Summary We assessed the impacts of low pH and 2.0 mM aluminum (Al) on the growth of sugar maple (Acer saccharum Marsh.) seedlings over a 13-week period. At Week 9, total leaf area of Al-treated seedlings was reduced by 27%; however, by Week 13, leaf area was similar for seedlings in all treatments. None of the other growth parameters examined were negatively affected by the treatments at either Week 9 or Week 13. The ABA concentration in the xylem sap, which is an indicator of tree stress in the field, was not affected by any of the treatments and was highest during periods of high evaporative demand in June and August. We conclude that the duration of exposure to Al is critical when assessing a threshold concentration for Al toxicity because plants can acclimate to an Al concentration previously considered toxic. Although Al stress did not appear to reduce the vigor of sugar maple seedlings directly, it could facilitate an inciting factor such as winter frost to induce tree decline.
Keywords: abscisic acid, Acer saccharum, acidic deposition, soil acidity.
Introduction
Sugar maple stands (Acer saccharum Marsh.) have shown symptoms of decline in eastern North America since the early 1980s (Lachance 1985), but the mechanisms underlying this decline have not been precisely defined. Acidic deposition is considered a contributing factor that weakens the trees (Foster 1989), and extreme climatic events are thought to be inciting factors that trigger tree decline (Robitaille et al. 1995). Others have postulated that the detrimental effects of acidic deposi-tion on trees are caused by mobilizadeposi-tion of aluminum (Al) in soil solution (Ulrich et al. 1980).
Aluminum can affect plant growth by causing an imbalance in plant mineral nutrition (Kelly et al. 1990). High Al concen-trations in the growth medium usually reduce concenconcen-trations of magnesium, calcium and often phosphorus in tree foliage below those required for normal metabolism. Aluminum is strongly adsorbed on cell walls of the root cortex and blocks the normal uptake of calcium and magnesium required in the aerial parts of the tree (Stienen and Bauch 1988). Aluminum can also lower phosphorus availability to the shoot by enhanc-ing its precipitation in the soil or on the plant root (Cummenhanc-ing et al. 1985). In greenhouse studies, a high Al concentration in
the nutrient solution has detrimental effects on the growth and mineral nutrition of many tree species (Thornton et al. 1986a, 1989, Kruger and Sucoff 1989).
Hormonal imbalances may also be involved in the Al effects on sugar maple seedling growth. According to Chapin (1990), the growth response to an inadequate nutrient supply cannot be explained by simple changes in the nutrient status of the plant but probably involves complex changes in hormonal balance, particularly in abscisic acid (ABA). Leaf ABA concentration increases in response to various mineral deficiencies (Gold-bach et al. 1975, Haeder and Beringer 1981, Radin 1984). High ABA concentrations are also a typical response to ion toxicity, but the increased ABA concentrations are generally explained by an indirect salinity effect induced by a water deficit (Marschner 1986). Few studies report a direct effect of high ion concentration, particularly Al, on the concentrations of growth regulators (Edwards et al. 1976).
We tested the hypothesis that low pH and high Al concentra-tion lower the vigor of sugar maple seedlings and are contrib-uting factors to sugar maple stand decline. The effects of these stresses on tissue mineral concentrations and various growth parameters were measured in roots and shoots over a 13-week period. We also measured the concentration of ABA in the xylem sap in response to Al over time to determine whether it could be used as an indicator of Al stress in sugar maple seedlings.
Materials and methods
Plant material and stress treatments
On April 20, 1993, 2-year-old sugar maple seedlings (50--90 cm in height), produced from seeds of a Saint-Georges-de-Beauce provenance (Québec, 46°10′ N, 70°37′ W), were planted in 16-liter pots containing washed silica (silica 16, Indusmin, Saint-Canut, Québec) and acclimated in a green-house at the Laurentian Forestry Centre, Sainte-Foy, Québec, for 7 weeks (day/night temperature of 23/20 °C and a 16-h photoperiod). The seedlings were fertilized weekly with half-strength Hoagland solution (Hoagland and Arnon 1938). At the beginning of June 1993, a shade cloth was installed in the greenhouse 2 m above the trees. The five treatments (n = 9 per treatment) were started on June 8, 1993, and consisted of irrigations with: (1) complete nutrient solution at pH 5.0
(con-Growth and ABA responses of maple seedlings to aluminum
ANNICK BERTRAND,
1GILLES ROBITAILLE,
1ROBERT BOUTIN
1and PAUL NADEAU
21 Natural Resources Canada, Canadian Forest Service-Québec Region, P.O. Box 3800, Sainte-Foy, Québec G1V 4C7, Canada 2
Agriculture and Agri-Food Canada Research Station, 2560 Hochelaga Blvd., Sainte-Foy, Québec G1V 2J3, Canada
Received November 17, 1994
trol), (2) complete nutrient solution at pH 4.0 (pH 4.0), (3) complete nutrient solution at pH 4.0 with the addition of 2.0 mM Al (pH 4.0 + Al), (4) complete nutrient solution at pH 3.5 (pH 3.5), and (5) complete nutrient solution at pH 3.5 with the addition of 2.0 mM Al (pH 3.5 + Al). The composition of the nutrient solution was similar to that described by Thornton et al. (1986a): 0.25 mM Ca(NO3)2, 0.5 mM KNO3, 0.5 mM
MgSO4, 0.5 mM NH4NO3, 0.04 mM NH4H2PO4, 10 µM Fe
EDTA, 0.8 µM Zn, 9.0 µM Mn, 0.3 µM Cu, 46 µM B, and 0.05 µM Mo. Aluminum was added as AlCl3 for Treatments 3 and
5. The Cl− concentrations of all treatments were equalized with NaCl. The pH of the solutions was adjusted with HCl. Irriga-tions with 0.7 l of each solution took place twice weekly during the 13-week experiment from June 8 (Day 0) to September 8 (Day 91), 1993. Once a week, all pots were also irrigated by a spray system with demineralized water. Four destructive har-vests were made: June 8 (Day 0), July 7--8 (Week 4), August 10--11 (Week 9) and September 7--8 (Week 13).
Growth parameters
At each harvest, the total weight and height (root collar to terminal bud) of each seedling were measured. We also deter-mined the weight of stems, leaves, and large (diameter > 1 mm) and fine (diameter < 1 mm) roots. The roots were thoroughly washed with demineralized water to remove adhering parti-cles. After blotting, the total volume of the root system was measured by a water displacement technique described by Burdett (1979). After fine root removal, the volume of roots larger than 1 mm was measured, as previously described, and the volume of fine roots (diameter < 1 mm) was calculated by the difference between the total root volume and the large root volume. The total leaf area of each seedling was measured with an image analyzer (AGVision, Pullman, WA). Each type of tissue was then dried for 72 h at 70 °C. The root/shoot ratio was calculated on the basis of tissue dry weight. The root relative growth rate (RGR) was calculated on the basis of root volume and expressed as a percentage: ((final root volume − initial root volume)/initial root volume) × 100.
Xylem sap extraction and ABA quantification
The terminal twig (approximately 12 cm long), including five to ten leaves (or the lateral twig when the terminal twig was missing), was removed and immediately placed in a pressure chamber (PMS Instruments Co., Corvallis, OR) with the bot-tom covered with a damp paper towel. A gradual pressure was exerted on the twig, and the pressure was then maintained between 2.0 and 3.0 MPa for approximately 5 min for the collection of 100 µl of xylem sap. The vials containing the sap were immediately put on ice and stored at −80 °C within 2 h of collection.
After a 30-min incubation with polyvinylpolypyrrolidone (0.1 mg per 100 µl), xylem sap was assayed for free ABA content by radioimmunoassay (RIA) as described by Bertrand et al. (1994). Antibodies specific to free (+) cis-trans ABA were prepared according to Weiler (1980). The antibody used showed a linear response from 0.1 to 10.0 pmol under RIA
conditions. The antiserum was highly specific with a cross-re-activity of 53% for (±) cis-trans ABA, 32% for (±) racemic ABA and 7% for methylated (±) ABA. The affinity constant for the antiserum was 2.47 × 10−9 l mol−1. All standards and samples were assayed in triplicate. Samples were diluted 1/10 in phosphate buffered saline solution (Weiler 1980).
Extraction and analysis of the silica solution
At each harvest date, 1 day after a fertilization treatment, 600 g of silica was centrifuged at 1000 g for 5 min to extract the silica solution. The samples were then placed in a cold room (2 °C) until they were prepared for chemical analysis. The samples were filtered at 0.45 µm, and the conductivity and pH ured. Total monomeric Al (with chrome azurol S) was meas-ured by colorimetry (Flow Injection Analysis, Tecator 5020, Sweden) with a detection limit of 2.0 µmol l−1; total Ca and Mg were measured by atomic absorption spectrophotometry.
Analysis of the mineral composition of plant tissues
The dried plant material was ground to pass through a 2-mm mesh. Subsamples of 0.5 g of leaf and root material from four replicates per treatment were analyzed according to the dry ashing method (NCASI 1983). Total P content of samples was determined by colorimetry (Colorimeter PC 800, Brinkman Instruments, Westbury, NY). Total tissue content of Al, Ca, Mg and Mn was analyzed by atomic absorption spectrophotometry with nitrous oxide for Al, and flame emission spectro-photometry for K and Na. The detection limits for the ions (in mg l−1) were: Ca, 0.1; Mn, 0.06; K, 0.05; Mg, 0.01; and Al, 1.1. The accuracy of tissue analysis was confirmed by comparison with the Quality Assurance Subgroup of the Federal-Provin-cial Research and Monitoring Coordinating Committee refer-ence material. For each ion under study, the results from our laboratory were less than ± 6% of the median results from 18 laboratories.
Statistical analysis
Results
Silica solution composition
Before the treatments began, the pH of the solution extracted from the silica growth medium was 6.9. At Week 4, the pH values of the silica solutions were less for all treatments than at Day 0, and the reductions were significantly greater in the Al treatments than in the other treatments (P ≤ 0.05) (Fig-ure 1A). The acidity of the silica solutions did not differ sig-nificantly between the two Al treatments (Treatments 3 and 5) or between the two pH treatments (Treatments 2 and 4) (Fig-ure 1A). The pH of the silica solutions for treatments without Al (Treatments 1, 2 and 4) decreased sharply to 4.5--5.2 be-tween Weeks 9 and 13. The conductivities of the silica
solu-tions were similar for all treatments (Figure 1B). The low conductivity of the silica solution on Day 0 corresponded to the nutrient solution used before the treatments began, i.e., before the addition of AlCl3 or NaCl. The highest conductivity
(Figure 1B) was reached at Week 9 and corresponded with a period of high evaporative demand in the greenhouse when soil water content was at a minimum (Figure 1C).
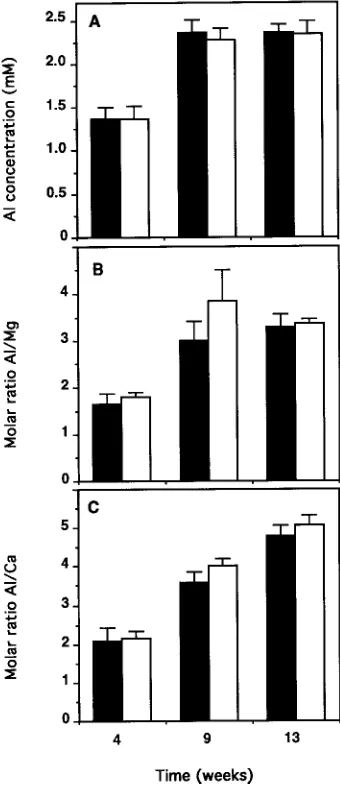
In the pH 4.0 + Al and pH 3.5 + Al treatments, the Al concentration of the silica solutions increased from 1.25 to 2.30 mM between Weeks 4 and 9, and then stabilized (Fig-ure 2A). The Al/Mg ratio followed the same trend and stabi-lized at approximately 3.4 (Figure 2B). The Al/Ca molar ratio doubled from 2 to 4 between Weeks 4 and 9, and then increased more gradually to reach about 5 by Week 13 (Figure 2C). There were no significant differences between the two Al treatments for these variables. Throughout the study, the Mg2+ and Ca2+ concentrations in the silica solution remained higher in the Al treatments than in the other treatments (Figures 3A and 3B).
Figure 1. (A) pH, (B) conductivity of the silica solution, and (C) soil water content (SWC) at Weeks 4, 9 and 13 (n = 9). Symbols: d,
control; n, pH 4.0; h, pH 4.0 + Al; m, pH 3.5; j, pH 3.5 + Al. Each curve represents the mean for nonsignificantly different treatments.
Seedling growth and ABA concentration
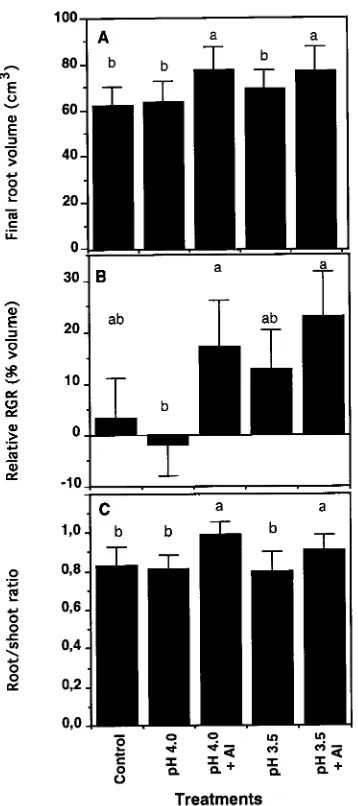
The 2.0 mM Al treatment stimulated root growth. By Week 13, the root volume of Al-treated seedlings was approximately 75 cm3 compared with a mean of ≈65 cm3 for seedlings in the
other treatments (P ≤ 0.05) (Figure 4A). In response to the presence of Al, the relative root growth rate ranged from 17 to 22% compared with 3% in the control seedlings (Figure 4B). The Al treatment significantly (P ≤ 0.05) enhanced the root/shoot ratio compared with the other treatments (mean value = 0.95 versus 0.81) (Figure 4C).
Leaf area was the only parameter negatively affected by the Al treatments (P ≤ 0.05). At Week 9, leaf area had decreased by about 27% in Al-treated seedlings compared with seedlings in the other treatments (Figure 5A); however, by Week 13, the total leaf area of seedlings in all treatments was about 1000 cm2. Between Weeks 4 and 13, the fine root volume/large root volume ratio decreased progressively and similarly for seed-lings in all treatments (Figure 5B).
The ABA concentration in the xylem sap was highest (403 pmol ml−1) at the beginning of the experiment when the soil water content was low. It decreased sharply to reach 144 pmol ml−1 at Week 4, and then increased to 210 pmol ml−1 between Weeks 4 and 9 as the soil dried (cf. Figures 1C and 5C). None of the treatments had a significant effect on xylem sap ABA concentration.
Tissue mineral concentration
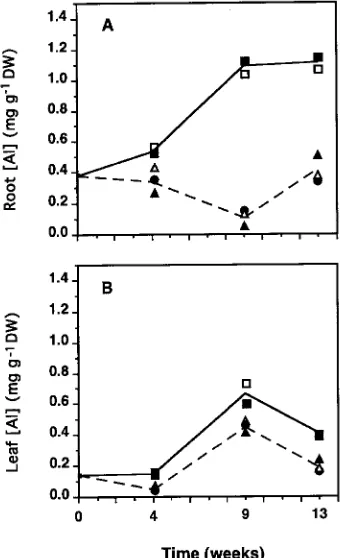
The mean Al concentration of roots of Al-treated seedlings increased from Week 4 to Week 13 reaching a mean of 1.1 mg gDW−1, which was three times the concentration found in roots
of control and pH-treated seedlings (Figure 6A). In leaves, a maximum Al concentration of ≈0.6 mg gDW−1 was found in
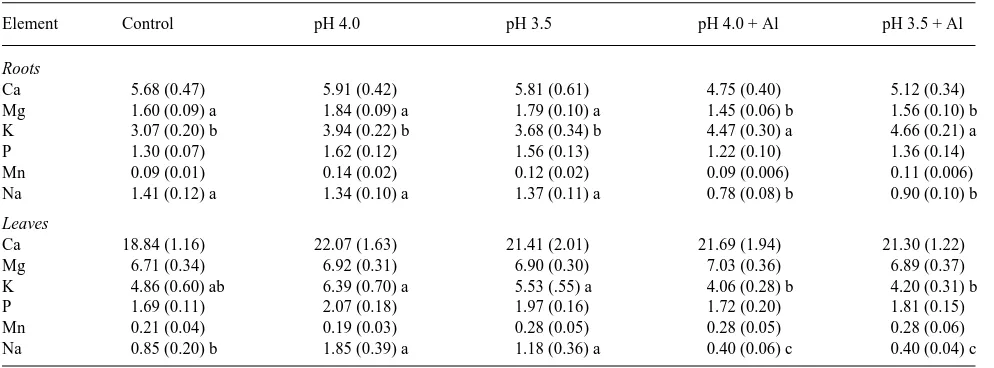
Al-treated seedlings at Week 9 (Figure 6B). Concentrations of Ca, P and Mn in roots and leaves were not affected by any of the treatments (Table 1). The Mg concentration was signifi-cantly lower in roots of Al-treated seedlings (P ≤ 0.05) than in roots of seedlings in the other treatments, whereas leaf Mg concentration was not affected by any of the treatments (6.7--7.0 mg gDW−1) (Table 1). Root K concentration was slightly
higher (P ≤ 0.05) in Al-treated seedlings than in seedlings in the other treatments (≈4.5 versus ≈3.5 mg gDW−1), whereas
foliar K concentration was lowest in Al-treated seedlings (≈4.0
Figure 3. (A) Mg, and (B) Ca concentrations in the silica solution at Weeks 4, 9 and 13 (n = 9). Symbols: d, control; n, pH 4.0; h, pH 4.0
+ Al; m, pH 3.5; j, pH 3.5 + Al. Each curve represents the mean for
nonsignificantly different treatments.
mg gDW−1) (Table 1). Root Na concentration in Al-treated
seedlings (≈0.7 mg gDW−1) was approximately half the
concen-tration found in the roots of seedlings in the other treatments. Foliar Na concentrations were lowest in Al-treated seedlings (0.4 mg gDW−1) and highest in pH-treated seedlings (1.6 mg
gDW−1) (Table 1).
Discussion
The negative effects of Al were more pronounced in the aerial part (shoots and leaves) than in the roots of sugar maple seedlings. The finding that the aerial part was more sensitive to Al than the roots may be a common feature of deciduous tree species (Thornton et al. 1986a, Kelly et al. 1990).
In our study, the Al concentration of the roots was much lower than the concentration reported by Thornton et al. (1986a) in response to a similar Al treatment (2.0 mM) and probably accounts for the absence of toxic Al effects on the root system. Reductions in root growth rate, root branching, or both that are generally associated with Al stress are usually explained by a decrease in Ca2+ or Mg2+ uptake by the root system in the presence of Al3+, resulting in a nutritional defi-ciency of these elements (Edwards et al. 1976, Thornton et al. 1987). The concentrations of Ca2+ and Mg2+ measured in the silica solution of Al-treated seedlings were higher than in the silica solution of control seedlings, which indicates that Al effectively blocked the entry of these ions into the root. We conclude that, under our experimental conditions, the blocking effect did not cause Ca or Mg deficiencies, because these elements were supplied in sufficient amounts in the nutrient medium (cf. Kelly et al. 1990). The absence of an Al effect on Ca concentration in leaves and roots differs from the observa-tions made on honeylocust, peaches and coniferous trees in which the tissue concentrations of Ca were depleted in the presence of Al (Edwards et al. 1976, Sucoff et al. 1990, Raynal et al. 1990). Kelly et al. (1990) concluded that the molar ratio of Al/Ca of the nutrient solution determines the interaction between the two elements. The Al/Ca ratio of our silica solu-tion was about 2 at Week 4 and increased to about 5 at Week 13. However, Kelly et al. (1990) observed that a wide range of Al/Ca ratios was ineffective in modifying the growth of red oak Figure 5. (A) Total leaf area per seedling, (B) fine/large root ratio, and
(C) xylem sap ABA concentration at Weeks 4, 9 and 13 (n = 9). Symbols: d, control; n, pH 4.0; h, pH 4.0 + Al; m, pH 3.5; j, pH
3.5 + Al. Each curve represents the mean for nonsignificantly different treatments.
Figure 6. (A) Root Al concentration, (B) leaf Al concentration, and (C) soil water content (SWC) at Weeks 4, 9 and 13 (n = 9). Symbols: d,
and suggested that the observed differences in Al sensitivity exhibited by tree species were associated with experimental conditions resulting in differences in either Ca2+ availability or ionic strength of the solution. Our results support the sugges-tion that Al sensitivity can be offset by the presence of suitable amounts of plant-available Ca.
We observed a stimulation of relative root growth rate by 2.0 mM Al3+. Thornton et al. (1986a) reported a similar observa-tion at 1.0 mM Al3+; however at 2.0 mm Al3+, these workers reported detrimental effects of Al3+ on roots growing in solu-tion culture, suggesting that the results obtained from plants in solution culture are not directly comparable with those ob-tained from plants in silica culture. Although a steady-state nutrient supply, as is possible in solution culture, cannot be achieved when silica is used as the growing medium, silica culture provides information about plant responses to stress such as exclusion mechanisms and root exudates (Taylor 1991). It has been shown for Glycine max (L.) Merill. that the mechanical impedance caused by sand culture increased toler-ance to Al stress as a result of reduced Al uptake into the root tips compared with uptake from solution culture (Horst et al. 1990). These authors suggest that enhanced release of exudates might account for the higher Al tolerance in sand culture than in solution culture. Plants could exclude Al from the symplasm if chelate ligands were released into the rhizosphere and these ligands formed stable complexes with Al, thus reducing the absorption of the metal ion across the plasma membrane (Tay-lor 1991). In addition to root exudates, there is the possibility that genotype may also strongly influence responses.
The Al treatments caused decreases in leaf area between Weeks 4 and 9 so that at Week 9, total leaf area per tree was reduced by more than 27% in Al-treated seedlings relative to seedlings in the other treatments. By Week 13, the total leaf area was similar for all treatments even though the leaf Al concentration was twice as high in Al-treated seedlings as in untreated seedlings. Thornton et al. (1986b) observed an abso-lute, but small (≤ 10% over a 30-day period), increase in
damage with increasing exposure time to Al. Our results show that plants can acclimate to a concentration of Al previously considered toxic and indicate that the duration of exposure to Al should therefore be taken into account when assessing a critical concentration for Al toxicity in silica culture. Possible mechanisms of acclimation include entrapment of excess Al in roots, reducing Al concentration in seedling tops (Foy et al. 1978) as observed at Week 13, and an enzyme reaction to Al stress in the leaves (Bowler et al. 1992).
Leaf K concentration decreased in response to the Al treat-ments. Translocation of K from roots to leaves may have been impaired in the presence of Al because the root K concentra-tion was significantly higher in Al-treated seedlings than in control seedlings. In our study, the leaf K concentration (≈4.0 mg gDW−1) of all the seedlings was below the critical
concen-tration of 6.0 mg g−1 needed for adequate nutrition of A. sac-charum (Bernier and Brazeau 1988) and lower than the concentration reported for mature sugar maple trees (Bernier et al. 1989) or for sugar maple seedlings growing in solution culture (Thornton et al. 1986a). We note that deficiencies in leaf K in sugar maple trees growing in the Beauce region (Québec, Canada), which is the provenance of our seedlings, have been associated with incidences of stand decline in this region (Ouimet and Fortin 1992). The numerous differences in nutrition requirements among varieties or lines of plants sug-gest genetic control of inorganic plant nutrition (Gerloff and Gabelman 1983). Moreover, a large range of variation in sen-sitivity to Al has been observed among various provenances of paper birch (Steiner et al. 1980), among Populus hybrids (Ste-iner et al. 1984), and among genotypes of loblolly pine (Raynal et al. 1990). The differential intraspecific sensitivity to toxic ions also reflects genetic differences in mineral nutrition, which could prove useful in breeding trees for tolerance to nutritional stress.
The treatments had no effect on ABA concentration in the xylem sap. Maximum ABA concentrations in the xylem sap were reached at the onset of the experiment and at Week 9, Table 1. Mineral concentration of sugar maple roots and leaves (mg gDW−1) in response to five treatments for the pooled sampling dates. Different
letters indicate significant differences among treatments at P ≤ 0.05; mean (± SE) for n = 27.
Element Control pH 4.0 pH 3.5 pH 4.0 + Al pH 3.5 + Al
Ca 18.84 (1.16) 22.07 (1.63) 21.41 (2.01) 21.69 (1.94) 21.30 (1.22)
Mg 6.71 (0.34) 6.92 (0.31) 6.90 (0.30) 7.03 (0.36) 6.89 (0.37)
K 4.86 (0.60) ab 6.39 (0.70) a 5.53 (.55) a 4.06 (0.28) b 4.20 (0.31) b
P 1.69 (0.11) 2.07 (0.18) 1.97 (0.16) 1.72 (0.20) 1.81 (0.15)
Mn 0.21 (0.04) 0.19 (0.03) 0.28 (0.05) 0.28 (0.05) 0.28 (0.06)
which corresponded to low soil water contents. A direct in-crease in ABA concentration of the xylem sap in response to soil water depletion has been observed in maize (Zhang and Davies 1990). At Week 9, when the soil water content was at its lowest value, the ABA concentration in the xylem sap was comparable to the concentration measured in mature sugar maple trees during a physiological drought stress caused by the freezing of soil water, whereas at Weeks 4 and 13, the ABA concentration in the xylem sap was similar to that in untreated trees with an adequate water supply (Bertrand et al. 1994). Based on this comparison of field and laboratory results, we conclude that water stress was the principal cause of increases in ABA concentration in the xylem sap and that Al had little or no effect on ABA concentration of the xylem sap.
Aluminum stress or other nutritional imbalances may facili-tate an inciting factor such as early or late winter frost to induce sugar maple tree decline. The impacts of high concentrations of Al and acid rain acting alone and in combination on the cold tolerance of sugar maple seedlings are under investigation.
Acknowledgments
We thank Mr. Pascal Aubé and Mr. Clément Aubin for their technical assistance, Mr. Alain LePage for his contribution to the chemical analysis, and Ms. Michèle Bernier-Cardou for discussions on statisti-cal analysis. This work was supported by the Long Range Transport of Atmospheric Pollutants (LRTAP).
References
Bernier, B. and M. Brazeau. 1988. Foliar nutrient status in relation to sugar maple dieback and decline in the Québec Appalachians. Can. J. For. Res. 18:754--761.
Bernier, B., D. Par and M. Brazeau. 1989. Natural stresses, nutrient imbalances and forest decline in southeastern Québec. Water Air Soil Pollut. 48:239--250.
Bertrand, A., G. Robitaille, P. Nadeau and R. Boutin. 1994. Effects of soil freezing and drought stress on abscisic acid content of sugar maple sap and leaves. Tree Physiol. 14:413--425.
Bowler, C., M. Van Montagu and D. Inz. 1992. Superoxide dismutase and stress tolerance. Annu. Rev. Plant Physiol. Plant Mol. Biol. 43:83--116.
Burdett, A.N. 1979. A nondestructive method for measuring the vol-ume of intact plant parts. Can. J. For. Res. 9:120--122.
Chapin III, F.S. 1990. Effects of nutrient deficiency on plant growth: evidence for a centralized stress-response system. In Importance of Root to Shoot Communication in the Response to Environmental Stress. Eds. W.J. Davies and B. Jeffcoat. British Society for Plant Growth Regulation, Lancaster, pp 135--148.
Cumming, J.R., R.T. Eckert and L.S. Evans. 1985. Effect of aluminum on 32P uptake and translocation by red spruce seedlings. Can. J. For. Res. 16:864--867.
Edwards, J.H., B.D. Horton and H.C. Kirckpatrick. 1976. Aluminum toxicity symptoms in peach seedlings. J. Am. Soc. Hortic. Sci. 101:139--142.
Foster, N.W. 1989. Acidic deposition: what is fact, what is needed? Water Air Soil Pollut. 48:299--306.
Foy, C.D., R.L. Chaney and M.C. White. 1978. The physiology of metal toxicity in plants. Annu. Rev. Plant Physiol. 29:511--566.
Gerloff, G.C. and W.H. Gabelman. 1983. Genetic basis of inorganic plant nutrition. In Encyclopedia of Plant Physiology. Vol. 15B. New Series. Eds. A. Lauchli and R.L. Bieleski. Springer-Verlag, Berlin, pp 453--480.
Goldbach, E., H. Goldbach, H. Wagner and G. Michael. 1975. Influ-ence of N-deficiency on the abscisic acid content of sunflower plants. Physiol. Plant. 34:138--140.
Haeder, H.E. and H. Beringer. 1981. Influence of potassium nutrition and water stress on the abscisic acid content in grains and flag leaves during grain development. J. Sci. Food Agric. 32:552--556. Hoagland, D.R. and D.I. Arnon. 1938. The water culture method for
growing plants without soil. Calif. Agric. Exp. Stn. Circ. 347, Univ. of California, Berkeley, CA, 39 p.
Horst, W.J., F. Klotz and P. Szulkiewicz. 1990. Mechanical impedance increases aluminum tolerance of soybean (Glycine max) roots. In Plant Nutrition----Physiology and Applications. Ed. M.L. van Beusichem. Kluwer Academic Publishers, Dordrecht, pp 351--355. Kelly, J.M., M. Schaedle, F.C. Thornton and J.D. Joslin. 1990. Sensi-tivity of tree seedlings to aluminum: II. Red oak, sugar maple, and European beech. J. Environ. Qual. 19:172--179.
Kruger, E. and E. Sucoff. 1989. Growth and nutrient status of Quercus rubra L. in response to aluminum and calcium. J. Exp. Bot. 40:653--658.
Lachance, D. 1985. Répartition géographique et intensité du dépéris-sement de l’érable à sucre dans les érablières au Québec. Phytopro-tection 66:83--90.
Marschner, H. 1986. Mineral nutrition in higher plants. Academic Press Inc., London, 674 p.
NCASI. 1983. Field study program elements to assess the sensitivity of soils to acidic deposition induced alterations in forest productiv-ity. Technical Bulletin No. 404. Nat. Counc. of the Paper Ind. for Air and Stream Improvement, New York, 88 p.
Ouimet, R., and J.-M. Fortin. 1992. Growth and foliar nutrient status of sugar maple: incidence of forest decline and reaction to fertiliza-tion. Can. J. For. Res. 22:699--706.
Radin, J.W. 1984. Stomatal responses to water stress and to abscisic acid in phosphorus-deficient cotton plants. Plant Physiol. 76:392--394.
Raynal, D.J., J.D. Joslin, F.C. Thornton, M. Schaedle and G.S. Hen-derson. 1990. Sensitivity of tree seedlings to aluminum: III. Red spruce and loblolly pine. J. Environ. Qual. 19:180--187.
Robitaille, G., R. Boutin and D. Lachance. 1995. Effects of soil freezing stress on sap flow and sugar content of mature sugar maples (Acer saccharum). Can. J. For. Res. 25:577--587. Steiner, K.C., J.R. Barbour and L.H. McCormick. 1984. Response of
Populus hybrids to aluminum toxicity. For. Sci. 30:404--410. Steiner, K.C., L.H. McCormick and D.S. Canavera. 1980. Differential
response of paper birch provenances to aluminum in solution cul-ture. Can. J. For. Res. 10:25--29.
Stienen, H. and J. Bauch. 1988. Element determination in tissues of spruce and fir seedlings from hydroponic and soil cultures simulat-ing acidification and deacidification. Plant Soil. 106:231--238. Sucoff, E., F.C. Thornton and J.D. Joslin. 1990. Sensitivity of tree
seedlings to aluminum: I. Honeylocust. J. Environ. Qual. 19:163--171. Taylor, G.J. 1991. Current views of the aluminum stress response; the physiological basis of tolerance. Curr. Top. Plant Biochem. Physiol. 10:57--93.
Thornton, F.C., M. Schaedle and D.J. Raynal. 1986a. Effect of alumi-num on the growth of sugar maple in solution culture. Can. J. For. Res. 16:892--896.
Thornton, F.C., M. Schaedle and D.J. Raynal. 1987. Effects of alumi-num on red spruce seedlings in solution culture. Environ. Exp. Bot. 27:489--498.
Thornton, F.C., M. Schaedle and D.J. Raynal. 1989. Tolerance of red oak and American and European beech seedlings to aluminum. J. Environ. Qual. 18:541--545.
Ulrich, B., R. Mayer and P.K. Khanna. 1980. Chemical changes due to acid precipitation in a loess-derived soil in central Europe. Soil Sci. 130:193--199.
Weiler, E.W. 1980. Radioimmunoassays for the differential and direct analysis of free and conjugated abscisic acid in plant extract. Planta 148:262--272.