E G U M M I N G
DEFINISI:
✓
Memisahkan/mengeluarkan gum (senyawa phosphate) dalam minyak kasar (crude oil).TUJUAN:
✓
Menmudahkan proses penyimpanan dan pengangkutan.✓
Memudahkan proses pemurnian berikutnya (caustic refining dan bleaching).✓
Menghasilkan lecithin.2
Jurusan Teknologi Hasil Pertanian ! Fakultas Pertanian Universitas Lampung
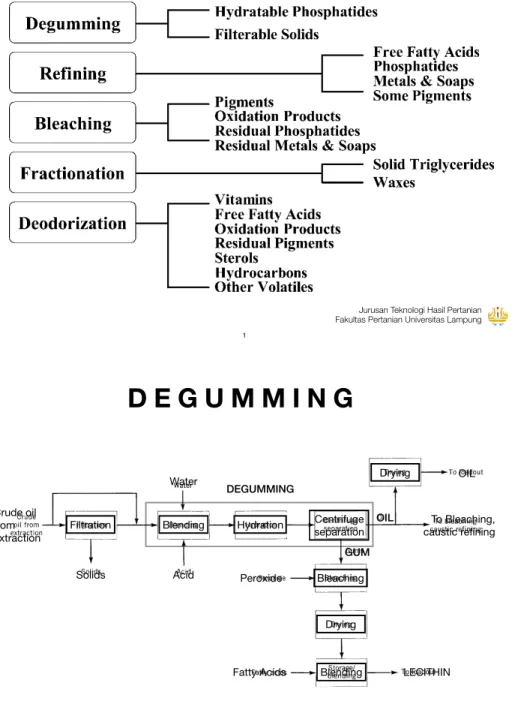
Figure 6. Degumming and lecithin processing.
17
DEGUMMING Crude oil
from extraction
Filtration Blending Hydration Centrifuge separation Bleaching Drying Blending Drying Solids Acid Water Peroxide OIL GUM Fatty Acids OIL LECITHIN To Bleaching, caustic refining
D E G U M M I N G
Jurusan Teknologi Hasil Pertanian ! Fakultas Pertanian Universitas Lampung
D E G U M M I N G
METODE DEGUMMING:1) Dry Degumming.
✓ Proses degumming dilakukan bersamaan dengan proses bleaching.
✓ Dilakukan pada crude oil yang kandungan gum-nya sangat rendah, seperti crude coconut oil dan crude
palm kernel oil.
2) Wet Degumming.
✓ Proses digumming pada minyak yang banyak mengandung gum (senyawa (hydratable phosphate).
✓ Gum yang dihasilkan dapat diproses menjadi lecithin.
✓ Prosesnya: Steam diinjeksikan ke dalam minyak, gum akan menyatu, dan dipisahkan dengan sentrifugasi.
Jurusan Teknologi Hasil Pertanian ! Fakultas Pertanian Universitas Lampung
D E G U M M I N G
METODE DEGUMMING:3) Acid Degumming.
✓ A c i d d e g u m m i n g d i l a k u k a n b e r s a m a a n menghilangkan senyawa phosphate (hydratable dan non hydrateable).
✓ Non-hydratable phosphate adalah jenis phospholipid yang mengandung ion Ca, Mg, dan Fe, dihasilkan dari ekstraksi biji-bijian yang telah mengalami kerusakan.
✓ Prosesnya: Asam encer (asam phosphate atau asam sitrat) ditambahkan ke dalam minyak, dilanjutkan dengan proses hidrasi (injeksi uap panas ke dalam minyak).
✓ Pemisahan gum dengan cara sentrifugasi.
✓ Hasil akhir minyak dengan kadar gum < 3 ppm. 5
Jurusan Teknologi Hasil Pertanian ! Fakultas Pertanian Universitas Lampung
D E G U M M I N G
6
D E G U M M I N G
Crude Soybean Oil Degummed Soybean Oil
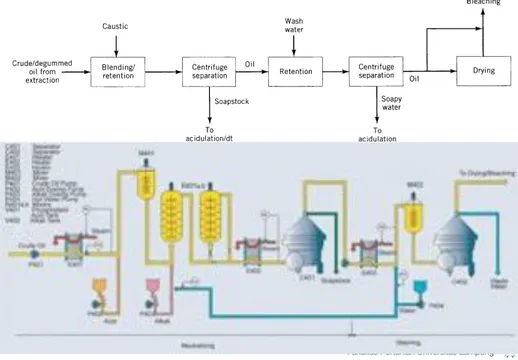
N E U T R A L I S I N G
➡ Bertujuan untuk menetralisasi atau menghilangkan
asam lemak bebas.
➡ Disebut juga: Caustic Refining atau Alkali Refining.
➡ Juga menghilangkan gum dan mineral kalsium dan
magnesium yang tersisa dari proses degumming.
➡ Proses netralisasi:
a. Larutan alkali (16-24 Be) dicampurkan ke dalam degummed oil, diaduk dan didiamkan selama 5 menit.
b. Minyak dan sabun dipisahkan dengan sentrifugasi.
c. Minyak masih mengandung sabun sekitar 500 ppm.
d. Minyak selanjutnya dicampur dengan air panas sebanyak 15-20%, untuk mencuci sabun.
e. Minyak dan air sabun di pisahkan dengan sentrifugasi.
Figure 7. Caustic refining.
22
Jurusan Teknologi Hasil Pertanian Fakultas Pertanian Universitas Lampung
DIAGRAM ALIR NETRALISASI
9
Jurusan Teknologi Hasil Pertanian Fakultas Pertanian Universitas Lampung
B L E A C H I N G
➡ Merupakan proses pembersihan minyak secara
absorpsi.
➡ Absorbent yang digunakan karbon aktif (activated
carbon) dan lempung aktif (activated clay).
➡ Bahan asing yang dipisahkan meliputi:
✓ Komponen warna (chlorophyll dan carotenoids),
✓ Gum (phospholipid), Sabun (soap stock),
✓ Logam (besi, Fe dan tembaga, Cu),
✓ Komponen hasil reaksi oksidasi.
➡ Pretreatment bleaching dengan ditambahkan asam
(sitrat dan fosfat) untuk mengikat logam, mencegah oksidasi.
10
Jurusan Teknologi Hasil Pertanian Fakultas Pertanian Universitas Lampung
DIAGRAM ALIR BLEACHING
Figure 8. Bleaching/dry degumming.
26
Jurusan Teknologi Hasil Pertanian Fakultas Pertanian Universitas Lampung
ABSORBENT FOR BLEACING
DIAGRAM ALIR BLEACHING
Jurusan Teknologi Hasil Pertanian Fakultas Pertanian Universitas Lampung 13
BLEACHING PROCESS
Jurusan Teknologi Hasil Pertanian Fakultas Pertanian Universitas Lampung 14
D E O D O R I S I N G
➡ Disebut juga sebagai physical refining.
➡ Ditujukan untuk mengeluarkan komponen volatile.
➡ Deodorisasi dapat digunakan sebagai pengganti
proses netralisasi (chemical refining).
➡ Komponen volatil yang dikeluarkan terdiri:
✓ Komponen aroma (flavor dan tastes dari bahan baku),
✓ Asam lemak bebas (physical refining).
➡ Selain menghilangkan komponen volatil, deodorisasi
juga memucatkan warna minyak, pigmen carotenoids rusak akobat pemanasan.
DIAGRAM ALIR DEODORIZATION
Figure 13. (b) Continuous deodorizer.
Jurusan Teknologi Hasil Pertanian Fakultas Pertanian Universitas Lampung
DIAGRAM ALIR DEODORIZATION
17
1. Deaeration:
Proses pengerluaran udara untuk mencegah oksidasi. Minyak dipanaskan pada suhu 80oC dalam kondisi vakum (50 mm Hg).
2. Stipping:
Minyak yang telah di deaerasi, dipanaskan dengan steam hingga suhu dan tekanan yang diinginkan untuk menguapkan komponen volatile impurities (FFA dan odor).
PROSES DEODORIZATION
phosphatide content after degumming (<15 ppm). If not, chemical refining will yield better results.
Another important factor is the free fatty acid content of the crude oil. In general, physical refining only becomes advantageous when the acidity of the crude oil is sufficiently high. For relatively cheap oils, like soybean oil, the higher oil yield with the physical refining is less important than the higher bleaching earth con-sumption, making chemical refining more attractive. For other unsaturated oils with a higher value, such as peanut oil and sunflower seed oil, physical refining will be more attractive.
2. DEODORIZATION PRINCIPLE
Although the process is commonly named deodorization, it is actually a combina-tion of three different effects on the oil: (1) stripping: Stripping of volatile compo-nents (free fatty acids, odorous compounds, tocopherols, sterols, and contaminants such as pesticides and light polycyclic aromatic hydrocarbons, etc.), (2) actual deodorization: Removal of different off-flavors, and (3) temperature effect: Thermal destruction of pigments and unwanted side reactions such as cis-trans-iso-merization, polycis-trans-iso-merization, conjugation, and so on.
Optimal stripping parameters (temperature, time, operating pressure, and amount of stripping gas) are governed by the properties of the ingoing product, the specifications of the outgoing product, equipment limitations, and the need to minimize costs. In Table 1, some typical deodorization conditions for edible oils are given. As observed, steam refining applied during physical refining requires more severe conditions than deodorization in case of chemical refining. This is mainly because of the removal of FFA by distillation, which is more significant in physical refining, as the initial FFA levels are considerably higher.
To obtain the required final FFA content of 0.03–0.05% by physical refining, it is necessary to adjust the operating conditions. The easiest way is to increase the
TABLE 1. Typical Operating Conditions for Deodorization of Vegetable Oils. Chemical
—————
———————————————— Physical Conditions U.S. Europe Europe Temperature (!C) 250–260 220–240 230–250
Pressure (mbar) 3–4 2–3 2 Sparge steam (%) 0.5–2(a)
0.5–1.5 1–2 Deodorization time (min) 20 –40 40–60 60–90 Final acidity (% FFA) """""""""""""""""""""" 0.03–0.05 """""""""""""""""""""""""! Trans fatty acids (%) """""""""""""""""""""" 0.5–1 """""""""""""""""""""""""! Tocopherol loss (%)(b) up to 60 max 25 max 25
aTo remove tocopherols, a higher amount of steam is required.
bFor example, for soybean oil in the United States, the minimum is 500 ppm; in Europe, it is 900 ppm.
DEODORIZATION PRINCIPLE 343
Jurusan Teknologi Hasil Pertanian Fakultas Pertanian Universitas Lampung 18
3. Retention:
• Menahan (membiarkan) minyak pada suhu dan tekanan deodorisasi (stripping) selama 10-30 menit, untuk menghilangkan pigmen (carotenoid).
• Disebut juga dengan Heat Bleaching (bleaching dengan panas).
4. Scrubbing (Condensation):
• Mengkondensasi dan merekoveri komponen volatil (FFA) menjadi Faty acid destilate.
5. Cooling:
• Mendinginkan minyak setelah proses deodorisasi.
• Ditambahkan asam sitrat untuk mengikat logam dan mencegah oksidasi.
PROSES DEODORIZATION
Jurusan Teknologi Hasil Pertanian
Fakultas Pertanian Universitas Lampung Fakultas Pertanian Universitas LampungJurusan Teknologi Hasil Pertanian
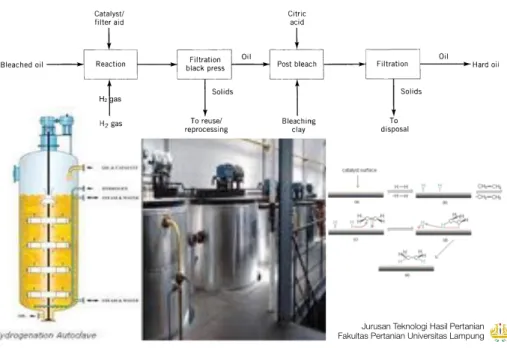
H Y D R O G E N A T I O N
Hidrogenasi adalah proses penambahan molekul hidrogen (H pada ikatan tak jenuh dengan bantuan katalisator.
➡ Tujuan Hidrogenasi:
1. Meningkatkan stabilitas rasa dan aroma, serta daya simpan minyak/lemak.
2. Mengubah sifat fungsional minyak/lemak untuk aplikasi tertentu.
➡ H2
atas 480 suhu rendah.
➡ Katalisator Hidrogrenasi: nickel, alumina, silica, platinum, palladium, rhodium, dan ruthenium.
Jurusan Teknologi Hasil Pertanian Fakultas Pertanian Universitas Lampung
DIAGRAM ALIR HIDROGENASI
Figure 10. Hydrogenation. 35 H2 gas ! ! 21
Jurusan Teknologi Hasil Pertanian Fakultas Pertanian Universitas Lampung
Efek Hidrogenasi Terhadap As. Lemak
As. Lemak KarbonRantai Titik Leleh ( OksidasiLaju Hidro-Laju genasi Linolenat C18:3 -13 150 40 Linoleat C18:2 -7 100 20 Oleat C18:1 cis 16 10 1 Stearat C18:0 70 1 -Oleat C18:1 trans 44 10 1 22
Jurusan Teknologi Hasil Pertanian Fakultas Pertanian Universitas Lampung
The manufacture of lightly hydrogenated, winterized soybean oil led to the
new terms ‘‘selective hydrogenation’’ and ‘‘selectivity catalyst.’’ ‘‘Selective
hydro-genation’’ technically defines the preferential conversion of 18:3
! 18:2 relative to
18:1
> 18:0. In practical terms, this process reflects the selective removal of double
bonds via hydrogen addition such that saturated fatty acid (stearic) formation is
minimized (7).
‘‘Catalyst selectivity’’ is somewhat meaningless unless the term is defined. There
also are selective catalysts that do not meet the technical or practical definition of
hydrogen selectivity. Such catalysts are sulfur-poisoned catalyst. Sulfided nickel
catalyst produces high trans-isomers, has lower activity than conventional nickel,
exhibits longer reaction times, and is used for specialty applications (e.g., coating
fats and hard butters).
Most unsaturated bonds in vegetable oils naturally occur in the cis-form. During
partial hydrogenation, part of the cis-isomers is changed to trans-isomers.
Trans-isomers have a dramatically higher melting point (42
"C) as compared with
cis-isomers (6
"C). The creation of trans-isomers is desirable in margarine oil in that
a higher melting point can be achieved without developing a higher level of
nutri-tionally undesirable saturated compounds. Altering hydrogenation conditions to
produce higher (or lower) trans-isomers is termed ‘‘trans-isomer selectivity.’’
Factors influencing cis-trans-isomerization are shown in Table 2.
A typical hydrogenation converter is shown in Figure 1. The converter is the
heart of the complete hydrogenation system. Proper design and maintenance of the
hydrogen gas distributor, the agitator, and the heating cooling coils are mandatory
for optimum productivity and consistency of basestocks produced. Most
conver-ters are 30,000-pound, 40,000-pound, or 60,000-pound batch sizes with some
now as large as 90,000 pounds. The common agitator design provides
approxi-mately 100 rpm, and radial flow impellers are used. The lower impeller is
posi-tioned slightly above the hydrogen gas distributor; therefore, the diameter of the
gas distributor and the tip-to-tip dimension of the lower impeller are critical.
Originally, the middle and top impellers were of the radial flow type also. Some
converters have now been operating for many years with an axial flow impeller
at the top position. Although the lower and middle radial flow impellers are ideally
suited for gas dispersion, the top impeller pumps the oil downward, and if
posi-TABLE 2. Factors Influencing cis-trans-isomerization. High trans Low trans
Temperature High Low
H2pressure Low High
Catalyst dosage Low High
Agitation Slow Fast
Catalyst Ni-S Ni
INTRODUCTION 387
Efek Hidrogenasi Terhadap Trans As.
Lemak
‣
Proses hidrogenasi, suhu 204oC, nickel katalisator 0,02%, tekanan H2 15 psia, menghasilkan asam lemak trans 44%.‣
Proses hidrogenasi, suhu 70oC, konsentrasi katalis 0,11%, dan tekanan H2 250 psia, menghasilkan as. lemak trans 22%.23
Jurusan Teknologi Hasil Pertanian Fakultas Pertanian Universitas Lampung
HIDROGENASI
Faktor mempengaruhi laju hidrogenasi:
a. Suhu awal minyak,
b. Suhu reaksi,
c. Aktivitas katalisator,
d. Konsentrasi katalisator,
e. Laju serapan hidrogen,
f. Kemurnian hidrogen,
g. Kualitas minyak,
h. Intensitas pengadukan.
Jurusan Teknologi Hasil Pertanian Fakultas Pertanian Universitas Lampung
INTERESTERIFICATION
‣
Didefinisikan sebagai:
Penataan ulang susunan asam lemak, baik secara random atau terarah, dalam trigliserida dengan tujuan untuk memodifikasi sifat minyak atau lemak tanpa mengubah komposisi asam lemaknya.
‣
Reaksi transesterifikasi dapat terjadi antara minyak/lemak dengan alkohol, asam, atau minyak/lemak.1. Alcoholysis: pertukaran dengan mono atau polihidrat
alkohol.
2. Acidolysis: pertukaran dengan asam karboksilat.
3. Methanolysis: pertukaran dengan methanol.
4. Interesterification: Pertukaran dengan trigliserida lainnya.
25
Jurusan Teknologi Hasil Pertanian Fakultas Pertanian Universitas Lampung
INTERESTERIFICATION
236 Modifying lipids for use in food
reaction can be completed within 30 minutes. The reaction isstopped by the introduction of water, and subsequent processing usually includes washing, bleaching and deodorization to obtain the final randomized product.
Figure 11.1 illustrates the difference between chemical and specific lipase-catalyzed interesterification in two triacylglycerols. For the chemical randomization of ACA and BBB governed by thermodynamics and statistics, theoretically all 18 isomers (27 if stereoisomers are included) might be detected in the end reaction mixture. For comparison, with 1,3-specific lipase-catalyzed reaction governed mainly by enzyme specificity and dynamics, only four new isomers (six if stereoisomers are included) are expected, apart from the original ACA and BBB. The formation of isomers with acyl group C at the 1- or 3-positions and A at the 2-position of the glycerol backbone is theoretically impossible if acyl migration can be ruled out. The randomization of triacylglycerols can also be driven by non-specific lipases, yielding similar results to chemical interesterification. In practice, only a very few lipases are strictly non-specific. Most lipases are specific to a certain extent (Matori et al., 1991). That is to say, the result of the reaction catalyzed even by a non-specific lipase will not be exactly the same as that with a chemical catalyst.
The widely used chemical catalysts are Na-K alloy or sodium alcoholate (e.g. CH3ONa). However, they are believed not to be the real catalysts due to their ease of consumption during reaction. Glycerate is the real catalyst of interesterification. This mechanism explains the formation of both monoalcohol ester and diacylglycerol, the latter being formed by glycerate hydrolysis (Ucciani and Debal, 1996). Dijkstra et al. (2005) proposed a new mechanism in order to explain the experimental observations.This mechanism assumes that the reaction of a base (such as sodium methanolate) with the oil will eventually lead to the abstraction of an α-hydrogen from a fatty acid moiety and that the enolate anion thus formed acts as the catalytic intermediate. This enolate can re-abstract a proton from the hydroxyl group of a partial glyceride, whereupon the resulting alcoholate attacks the carbonyl group. This leads to a new ester and a glycerolate anion which then regenerates a new enolate anion.
1,3-specific lipase chemical catalyst or nonspecific lipase A C + A B B B A C + A B B + B A C + B A B + A B B + A B C B C C A C C B C A C C B C B B C B B A* B A B B C B* A A B A A C A B A* A C A* A B C A C B* B A C A A A B B B* C C C + + + +
Fig. 11.1 Schematic illustration of the difference between randomization and
1,3-specific interesterification, exemplified by the reaction between ACA- and BBB-type triacylglycerols. Underlined molecules are starting materials and those with star are
possible products for 1,3-specific lipase-catalyzed reaction.
236 Modifying lipids for use in food
reaction can be completed within 30 minutes. The reaction isstopped by the introduction of water, and subsequent processing usually includes washing, bleaching and deodorization to obtain the final randomized product.
Figure 11.1 illustrates the difference between chemical and specific lipase-catalyzed interesterification in two triacylglycerols. For the chemical randomization of ACA and BBB governed by thermodynamics and statistics, theoretically all 18 isomers (27 if stereoisomers are included) might be detected in the end reaction mixture. For comparison, with 1,3-specific lipase-catalyzed reaction governed mainly by enzyme specificity and dynamics, only four new isomers (six if stereoisomers are included) are expected, apart from the original ACA and BBB. The formation of isomers with acyl group C at the 1- or 3-positions and A at the 2-position of the glycerol backbone is theoretically impossible if acyl migration can be ruled out. The randomization of triacylglycerols can also be driven by non-specific lipases, yielding similar results to chemical interesterification. In practice, only a very few lipases are strictly non-specific. Most lipases are specific to a certain extent (Matori et al., 1991). That is to say, the result of the reaction catalyzed even by a non-specific lipase will not be exactly the same as that with a chemical catalyst.
The widely used chemical catalysts are Na-K alloy or sodium alcoholate (e.g. CH3ONa). However, they are believed not to be the real catalysts due to their ease of consumption during reaction. Glycerate is the real catalyst of interesterification. This mechanism explains the formation of both monoalcohol ester and diacylglycerol, the latter being formed by glycerate hydrolysis (Ucciani and Debal, 1996). Dijkstra et al. (2005) proposed a new mechanism in order to explain the experimental observations.This mechanism assumes that the reaction of a base (such as sodium methanolate) with the oil will eventually lead to the abstraction of an α-hydrogen from a fatty acid moiety and that the enolate anion thus formed acts as the catalytic intermediate. This enolate can re-abstract a proton from the hydroxyl group of a partial glyceride, whereupon the resulting alcoholate attacks the carbonyl group. This leads to a new ester and a glycerolate anion which then regenerates a new enolate anion.
1,3-specific lipase chemical catalyst or nonspecific lipase A C + A B B B A C + A B B + B A C + B A B + A B B + A B C B C C A C C B C A C C B C B B C B B A* B A B B C B* A A B A A C A B A* A C A* A B C A C B* B A C A A A B B B* C C C + + + +
Fig. 11.1 Schematic illustration of the difference between randomization and
1,3-specific interesterification, exemplified by the reaction between ACA- and BBB-type triacylglycerols. Underlined molecules are starting materials and those with star are
possible products for 1,3-specific lipase-catalyzed reaction.
236 Modifying lipids for use in food
reaction can be completed within 30 minutes. The reaction isstopped by the introduction of water, and subsequent processing usually includes washing, bleaching and deodorization to obtain the final randomized product.
Figure 11.1 illustrates the difference between chemical and specific lipase-catalyzed interesterification in two triacylglycerols. For the chemical randomization of ACA and BBB governed by thermodynamics and statistics, theoretically all 18 isomers (27 if stereoisomers are included) might be detected in the end reaction mixture. For comparison, with 1,3-specific lipase-catalyzed reaction governed mainly by enzyme specificity and dynamics, only four new isomers (six if stereoisomers are included) are expected, apart from the original ACA and BBB. The formation of isomers with acyl group C at the 1- or 3-positions and A at the 2-position of the glycerol backbone is theoretically impossible if acyl migration can be ruled out. The randomization of triacylglycerols can also be driven by non-specific lipases, yielding similar results to chemical interesterification. In practice, only a very few lipases are strictly non-specific. Most lipases are specific to a certain extent (Matori et al., 1991). That is to say, the result of the reaction catalyzed even by a non-specific lipase will not be exactly the same as that with a chemical catalyst.
The widely used chemical catalysts are Na-K alloy or sodium alcoholate (e.g. CH3ONa). However, they are believed not to be the real catalysts due to
their ease of consumption during reaction. Glycerate is the real catalyst of interesterification. This mechanism explains the formation of both monoalcohol ester and diacylglycerol, the latter being formed by glycerate hydrolysis (Ucciani and Debal, 1996). Dijkstra et al. (2005) proposed a new mechanism in order to explain the experimental observations.This mechanism assumes that the reaction of a base (such as sodium methanolate) with the oil will eventually lead to the abstraction of an α-hydrogen from a fatty acid moiety and that the enolate anion thus formed acts as the catalytic intermediate. This enolate can re-abstract a proton from the hydroxyl group of a partial glyceride, whereupon the resulting alcoholate attacks the carbonyl group. This leads to a new ester and a glycerolate anion which then regenerates a new enolate anion.
1,3-specific lipase chemical catalyst or nonspecific lipase A C + A B B B A C + A B B + B A C + B A B + A B B + A B C B C C A C C B C A C C B C B B C B B A* B A B B C B* A A B A A C A B A* A C A* A B C A C B* B A C A A A B B B* C C C + + + +
Fig. 11.1 Schematic illustration of the difference between randomization and
1,3-specific interesterification, exemplified by the reaction between ACA- and BBB-type triacylglycerols. Underlined molecules are starting materials and those with star are
possible products for 1,3-specific lipase-catalyzed reaction. Tujuan:
Merubah titik leleh, memperlambat oksidasi, menghasilkan minyak/lemak yang cocok untuk deep frying dan pembuatan margarin.
Metode:
➡ Interesterifikasi Kimia: Menggunakan katalis sodium
metoksi dan logam alkali.
➡ Interesterifikasi enzim: Menggunakan enzim lipase. 26
Jurusan Teknologi Hasil Pertanian Fakultas Pertanian Universitas Lampung 246 Modifying lipids for use in food
11.3.2 Enzymatic acidolysis for the production of structured lipids
Structured lipids (SL), in this text, means fats that are modified or restructured from natural oils and fats, or the fatty acids therefrom, with regio-positional preference of each fatty acid or each group of fatty acids in the glycerol backbone. Structured lipids are designed for a particular functionality or nutritional property for edible or pharmaceutical purposes. This definition
Fig. 11.4 Morphology of the blend (palm stearin/coconut oil, 70/30) and
enzymatically interesterified products at different degrees of conversion (measured by HPLC). (Samples with 50 % added rapeseed oil were melted at 70 °C, and one drop was put on a slide. They were then cooled to room temperature. The morphology was
observed after one hour at room temperature by polar light microscopy) (adapted from Zhang et al., 2004b).
58 % conversion 31 % conversion 71 % conversion conversion100 % BlendINTERESTERIFICATION
246 Modifying lipids for use in food
11.3.2 Enzymatic acidolysis for the production of structured lipids
Structured lipids (SL), in this text, means fats that are modified or restructured from natural oils and fats, or the fatty acids therefrom, with regio-positional preference of each fatty acid or each group of fatty acids in the glycerol backbone. Structured lipids are designed for a particular functionality or nutritional property for edible or pharmaceutical purposes. This definition
Fig. 11.4 Morphology of the blend (palm stearin/coconut oil, 70/30) and
enzymatically interesterified products at different degrees of conversion (measured by HPLC). (Samples with 50 % added rapeseed oil were melted at 70 °C, and one drop was put on a slide. They were then cooled to room temperature. The morphology was
observed after one hour at room temperature by polar light microscopy) (adapted from Zhang et al., 2004b).
58 % conversion 31 % conversion 71 % conversion 100 % conversion Blend
246 Modifying lipids for use in food
11.3.2 Enzymatic acidolysis for the production of structured lipids
Structured lipids (SL), in this text, means fats that are modified or restructured from natural oils and fats, or the fatty acids therefrom, with regio-positional preference of each fatty acid or each group of fatty acids in the glycerol backbone. Structured lipids are designed for a particular functionality or nutritional property for edible or pharmaceutical purposes. This definition
Fig. 11.4 Morphology of the blend (palm stearin/coconut oil, 70/30) and
enzymatically interesterified products at different degrees of conversion (measured by HPLC). (Samples with 50 % added rapeseed oil were melted at 70 °C, and one drop was put on a slide. They were then cooled to room temperature. The morphology was
observed after one hour at room temperature by polar light microscopy) (adapted from Zhang et al., 2004b).
58 % conversion 31 % conversion 71 % conversion 100 % conversion Blend
246 Modifying lipids for use in food
11.3.2 Enzymatic acidolysis for the production of structured lipids
Structured lipids (SL), in this text, means fats that are modified or restructured from natural oils and fats, or the fatty acids therefrom, with regio-positional preference of each fatty acid or each group of fatty acids in the glycerol backbone. Structured lipids are designed for a particular functionality or nutritional property for edible or pharmaceutical purposes. This definition
Fig. 11.4 Morphology of the blend (palm stearin/coconut oil, 70/30) and
enzymatically interesterified products at different degrees of conversion (measured by HPLC). (Samples with 50 % added rapeseed oil were melted at 70 °C, and one drop was put on a slide. They were then cooled to room temperature. The morphology was
observed after one hour at room temperature by polar light microscopy) (adapted from Zhang et al., 2004b).
58 % conversion 31 % conversion 71 % conversion 100 % conversion Blend
246 Modifying lipids for use in food
11.3.2 Enzymatic acidolysis for the production of structured lipids
Structured lipids (SL), in this text, means fats that are modified or restructured from natural oils and fats, or the fatty acids therefrom, with regio-positional preference of each fatty acid or each group of fatty acids in the glycerol backbone. Structured lipids are designed for a particular functionality or nutritional property for edible or pharmaceutical purposes. This definition
Fig. 11.4 Morphology of the blend (palm stearin/coconut oil, 70/30) and
enzymatically interesterified products at different degrees of conversion (measured by HPLC). (Samples with 50 % added rapeseed oil were melted at 70 °C, and one drop was put on a slide. They were then cooled to room temperature. The morphology was
observed after one hour at room temperature by polar light microscopy) (adapted from Zhang et al., 2004b).
58 % conversion 31 % conversion 71 % conversion conversion100 % Blend
Palm stearin/coconut oil (70/30) 31% conversion
58% conversion 71% conversion 100% conversion
27
Jurusan Teknologi Hasil Pertanian Fakultas Pertanian Universitas Lampung
of fats). This process has great potential as the ramifications of natural versus mod-ified fats continue to be debated.
Figure 11 depicts a typical interesterification process. This random inter-esterification uses a reactor quite similar in design (if not identical) to the hydro-genation dead-end reactor. Oil is heated after being neutralized and dried to around
90–120!C and is blended in the reactor with a catalyst, such as sodium methoxide
(0.2–0.3%) or an alkali metal (0.1–0.2%). During reaction, the reactants become orange-brown in color (the first quality check of reaction process). Once the color develops, the reaction is normally completed within 30 min, where an equilibrium exists of the random distribution of fatty acids on the glycerol molecule. When completed, water is introduced to stop the reaction, vacuum is released, and the oil discharged to a holding tank. As a soap residue is normally present in the oil, the mixture may be washed and centrifuged from the oil. The oil is dried and bleached and deodorized in the normal manner.
Continuous interesterification processes exist but to date none have been com-mercialized. Interesterification is generally performed in small batches by specialty processors. An alternate method of increasing interest is directed interesterification using enzymes. The process is generally applied to palm-oil-based materials such as cocoa butter substitutes and to coconut oils. There is some concern about the effectiveness of interesterification with physical refined oils as low levels of FFA must be present or the reaction will not proceed as planned (5). Although not a hazardous process, interesterification is often included in the hydrogenation section of the refinery because of the similarity of the reactors.
As the debate concerning health effects of saturated products and that of trans isomers generated during hydrogenation continues, interesterification may offer a viable alternative to the refiner. Outside the United States interesterification is used to produce hardened fats without trans isomers. These products are available in Canada and continental Europe. This technology has been available for quite some time, as a patent on the product was granted to Unilever in 1961 (22). The ability
Figure 11. Interesterification.
38 A PRIMER ON OILS PROCESSING TECHNOLOGY
Proses:
Minyak (degummed, refined), dipanaskan (90-120oC) dalam vakum, ditambah katalisator (sodium metoksi, 0,2-0,3%: metal alkali, 0,1-0,2%), reaksi dimulai saat timbul warna kuning-kecoklatan. Reaksi (30 menit) dihentikan dengan penambahan air dan pendinginan.