Summary For 20 weeks during the growing season, cuttings of one birch clone (Betula pendula Roth.) were exposed in the Birmensdorf fumigation chambers to O3-free air (control) or 75 nl O3 l−1. Ozone was supplied either from 1900 until 0700 h (nighttime regime), from 0700 until 1900 h (daylight regime), or all day (24-h regime). By autumn, reductions in whole-plant biomass production, root/shoot biomass and stem weight/length ratios were evident in all three O3 regimes. The reductions in cuttings receiving the 24-h O3 treatment were about twofold larger than in cuttings receiving the daylight O3 treatment. Stomata were open at night, and stomatal conduc-tance was about 50% of its maximum daytime value. We calculated that the rate of O3 uptake into leaves in the dark approached 4 nmol m−2 s−1. Whole-plant production and carb-on allocaticarb-on were more sensitive to O3 during the night than during the day; however, O3 exposure caused similar visible leaf injury in both of the 12-h regimes, although the leaves exposed to O3 at night exhibited delayed O3-induced shedding. Overall, changes in production and carbon allocation were determined by the external O3 dose rather than by the kind of O3 exposure, indicating that, at the seasonal scale, the internal dose of ozone that was physiologically effective was a constant fraction of the external O3 dose. We conclude that nighttime O3 exposures should be included in the daily time period for determining critical concentrations of O3 causing injury in trees.
Keywords: biomass production, carbon allocation, foliage de-velopment, stomatal conductance.
Introduction
Ozone can limit tree development in controlled experiments with young plants (Reich 1987, Pye 1988), but O3 effects on mature trees vary between sites and species (Miller 1973, Peterson et al. 1987, Schulze et al. 1989). Geographical infor-mation systems (GIS) are gaining importance as a means of assessing large-scale ecological risk, e.g., for estimating the constraint ozone poses on trees and forests (Hogsett et al.
1995). However, implementation of GIS for risk assessment of O3 injury (see UNECE 1994) requires that critical levels of O3 exposure (CL) be established below which no adverse effects are assumed to occur in plants (UNECE 1988). Thus, CLs need to be defined for the time periods during which trees are sensitive to ozone. It has not been determined whether ozone-sensitive periods are restricted to the daylight hours of the growing season for trees as proposed for agricultural crop plants (Fuhrer 1994). Forest sites that are not continuously affected by ozone from local urban sources can experience high O3 concentrations in the evening and early morning hours (Lefohn and Jones 1986).
To determine whether trees are sensitive to ozone during the night, we analyzed the influence of nighttime exposure to ozone on foliage development and annual biomass production of a birch clone (Betula pendula Roth.) relative to the influence of ozone exposures during daylight hours or throughout the day. In a previous study with this clone, continuous O3 expo-sure throughout the growing season caused declines in CO2 assimilation and biomass production that were accompanied by marked changes in leaf differentiation and whole-plant carbon allocation (Matyssek et al. 1991, 1992, Günthardt-Go-erg et al. 1993). Although O3 sensitivity of deciduous tree species is restricted to the growing season, it is not known whether ozone is taken up at night. The capacity of stomata to open without light stimulation (Tobiessen 1982) may be fa-vored under warm and humid conditions (Lösch 1979, Schulze and Hall 1982), depend on leaf age (Field 1987), or be caused by sluggishness in stomatal regulation under O3 stress (Keller and Häsler 1987). To distinguish among these possibilities, we examined leaf gas exchange during the nighttime.
Materials and methods
Plants and treatments
From April 17 until September 27, 1990, cuttings of one birch clone (Betula pendula) were each grown in a 10-l pot filled with sand and a basal layer of inert synthetic clay beads. The
Nighttime exposure to ozone reduces whole-plant production in
Betula
pendula
RAINER MATYSSEK,
1,2MADELEINE S. GÜNTHARDT-GOERG,
1STEFAN MAURER
1and
THEODOR KELLER
11 Swiss Federal Institute for Forest, Snow and Landscape, Research, Zürcherstrasse 111, CH-8903 Birmensdorf ZH, Switzerland 2 New address: Department of Forest Botany, University of Munich, Hohenbachernstr. 22, D-85354 Freising, Germany
Received April 4, 1994
cuttings were fertilized and well watered. On May 17, 1990, when the shoot length of the plants was about 3 cm, they were transferred to the Birmensdorf field fumigation chambers and subjected to one of four O3 treatments (five plants per treat-ment, one plant per chamber). The O3 concentrations were < 0.003 µl l−1 (control, charcoal-filtered air, regarded as O3-free, 24 h day−1) and 0.075 µl l−1, the latter being applied daily either from 0700 until 1900 h (daylight regime), from 1900 until 0700 h (nighttime regime, including dawn and dusk), or throughout the day (24-h regime). Ozone was generated from pure oxygen (Fischer, model 502) and added to charcoal-fil-tered air. All treatments were continuously monitored with a Monitor Labs 8810 instrument. The birch clone, cultural prac-tices and fumigation chambers have been described by Matyssek et al. (1991, 1992, see also Landolt et al. 1989). On clear sunny days, a shade roof limited the photon flux density to a maximum of about 600 µmol m−2 s−1 to prevent over-heat-ing in the open-top chambers. The roof was not used under overcast and cloudy conditions, or at dawn and dusk.
Macroscopic leaf injury
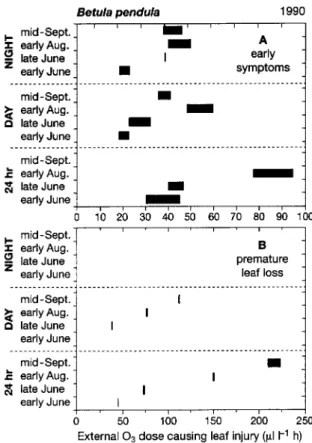
Visible symptoms of O3-induced leaf injury were classified on the basis of leaf color, which progressed from dark-green to the occurrence of light-green dots, brownish-bronze discoloration, necroses and premature leaf loss. The early symptoms (i.e., light-green dots spread over the leaf lamina) were assigned to Class 1 and premature leaf loss was assigned to Class 4, following Günthardt-Goerg et al. (1993). The trees were exam-ined for symptoms of visible injury in early and late June, early August and mid-September.
Biomass analysis
On September 27, 1990, the five trees in each treatment were harvested. Whole-plant foliage area was determined with a leaf area meter (MK2, Delta-T Devices, U.K.) by summing the one-sided area of all leaves. Attached and prematurely shed leaves were counted. All tree organs (leaves, branches, stem, root and initially planted cutting) were dried at 65 °C to constant weight. The ratio of root/shoot biomass comprised the plant organs that had developed from the initial cutting (about 13 cm long, 0.6 g in April), otherwise the cutting increment was part of the whole-plant dry weight.
Measurements of leaf gas exchange
Measurements are not available from the 1990 experiment, but measurements were conducted in 1992 with the same birch clone. In 1992, the daily O3 regime was 0.09 µl l−1 from 0700 until 2100 h and 0.04 µl l−1 during the rest of the day (the control treatment and all other practices were the same as in 1990). Attached complete leaves were measured with a ther-moelectrically climate-controlled cuvette system (Walz, Ger-many) as described by Matyssek et al. (1991). Diurnal courses of leaf gas exchange were recorded from the plants exposed in the fumigation chambers. Temperature and air humidity were reproduced inside the gas exchange cuvette at any instant of time, and the photon flux density was reduced by about 8% by the cuvette lid (CO2 concentration of the ambient air was about
340 µl l−1). Gas exchange rates were based on the one-sided leaf area. The rate of O3 uptake into the intercellular spaces of the leaf mesophyll was calculated according to the water vapor surrogate method (Laisk et al. 1989). That is, by converting stomatal conductance for water vapor (gH2O) to stomatal
con-ductance for ozone (gO3), based on the ratio of the diffusion
coefficients (DH2O /DO3 = 1.68), and assuming that the O3
concentration in the intercellular spaces of the mesophyll was zero. The expression ‘‘external O3 dose’’ denotes the O3 con-centration of the ambient air multiplied by the duration of exposure.
Stomatal apertures were investigated by low-temperature scanning electron microscopy (Scheidegger et al. 1991).
Results
The tolerance of leaves to O3 stress varied with both season and the daily period of O3 exposure. Leaves developing in summer generally tolerated higher external O3 doses before exhibiting visible injury than leaves that were formed in the spring (Fig-ure 1A). The O3 tolerance of leaves in the daylight and night-time O3 regimes increased similarly during the growing
season, but O3 tolerance was lower than in leaves in the 24-h O3 regime. In the daylight O3 regime, no leaves were shed by early June, and in the nighttime O3 regime, no leaves were lost by mid-September (Figure 1B). The external O3 dose that caused premature abscission was higher in leaves that had developed during the second half of the growing season than in leaves formed in the spring (Figure 1B). The dose range that induced leaf shedding was small.
The total number of leaves formed per plant throughout the growing season did not differ among treatments (Figure 2A). In the nighttime regime, premature leaf loss did not occur until the end of September, and the number of leaves shed was less than in the other two O3 regimes (Figure 2A). In both the daylight and nighttime regimes, leaf loss was too small to cause a perceptible reduction in crown foliage area relative to that of the control trees (Figure 2B). Although leaf loss was similar in trees in the daylight and 24-h O3 regimes, only trees in the 24-h O3 regime displayed an apparent reduction in
whole-plant foliage area as the result of a decrease in stem rather than branch foliage area (Figure 2B). A reduction in the size of the stem leaves, but not of the branch leaves, in response to 24-h O3 exposure (Figure 2C) resulted in the decreased crown foliage area.
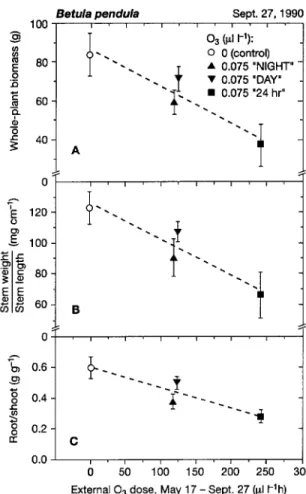
Ozone had a greater effect on whole-plant biomass produc-tion than on foliage area during the growing season (Fig-ure 3A). The decline in biomass production was accompanied by marked changes in whole-plant carbon allocation. With increasing external O3 dose, annual stem production, based on the increment in stem length, was reduced (Figure 3B, stem weight/stem length) as was the root/shoot biomass ratio (Fig-ure 3C). The ozone sensitivity of biomass production and carbon allocation was higher in trees in the nighttime than in the daylight O3 regime, but the combined effects of the treat-ments were similar to those of the 24-h treatment (Figures 3A--C).
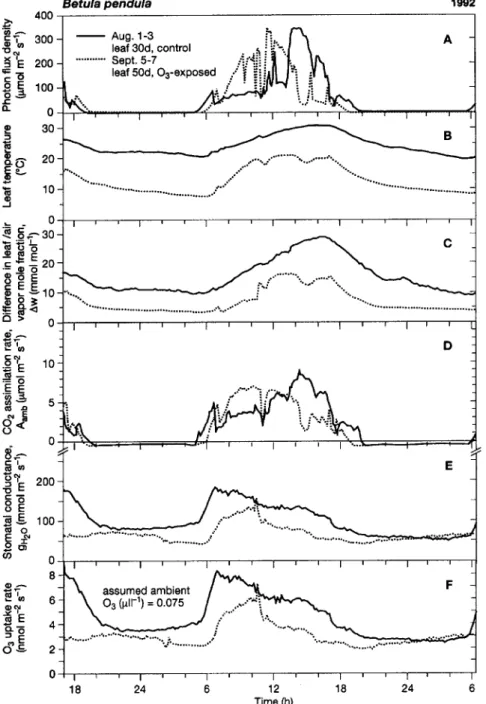
We compared two 37-h sequences of leaf gas exchange that differed in weather conditions, leaf age and O3 exposure (Fig-ure 4). Gas exchange of the 4-week-old leaf, which had been continuously exposed to O3-free air, is shown for a period of hot midsummer weather (Figure 4, solid line). Day
tempera-Figure 2. Whole-plant leaf number and prematurely shed leaves (A), whole-plant foliage area on stem and branches (B), and mean area of the stem and branch leaves (C) at the end of the growing season as related to the external O3 dose applied during the growing season. Data represent means of five plants per treatment ± SD; for graphical reasons, the symbols of the NIGHT and DAY regimes were grouped around the O3 dose of 120 µl l−1 h in both treatments. Dashed lines illustrate the overall trend in the response to the ozone dose.
tures reached 33 °C, nighttime temperatures did not drop below 20 °C, and the air was moderately dry during the night (leaf/air mole fraction difference of water vapor, ∆w > 10 mmol mol−1; Figures 4B and 4C). During the day, stomatal conductance (gH2O) allowed CO2 uptake to follow the time
course of irradiance; however, at night gH2O still reached about
50% of the daily maximum value (Figures 4A, 4D and 4E), indicating that stomata remained open in the dark. Gas ex-change of the 7-week-old leaf (Figure 4, dotted line), which had been continuously exposed to ozone since bud break, is shown for a period during late summer when night tempera-tures fell to 7 °C at ∆w = 4 mmol mol−1 (Figures 4B and C). Stomatal conductance remained high at night (Figure 4E), even though stomata may narrow in the light in aging leaves or
under prolonged O3 exposure (cf. Matyssek et al. 1991). Based on the assumption that the O3 concentration of the ambient air remained constant at 0.075 µl l−1 (corresponding to the 24-h O3 regime in 1990), the calculated rate of O3 uptake, which then parallels the time course of gH2O, could have reached 3--4
nmol m−2 s−1 at night in both the 4- and 7-week-old leaves (Figure 4F), indicating that O3 uptake at night was substantial relative to that during the day. The stomatal behavior shown in Figure 4E was representative of the daily leaf gas exchange of the birch clone investigated (Maurer and Matyssek, unpub-lished data), and was confirmed by microscopic analysis (Fig-ure 5). Stomatal pores were found to be open in the dark, but less so than during the day, regardless of the O3 regime.
Discussion
Nighttime exposure to O3caused leaf injury, limited biomass
production and decreased the root/shoot biomass ratio (R/S)
as a result of changed carbon allocation. ReducedR/Shas
often been observed in response to the growth-limiting
in-fluence of O3or SO2(Freer-Smith 1985, Mahoney et al. 1985,
Tsukahara et al. 1986, Chappelka et al. 1988, Schier et al.
1990, Matyssek et al. 1992, 1993b). LowR/Sratios may
re-sult from impeded assimilate translocation caused by either cell collapse in the mesophyll or impaired phloem structure and function (Michin and Gould 1986, Günthardt-Goerg et al. 1993); however, it may also indicate acclimation to ozone. During the acclimation process, assimilates can be used in the leaves for repair and detoxification processes (enhanced demand for energy and antioxidants; Reich 1983, Polle 1994) instead of being allocated to roots. Such behavior tends to maintain set-points between the plant’s
internal fluxes of carbon and nutrients during O3 stress
(Mooney and Winner 1991), and may explain the insensitivity of leaf formation to the ozone treatments (cf. Figure 2A, Mooi
1980, Matyssek et al. 1993a, 1993b). Leaf formation in birch
inherently relates to the length increment of stem and branch axes, which precedes radial growth (i.e., biomass increment).
Radial growth becomes limited (cf. Figure 3B) as CO2
assimi-lation declines with O3dose (i.e., exposure time), and may
cease before latewood formation is initiated (Matyssek et al
1991, 1993a).
Birch stomata remained open at night thus allowing pollut-ant uptake. Open stomata at night have also been observed in spruce (Wieser and Havranek 1993) and herbaceous plants (Goknur and Tibbitts 1984, Aben et al. 1989). Also, in turnip plants, nighttime exposure to ozone reduced production
(Win-ner et al. 1989); however, the O3concentration must remain
enhanced at night to limit plant growth (cf. Kress et al. 1989). These observations suggest that a 24-h period may provide the most satisfactory daily basis for defining critical ozone
expo-sure for both agricultural crop plants (cf. Fuhrer 1994) and trees.
Although the stomata of birch were open in the dark,gH O2
and thus O3uptake were lower than during the day (cf. Figure
4); however, whole-plant production and carbon allocation tended to be more sensitive to ozone at night than during the day (cf. Figure 3). Reactive oxygen species, which are re-leased in the leaves by ozone as well as by photosynthetic pro-cesses, may be detoxified more efficiently in the light (Menser 1964, Gillham and Dodge 1987, Schupp and Rennenberg 1988). Both ascorbate and glutathione require NADPH, generated by photosynthetic electron transport, to stay re-duced and scavenge oxidants (Foyer et al. 1991). Ascorbate can be exchanged between chloroplasts and cytosol and be de-livered to the plasmalemma and apoplast, where ozone, when occurring in low concentrations as applied in this study, mainly attacks the cell (Laisk et al. 1989, Urbach et al. 1989, Luwe et al. 1993). Consequently, the absence of light may
ren-der plants susceptible to O3stress. However, the increased
nighttime sensitivity to ozone was not reflected in a reduction in foliage area or in substantially lowered tolerance to macro-scopic leaf injury, indicating that the amount and appearance of foliage were not closely coupled with a decline in photosynthetic capacity. Limited photosynthesis, however, must have impeded the overall carbon flux in the plant
result-ing in O3-induced changes in whole-plant production and
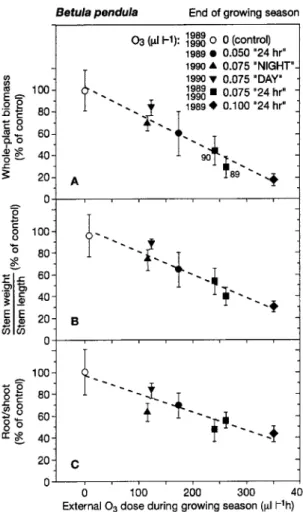
car-bon allocation (cf. Figure 3, Mooney and Winner 1991). Overall, the proportional decline in biomass production,
R/Sand stem weight/length relative to plants in O3-free air
was determined by the external O3dose rather than by the kind
of O3exposure (daily time interval, O3concentration, different
growing seasons; Figure 6). The relationships suggest that, at the seasonal scale, the internal dose of ozone that is
physiolog-ically effective was a constant fraction of the external O3dose
in each O3regime.
Although the nonlimiting water supply of the experimental
plants may have favored stomatal opening at night, the plants showed a capacity for substantial O3 uptake at night and, as a consequence, a reduction in biomass production. Tropospheric O3 concentration is often highest in the afternoon and can remain enhanced during the night or may be permanently elevated at high altitudes (Lefohn and Jones 1986, Krupa and Manning 1988, NAPAP 1991). We conclude that for defining critical exposure for ozone in trees, a daily time basis of 24 h is justified.
Acknowledgments
We gratefully acknowledge the technical assistance of Mr. U. Bühl-mann, Mr. P. Bleuler, Mr. R. Gall and Mr. A. Burkart in tending the plants and operating the O3 fumigation chambers. We also thank Dr. C. Scheidegger for his support in scanning electron microscopy. The helpful discussions with Dr. W. Landolt, Dr. J.B. Bucher and Dr. A. Polle and the stylistic editing of the English text by Mrs. M. Sieber are
highly appreciated. Part of the study was financed through the EUREKA 447 EUROSILVA program by the Swiss Bundesamt für Bildung und Wissenschaft.
References
Aben, J., F. Thiel and A. Boekestein. 1989. Are the stomata of Vicia faba L. closed in the dark? Ultramicroscopy 31:457.
Chappelka, A.H., B.I. Chevone and T.E. Burk. 1988. Growth response of green and white ash seedlings to ozone, sulfur dioxide, and simulated acid rain. For. Sci. 34:1016--1029.
Field, C.B. 1987. Leaf-age effects on stomatal conductance. In Sto-matal Function. Eds. E. Zeiger, G.D. Farquhar and I.R. Cowan. Stanford University Press, Stanford, pp 367--384.
Foyer, C.H., M. Lelandais, E.A. Edwards and P.M. Mullineaux. 1991. The role of ascorbate in plants, interactions with photosynthesis, and regulatory significance. In Active Oxygen/Oxidative Stress and Plant Metabolism. Eds. E. Pell and K. Steffen. Am. Soc. Plant Physiol., pp 131--144.
Freer-Smith, P.H. 1985. The influence of SO2 and NO2 on the growth, development and gas exchange of Betula pendula Roth. New Phy-tol. 99:417--430.
Fuhrer, J. 1994. The critical level for ozone to protect agricultural crops----an assessment of data from European open-top chamber experiments. In UNECE Workshop Report, Bern, Switzerland, 1993. Eds. J. Fuhrer and B. Achermann. Schriftenreihe FAC 16:42--57.
Gillham, D.J. and A.D. Dodge. 1987. Chloroplast superoxide and hydrogen peroxide scavenging systems from pea leaves: seasonal variations. Plant Sci. 50:105--109.
Goknur, A.B. and T.W. Tibbitts. 1984. Dark opening of stomata to SO2 sensitivity of potatoes. HortScience 19:548.
Günthardt-Goerg, M.S., R. Matyssek, C. Scheidegger and T. Keller. 1993. Differentiation and structural decline in the leaves and bark of birch (Betula pendula) under low ozone concentration. Trees 7:104--114.
Hogsett, W.E., A.A. Herstrom, J.A. Laurence, E.H. Lee, J.E. Weber and D.T. Tingey. 1995. Risk characterization of tropospheric ozone to forests. In Comparative Risk Analysis and Priority Setting for Air Pollution Issues. Publ. Air and Waste Management Assoc., Pittsburg, PA. In press.
Keller, T. and R. Häsler. 1987. The influence of a fall fumigation with ozone on the stomatal behavior of spruce and fir. Oecologia 64:284--286.
Kress, L.V., B.L. Allen, J.E. Mudano and W.W. Heck. 1989. Ozone effects on the growth of loblolly pine. In Air Pollution and Forest Decline. Eds. J.B. Bucher and I. Bucher-Wallin. Birmensdorf, pp 153--158.
Krupa, S.V. and W.J. Manning. 1988. Atmospheric ozone: formation and effects on vegetation. Environ. Pollut. 50:101--137.
Laisk, A., O. Kull and H. Moldau. 1989. Ozone concentration in leaf intercellular air spaces is close to zero. Plant Physiol. 90:1163--1167.
Landolt, W., I. Pfenninger and B. Lüthy-Krause. 1989. The effect of ozone and season on the pool sizes of cyclitols in Scots pine (Pinus sylvestris). Trees 3:85--88.
Lefohn, A.S. and C.K. Jones. 1986. The characterization of ozone and sulfur dioxide air quality data for assessing possible vegetation effects. J. Air Pollut. Control Assoc. 36:1123--1129.
Lösch, R. 1979. Stomatal responses to changes in air humidity. In
Structure, Function and Ecology of Stomata. Eds. D.N. Sen, D.D. Chawan and R.P. Bansal. Dehra Dun, pp 189--216.
Luwe, M.W.F., U. Takahama and U. Heber. 1993. Role of ascorbate in detoxifying ozone in the apoplast of spinach (Spinacea oleracea L.) leaves. Plant Physiol. 101:969--976.
Mahoney, M.J., B.I. Chevone, J.M. Skelly and L.D. Moore. 1985. Influence of mycorrhizae on the growth of loblolly pine Pinus taeda
seedlings exposed to ozone and sulphur dioxide. Phytopathology 75:679--682.
Matyssek, R., M.S. Günthardt-Goerg, T. Keller and C. Scheidegger. 1991. Impairment of the gas exchange and structure in birch leaves (Betula pendula) under low ozone concentrations. Trees 5:5--13. Matyssek, R., M.S. Günthardt-Goerg, M. Saurer and T. Keller. 1992.
Seasonal growth, δ13C of leaves and stem, and phloem structure in birch (Betula pendula) under low ozone concentrations. Trees 6:69--76.
Matyssek, R., T. Keller and T. Koike. 1993a. Branch growth and leaf gas exchange of Populus tremula exposed to low ozone concentra-tions throughout two growing seasons. Environ. Pollut. 79:1--7. Matyssek, R., M.S. Günthardt-Goerg, W. Landolt and T. Keller. 1993b.
Whole-plant growth and leaf formation in ozonated hybrid poplar (Populus × euramericana). Environ. Pollut. 81:207--212. Menser, H.A. 1964. Response of plants to air pollutants. III. A relation
between ascorbic acid levels and ozone susceptibility of light-pre-conditioned tobacco plants. Plant Physiol. 39:564--567.
Michin, P.E.H. and R. Gould. 1986. Effect of SO2 on phloem loading. Plant Sci. 43:179--183.
Miller, P.R. 1973. Oxidant-induced community change in a mixed conifer forest. In Air Pollution Damage to Vegetation. Advances in Chemistry Series, No. 122. Ed. J.A. Naegele. Am. Chem. Soc., Washington DC, pp 101--117.
Mooi, J. 1980. Influence of ozone on growth of two poplar cultivars. Plant Diss. 64:772--773.
Mooney, H.A. and W.E. Winner. 1991. Partitioning response of plants to stress. In Response of Plants to Multiple Stresses. Eds. H.A. Mooney, W.E. Winner, and E.J. Pell. Academic Press, San Diego, pp 129--141.
NAPAP. 1991. Acidic deposition: state of science and technology. Ed. P.W. Irving. US Natl. Acid Precip. Assess. Prog., 265 p.
Peterson, D.L., M.J. Arbaugh, V.A. Wakefield and P.R. Miller. 1987. Evidence of growth reduction in ozone-stressed Jeffrey pine (Pinus jeffreyi Grev. and Balf.) in Sequoia and Kings Canyon National Parks. J. Air Pollut. Control Assoc. 37:906--912.
Polle, A. 1994. Protection from oxidative stress in trees as affected by elevated CO2 and environmental stress. In Terrestrial Ecosystem Response to Elevated CO2. Eds. H.A. Mooney and G.W. Koch. Academic Press, Physiological Ecology Series. In press.
Pye, J.M. 1988. Impact of ozone on the growth and yield of trees: a review. J. Environ. Qual. 17:347--360.
Reich, P.B. 1983. Effects of low concentrations of O3 on net photosyn-thesis, dark respiration, and chlorophyll contents in aging hybrid poplar leaves. Plant Physiol. 73:291--296.
Reich, P.B. 1987. Quantifying plant response to ozone: a unifying theory. Tree Physiol. 3:63--91.
Scheidegger, C., M.S. Günthardt-Goerg, R. Matyssek and P. Hatvani. 1991. Low temperature SEM of birch leaves after exposure to ozone. J. Microsc. 161:85--95.
Schier, G.A., C.J. McQuattie and K.F. Jensen. 1990. Effects of ozone and aluminum on pitch pine (Pinus rigida) seedlings growth and nutrient relations. Can. J. For. Res. 20:1714--1719.
Schulze, E.-D. and A.E. Hall. 1982. Stomatal responses, water loss, and nutrient relations in contrasting environments. In Encyclopedia of Plant Ecology 12B, Physiological Plant Ecology II. Eds. O.L. Lange, P.S. Nobel, C.B. Osmond and H. Ziegler. Springer, Berlin, Heidelberg, New York, pp 182--230.
Schulze, E.-D., O.L. Lange and R. Oren. 1989. Forest decline and air pollution. Ecological Studies 77, Springer-Verlag, Berlin, Heidel-berg, 475 p.
Schupp, R. and H. Rennenberg. 1988. Diurnal changes in the glu-tathione content of spruce needles (Picea abies L.). Plant Sci. 57:113--117.
Tobiessen, P. 1982. Dark opening of stomata in successional trees. Oecologia 52:356--359.
Tsukahara, H., T.T. Kozlowski and J. Shanklin. 1986. Effects of SO2 on growth of two age classes of Chamaecyparis obtusa seedlings. J. Jpn. For. Soc. 68:349--353.
UNECE. 1988. Critical levels workshop, final report. Bad Harzburg (Germany), 146 p.
UNECE. 1994. Critical level for ozone. In UNECE Workshop Report. Eds. J. Fuhrer and B. Achermann. Schriftenreihe FAC 16, 328 p. Urbach, W., W. Schmidt, J. Kolbowski, S. Rümmele, E. Reisberg, W.
Steigner and U. Schreiber. 1989. Wirkungen von Umweltschad-stoffen auf Photosynthese und Zellmembranen von Pflanzen. In 1. Statusseminar der PBWU zum Forschungsschwerpunkt Wald-schäden. Eds. M. Reuther and M. Kirchner. GSF München, pp 195--206.
Wieser, G. and W.M. Havranek. 1993. Ozone uptake in the sun and shade crown of spruce: quantifying the physiological effects of ozone exposure. Trees 7:227--232.