Derivation of Class II Force Fields. VIII. Derivation of a General
Quantum Mechanical Force Field For Organic Compounds
Carl S. Ewig, Rajiv Berry, Uri Dinur, Jörg-Rüdiger Hill, Ming-Jing Hwang, Haiying Li, Chris
Liang, Jon Maple, Zhengwei Peng, Thomas P. Stockfisch, Thomas S. Thacher,
Lisa Yan, Xiangshan Ni, and Arnold T. Hagler
Table 1. Compounds Comprising the Training Set of Molecular Structures Used for Parameterizing the Force Field.
Acetals/hemiacetals
1,1,2-trihydroxyethane 1,1,3-trihydroxypropane
1,1,4-trihydroxyethylmethyl ether 1,1-dihydroxybutane
1,2,2-trihydroxypropane 1,3,5,7-tetraoxacane 1,3,5-trioxane
1,3-dihydroxymethylethyl ether 1,3-dioxane
1,3-dioxolane
2,2,3-trihydroxybutane
2-deoxy--D-glycerotetrafuranose 2-hydroxytetrahydrofuran
3-hydroxymethylethyl ether dihydroxymethyl ether dimethoxymethane hydroxyethoxymethane hydroxymethoxymethane Alcohols
Aldehydes/ketones acetaldehyde acetone butanone cyclobutanone cyclopropanone
cyclopropylcarboxaldehyde formaldehyde
isobutyraldehyde n-butyraldehyde propionaldehyde succinaldehyde Alkanes
1,1-dimethylcyclopropane 1,2-dimethylcyclopropane cyclobutane
cyclohexane cyclopentane cyclopropane ethane isobutane isopentane methane
methylcyclobutane methylcyclopropane n-butane
neopentane n-pentane propane Alkenes
1,3,6,8-nonatetraene 1,3-hexadiene 1,3-pentadiene 1,4-pentadiene 1,5-hexadiene 1-butene
1-methyl-cyclopentadiene 1-pentene
1-vinyl-cyclohexene 2-(isopropenyl)butadiene 2,3-dimethylbutadiene 2-butadienyl-butadiene 2-butene
2-ethyl-butadiene 2-methyl-1-butene 2-methyl-2,4-pentadiene 2-methyl-2-butene 2-pentene
2-vinyl butadiene
3-methyl-penta-1,3-diene 4,5-dimethyloctatetraene 4-methyl-1,3,5,8-octatetraene 4-methyl-1,3,6,8-nonatetraene 4-methyl-1,3-hexadiene butadiene
dodecahexaene ethylene
hexadecaoctaene hexatriene isobutene octatetraene propene
tetradecaheptaene Alkynes
1-butyne acetylene phenyl ethyne propyne Amides
acetamide
azacyclopropanone butyrolactam formamide
methylazacyclopropanone N,N-dimethylacetamide N,N-dimethylformamide N-ethylformamide N-formylalanineamide N-formylazeridine N-formylformamide N-formylglycineamide
N-formyl-N-methylglycineamide N-methylacetamide
Amines
azetidine aziridine
cyclobutylamine cyclopropylamine dimethylamine ethylamine ethylenediamine isopropylamine methylamine Ammonium cations
ammonium choline
dimethylammonium ethanolammonium methylethylammonium methylphosphorylcholine n-propylammonium t-butylammonium tetramethylammonium trimethylammonium Aromatic acids/esters
2-imidazolecarboxylic acid 2-methylbenzoic acid 2-pyridinecarboxylic acid 2-pyrimidinecarboxylic acid 4-imidazolecarboxylic acid
4-methyl-5-imidazolecarboxylic acid 5-imidazolecarboxylic acid
benzoic acid methyl benzoate
methyl-4-imidazolecarboxylate Aromatic alcohols/ethers
2-ethoxy pyridine 2-hydroxy imidazole 2-hydroxy pyridine 2-hydroxy pyrimidine 2-methoxy imidazole 2-methoxy pyridine 2-methoxy pyrimidine 4-ethoxy imidazole 4-hydroxy imidazole 4-methoxy imidazole 5-hydroxy imidazole anisole
ethoxy benzene Aromatic amides
1,2,3,5-tetrazole-4-carboxamide 2-furamide
2-methylbenzamide 2-pyridinecarboxamide 2-pyrimidinecarboxamide
3-methyl-pyridine-2-carboxamide 4-methyl-imidazole-5-carboxamide 5-methyl-imidazole-4-carboxamide benzamide
N-methyl-2-pyridinecarboxamide N-methyl-2-pyrimidinecarboxamide N-methyl-benzamide
N-methyl-imidazole-2-carboxamide N-methyl-imidazole-5-carboxamide N-methyl-oxazole-2-carboxamide N-methyl-pyrrole-3-carboxamide N-methyl-thiazole-2-carboxamide N-phenyl-acetamide
N-phenyl-benzamide N-phenyl-urea
oxazole-2-carboxamide pyrimidine-5-carboxamide pyrrole-3-carboxamide thiazole-2-carboxamide thiophene-2-carboxamide Aromatic amines
(2-methyl-phenyl) methyl amine (5-methyl-4-imidazolyl) methyl amine 2-imidazolyl ethyl amine
2-pyridyl methyl amine 2-pyrimidinyl methyl amine 4-imidazolyl ethyl methyl amine 4-imidazolyl methyl amine 5-imidazolyl methyl amine phenyl ethyl amine
phenyl ethyl methyl amine phenyl methyl amine Aromatic ammonium
2-methyl-anilinium 2-pyridyl ammonium 2-pyrimidinyl ammonium 3-pyridyl ammonium 4-imidazolyl ammonium
4-methyl-imidazolyl-5-ammonium 5-imidazolyl ammonium
5-methyl-imidazolyl-4-ammonium anilinium
N-methyl-imidazolyl-2-ammonium N-methyl-imidazolyl-4-ammonium N-methyl-imidazolyl-5-ammonium N-methyl-pyridyl-2-ammonium N-methyl-pyrimidinyl-2-ammonium Aromatic amidine cation
2-imidazole-carboxamidine 2-pyridine-carboxamidine 2-pyrimidine-carboxamidine
3-methyl-pyridine-2-carboxamidine 3-pyrrole-carboxamidine
4-imidazole-carboxamidine
4-methyl-imidazole-5-carboxamidine 5-imidazole-carboxamidine
5-pyrimidine-carboxamidine benzamidine
Aromatic rings
1,3,4-oxadiazole 1,3,4-thiadiazole benzene
imidazolium cation oxazole
pyrazine pyrazole pyridazine pyridine pyrimidine pyrrole thiazole thiophene triazine
Aromatic sulfones/sulfonamides 2-(methylsulfonyl)imidazole 2-(methylsulfonyl)pyridine 2-(methylsulfonyl)pyrimidine 3-(methylsulfonyl)pyrrole 4-(ethylsulfonyl)imidazole 4-(methylsulfonyl)imidazole 5-(methylsulfonyl)imidazole benzenesulfonamide
imidazole-2-sulfonamide imidazole-4-sulfonamide imidazole-5-sulfonamide methylphenyl-sulfone
N-ethyl-methanesulfonamide
N-ethyl-N-methyl-methanesulfonamide N-methyl-benzenesulfonamide
N-phenyl-methanesulfonamide pyridine-2-sulfonamide
Azoalkanes
1-pyrazoline azomethane
di(diazenyl)-ethane di(diazenyl)-methane diazene
ethyldiazene isopropyldiazene methyldiazine propyldiazene t-butyldiazene
tetrahydro-pyradazine Bridged aromatics
2-(fur-2-yl)pyrrole 2-(pyridin-2-yl)furan 2-(pyridin-2-yl)pyrrole 2-(pyridin-2-yl)thiophene 2-(thien-2-yl)furan 2-(thien-2-yl)pyrrole 2,2’-bifuran
5-(thien-2-yl)pyrazole 5,5’-bithiazole
biphenyl Carbamates
carbamic acid methylcarbamate
N,N-dimethyl carbamate N,N-dimethyl methylcarbamate N-methyl carbamate
N-methyl methylcarbamate Carbonates
carbonic acid dimethyl carbonate methyl carbonate Carboxylate anions
acetate formate propionate Carboxylic acids
acetic acid formic acid glycolic acid glyoxylic acid propionic acid pyruvic acid Clorinated hydrocarbons
1,2-dichlorobenzene
1,5-dimethyl-4-chloro-imidazole 2-chloroimidazole
4-chloroimidazole
4-methyl-5-chloro imidazole 4-trichloromethyl imidazole
4-trichloromethyl-5-methyl imidazole 5-chloroimidazole
5-trichloromethyl imidazole benzotrichloride
chlorobenzene chlorotoluene Dialkyl phosphate anions
dimethyl phosphate methylethyl phosphate Disulfides/sulfides/thiols
1,2-dithiethane 1,2-dithiirane
1,3-dithiacyclopentane
1-methyl-1-methylthiocyclopropane 1-methylcyclopropane-1-thiol 2-methylthioethane-1-thiol 2-methylthiopropane cyclobutanethiol cyclopentanethiol cyclopropanethiol dimethyl disulfide ethane-1,2-dithiol ethanethiol
ethyl hydrogen disulfide hydrogen disulfide methanethiol
methylthiocyclopentane methylthiocyclopropane methylthioethane methylthiomethane methylthiomethanethiol methylthiopropane propane-2-thiol propanethiol
tetrahydrothiophene thietane
thiirane Esters
3-formyl propanal acetolactone ethyl formate methyl acetate methyl formate methyl glycolate propiolactone Ethers
dimethyl ether methyl ethyl ether methyl isopropyl ether oxetane
oxirane
Fluorinated hydrocarbons 1,2-difluorobenzene
1-trifluoromethyl-2-methylbenzene 2-fluoro imidazole
4-fluoroimidazole
4-methyl-5-fluoroimidazole 4-trifluoromethyl imidazole
4-trifluoromethyl-5-methyl imidazole 5-fluoroimidazole
5-trifluoromethyl imidazole benzotrifluoride
fluorobenzene fluorotoluene Hydrazines
hydrazine
methyl hydrazine
N,N,N’-trimethyl hydrazine N,N’-dimethyl hydrazine N,N’-methylethyl hydrazine N,N-dimethyl hydrazine N,N-methylethyl hydrazine N-isopropyl hydrazine pyrazolidine
tetramethyl hydrazine Nitroaromatics
1-nitro-2-methyl benzene 2-nitroimidazole
4-methyl-5-nitroimidazole 4-nitro-5-methylimidazole 4-nitroimidazole
5-nitroimidazole nitrobenzene Phosphate dianions
Table 2. Quantum Mechanical Force Field Parameters.
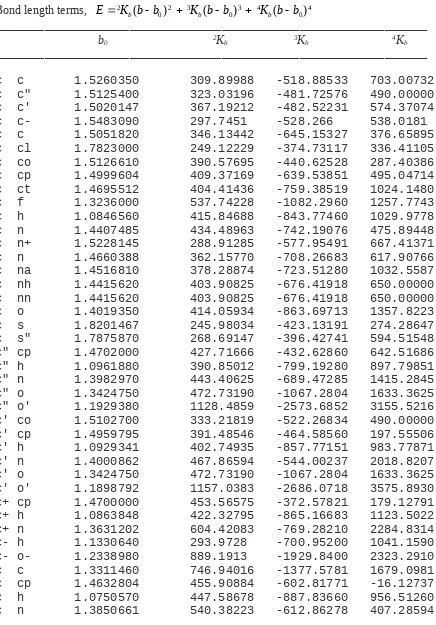
_____________________________________________________________ Bond length terms, E2K b bb K b bb K b bb
0 2 3 0 3 4 0 4
( ) ( ) ( )
_____________________________________________________________
b0 2Kb 3Kb 4Kb
_____________________________________________________________
np sp 1.6485995 364.49484 -545.62257 650.00000 o p 1.6354935 398.67842 -622.23348 826.84440 o p- 1.6997274 341.95620 -557.68919 360.06776 o' s" 1.435736 814.84012 -1897.2198 2627.9370 o- p 1.4703638 785.88168 -1629.6214 2056.5623 o- p- 1.4968054 683.42789 -1401.9900 1933.7442 s s 2.0818932 212.21289 -102.04010 820.35444
_____________________________________________________________
Bond angle terms, E2K K K
0 2 3 0 3 4 0 4
( ) ( ) ( )
__________________________________________________________________
0 2K 3K 4K
__________________________________________________________________
cp sp cpb 91.936733 105.41543 -1.9188303 -21.287306 cp sp np 92.774216 86.161137 -2.2523088 -17.443818 np sp np 92.134128 214.89015 -4.3097771 -43.389864
_____________________________________________________
Torsion terms, E 1K 1 2K 1 2 3K 1 3 ( cos ) ( cos ) ( cos )
_____________________________________________________
1K
2K 3K
_____________________________________________________
cp np np cp 0.0000000 6.5781347 0.0000000 cp np np nh 0.0000000 12.174899 0.0000000 cp np np np 0.0000000 11.553981 0.0000000 cp np np op 0.0000000 7.9409292 0.0000000 cp np np sp 0.0000000 9.4254789 0.0000000 cpb np np nh 0.0000000 12.174899 0.0000000 nh np np np 0.0000000 12.189261 0.0000000 np np np np 0.0000000 17.747821 0.0000000 cp np op cp 0.0000000 4.7482919 0.0000000 cp np op np 0.0000000 7.5296380 0.0000000 cpb np op cp 0.0000000 4.7482919 0.0000000 np np op cp 0.0000000 8.7762279 0.0000000 cp np sp cp 0.0000000 1.7246826 0.0000000 cp np sp np 0.0000000 7.8825686 0.0000000 np np sp cp 0.0000000 12.302920 0.0000000 c o p o 0.2930291 0.6805675 -0.1131336 c o p o- 0.2227508 1.0763550 -0.0456873 c o p- o- 2.1848923 1.9290247 0.0135564 cp o p- o- 0.2972287 0.4133854 0.6403414 h o p- o- -0.1640977 0.0892636 -0.0876827 c s s c -0.9068356 -3.3890910 -0.6594178 c s s h -0.3561881 -3.3826880 -0.3484207 h s s h -0.1274146 -3.5179435 -0.2618985
__________________________________________________________________ Electrostatic energy, for atom pair i and j, E 332q q ri j/ ij
where qi ik k
and ik >0 for charge donated from atom i to atom k
_______________________________________________________________________________
i k ik
__________________________
br br 0.0000 br c -0.1920 br c" -0.0800 br c' -0.0800 br c- -0.0281 br c -0.0800 br cl 0.0507 br cp -0.0800 br ct 0.0173 br f 0.2099 br h -0.1978 br i -0.0110 br n 0.1422 br n+ 0.2452 br n 0.1422 br na 0.0601 br nh -0.0438 br nn 0.1422 br np 0.1422 br o 0.0818 br o' 0.3140 br op 0.3140 br p -0.2156 br s -0.0437 br s" 0.0034 br sp 0.0034 c c 0.0000 c c" 0.0000000 c c' 0.0000000 c c- -0.2300 c c 0.0000000 c cl -0.011 c co 0.0000000 c cp 0.0000000 c ct 0.0441500 c f 0.13275 c h -0.0530 c hc+ -0.2270000 c i 0.1120 c n 0.0000000 c n+ 0.205
c op 0.3957 c p 0.0110 c s 0.065000 c s" -0.0500000 c sp 0.1180 c" c" 0.0000 c" c' 0.0000 c" c- -0.1368 c" c 0.0000 c" cl 0.1020 c" co 0.0000000 c" cp 0.065 c" ct -0.0927 c" f 0.1300 c" h -0.0456000 c" i -0.1291 c" n 0.0000000 c" n+ 0.1331
c+ cp 0.0 c+ h -0.1268 c+ n -0.0680 c+ nr -0.0680 c- c- 0.0000 c- cl 0.0747 c- cp -0.0424 c- ct 0.0432 c- f 0.2241 c- h -0.0530 c- i 0.0185 c- n 0.1607 c- n+ 0.2597
c- n 0.1607 c- nh -0.0176 c- nn 0.1607 c- np 0.1607 c- o 0.1012 c- o' 0.3241 c- o- 0.0337 c- op 0.3241 c- p -0.0857 c- s -0.0087 c- s- -0.1223
c c 0.0000000 c cl 0.1020 c cp 0.03 c ct 0.0852 c f 0.1300 c h -0.1268000 c i 0.0642 c n 0.07
c n+ 0.2989 c n 0.36
cl i -0.0623 cl n 0.0897 cl n+ 0.1858 cl n 0.0897 cl na 0.0117 cl nh -0.0854 cl nn 0.0897 cl np 0.0897 cl o 0.0367 cl o' 0.2585 cl op 0.2585 cl p -0.2544 cl s -0.0898 cl s" -0.0457 cl sp -0.0457 co h -0.0530000 co na 0.0827000 co nh 0.2108000 co o 0.1133000 cp cp 0.0000000 cp cpb 0.0000000 cp ct 0.0790400 cp f 0.116 cp h -0.14 cp i 0.0642 cp n 0.1746000 cp n' 0.2 cp n+ 0.2989
cp n 0.1380000 cp nh 0.16 cp nn 0.04 cp np 0.18 cp o 0.1500000 cp o' 0.3964 cp op 0.11 cp s -0.0120 cp s" 0.0732 cp sp -0.02 cpb cpb 0.0000000 cpb nh 0.16 cpb nn 0.0000000 cpb np 0.18 cpb op 0.11 cpb sp -0.02 cr n 0.1380 cr nr .1746
ct f 0.1873 ct h -0.2183300 ct i -0.0281 ct n 0.1204 ct n+ 0.0992
ct n 0.1204 ct na -0.0636 ct nh -0.0568 ct nn 0.0920 ct np 0.1204 ct o 0.0675 ct o' 0.2874 ct op 0.2874 ct p -0.1335 ct s -0.0581 ct s" -0.0135 ct sp -0.0135 f f 0.0000 f h -0.3823 f i -0.2234 f n -0.0731 f n+ 0.0062 f n -0.0731 f na -0.1415 f nh -0.2220 f nn -0.0731 f np -0.0731 f o -0.1077 f o' 0.0888 f op 0.0888 f p -0.3869 f s -0.2380 f s" -0.2011 f sp -0.2011 h h 0.0000 h i 0.1923 h o' 0.4943 h op 0.4943 h p -0.0356 h s 0.1900000 h s" 0.0500000 h sp 0.1932 h n 0.0000000 h n' 0.4
h nn 0.37 h np 0.3278 h nr 0.4068 h o 0.4241000 h o 0.3991 h+ n+ .2800000 h+ nh 0.3700 i i 0.0000 i n 0.1554 i n+ 0.2615 i n 0.1554 i na 0.0714 i nh -0.0356 i nn 0.1554 i np 0.1554 i o 0.0924 i o' 0.3297 i op 0.3297 i p -0.2110 i s -0.0345 i s" 0.0140 i sp 0.0140 n n 0.0000 n n+ 0.0883
n+ s -0.2755 n+ s" -0.2341 n+ sp -0.2341
__________________________________________________________________ Van der Waals interaction energy, for atom pair i and j,
E[ ( */ )2 r r 9 3( */ ) ]r r 6 where
r* [( * ri 6rj* )/ ] ,6 21 6/ ( i j)1 2/ 2( * *) /( *r ri j 3 ri 6 rj* )6
__________________________________________________________________
atom r*
______________________________________
nn 3.7000000 0.1700000 np 3.7000000 0.1700000 o 3.5350000 0.2400000 o' 3.5350000 0.2670000 o 3.6080 0.2740 o- 3.5960000 0.1670000 op 3.5350000 0.1090000 p 4.2000000 0.2000000 p- 4.2000000 0.2000000 s 4.0270000 0.0710000 s" 4.0270000 0.0710000 sp 4.0270000 0.0710000 ______________________________________ Bond-bond interaction terms,
EKbb´(b b b b 0)( ´ 0´)
______________________________________
Kbb´
______________________________________
_______________________________
Bond-angle interaction terms, _______________________________
EK b bb( 0)( 0)
The first two atoms listed form the bond. _______________________________
Kb
_______________________________
h s" o' -2.6941575 n s" c 34.057933 n s" cp 26.702044 n s" h 64.795392 n s" o' 82.126216 o' s" c 64.225460 o' s" cp 70.282762 o' s" h 70.658014 o' s" n 71.605035 o' s" o' 62.917644 cp sp cp 0.0000000 cp sp cpb 0.0000000 cp sp np 4.9599678 cpb sp cp 0.0000000 np sp cp 86.829685 np sp np 145.75877
_______________________________
Angle-angle interaction terms, EK´( 0)(´0´)
The first 3 atoms define the first angle. the second 3 atoms the second angle.
_______________________________ K´
_______________________________ c c c c -0.2010870
c s" cp o' 12.472972 n s" cp o' 31.425422 o' s" cp o' 31.313629 c s" h o' 73.817972 h s" h o' 81.357701 n s" h o' 70.343039 o' s" h o' 94.781438 c s" n o' 43.084921 cp s" n o' 23.902135 h s" n o' 39.712683 o' s" n o' 66.606522 c s" o' c 22.014898 c s" o' cp 12.786932 c s" o' h 26.903645 c s" o' n 21.950105 c s" o' o' 14.372797 cp s" o' n 11.397878 cp s" o' o' 10.909840 h s" o' h 33.246240 h s" o' n 32.458035 h s" o' o' 16.745065 n s" o' o' 10.946279
___________________________________________________
Angle-torsion interaction terms,
E( 0)(1K(1 cos ) 2K(1 cos )2 3K(1 cos )3
The first 3 atoms denote the angle.
___________________________________________________
1K
2K
3K
___________________________________________________
cp np sp cp 0.0000000 -18.067722 0.0000000 cp sp np cp 0.0000000 0.0000000 0.0000000 cp np sp np 0.0000000 -32.154493 0.0000000 np sp np cp 0.0000000 -92.681625 0.0000000 cp sp np np 0.0000000 9.6677462 0.0000000 np np sp cp 0.0000000 0.0000000 0.0000000 c o p o -.7624835 .0000000 -.1917483 o p o c .7025883 1.4881885 .6918788 c o p o- .2447691 .0000000 -.1490948 o- p o c 1.0224856 2.7888059 -.0455052 c o p- o- 3.9202442 2.6763874 -0.7781807 o- p- o c 4.9698831 5.8822768 -1.3708918 cp o p- o- -1.1851771 -0.1080315 -0.7516878 o- p- o cp 0.1471697 2.1542669 -2.5203351 h o p- o- 0.5457203 -0.3040876 -0.2517514 o- p- o h -3.2598969 1.4348270 -0.3098329 c s s c -0.8891659 4.4206322 -0.8057607 c s s h -2.8675863 4.7025788 -0.2965184 h s s c -1.4888296 3.6333475 0.3498797 h s s h -2.2328295 2.7400153 -0.6892629
___________________________________________________
Central bond-torsion interaction terms, E (b b0)(1Kb(1 cos ) 2Kb(1 cos )2 3Kb(1 cos )3
___________________________________________________
1K
b
2K
b
3K
b
___________________________________________________
________________________________________________________ Terminal bond-torsion interaction terms,
E(b b´ 0´)(1Kb´(1 cos ) 2Kb´(1 cos )2 3Kb´(1 cos )3
The first 2 atoms denote the bond.
________________________________________________________
1K
b
´ 2Kb´ 3Kb´
cp np np sp 0.0000000 -15.718746 0.0000000 sp np np cp 0.0000000 0.0000000 0.0000000 cpb np np nh 0.0000000 -48.580227 0.0000000 nh np np cpb 0.0000000 0.5298679 0.0000000 nh np np np 0.0000000 -17.936306 0.0000000 np np np nh 0.0000000 -35.999054 0.0000000 np np np np 0.0000000 -20.659569 0.0000000 cp np op cp 0.0000000 -16.867844 0.0000000 cp op np cp 0.0000000 0.0000000 0.0000000 cp np op np 0.0000000 -2.1974554 0.0000000 np op np cp 0.0000000 11.286416 0.0000000 cp op np cpb 0.0000000 0.0000000 0.0000000 cpb np op cp 0.0000000 -16.867844 0.0000000 cp op np np 0.0000000 0.0000000 0.0000000 np np op cp 0.0000000 0.0000000 0.0000000 cp np sp cp 0.0000000 12.554767 0.0000000 cp sp np cp 0.0000000 17.193865 0.0000000 cp np sp np 0.0000000 26.007690 0.0000000 np sp np cp 0.0000000 0.0000000 0.0000000 cp sp np np 0.0000000 0.0000000 0.0000000 np np sp cp 0.0000000 66.445782 0.0000000 c o p o -.4593485 -1.1087339 -.2708256 o p o c -6.0582395 1.6908438 .3746805 c o p o- -3.0190987 -1.3092218 -.0912146 o- p o c -5.7884348 .5788403 -.2691330 c o p- o- -5.3689239 -2.1094737 -3.3588287 o- p- o c -5.2084509 0.3831170 -0.7032106 cp o p- o- -2.8580464 -2.5796830 -3.4045218 o- p- o cp -4.8894572 0.2397342 -3.2676444 h o p- o- 1.2818410 1.3346600 1.4934464 o- p- o h -3.6855186 -0.2406764 -0.0277279 c s s c 0.1928236 0.0700661 -0.1260744 c s s h 0.3317717 1.1555102 -0.0994404 h s s c -0.0733998 0.4863718 0.2161753 h s s h 0.1527093 0.1702318 0.1954227 ______________________________________________
Angle-angle-torsion interaction terms EK ´ ( 0)( ´ 0´)cos
______________________________________________ K ´
______________________________________________
c o p o- -12.755978 c o p- o- -28.006783 cp o p- o- -7.8604649 h o p- o- -2.7406064 c s s c -53.231114 c s s h -37.742894 h s s h -23.899085