Principles of Bacterial Detection:
Biosensors, Recognition
Receptors and Microsystems
Edited by
MOHAMMED ZOUROB
Biophage Pharma Inc.Montreal, Canada
SOUNA ELWARY
Consultant to Biophage Pharma Inc. Montreal, CanadaANTHONY TURNER
Cranfield UniversityBedfordshire, UK
Mohammed Zourob Souna Elwary
Biophage Pharma Inc. Consultant to Biophage Pharma Inc.
Montreal Montreal
Canada Canada
[email protected] [email protected]
Anthony Turner Cranfield University Bedfordshire UK
ISBN: 978-0-387-75112-2 e-ISBN: 978-0-387-75113-9
Library of Congress Control Number: 2007941938
© 2008 Springer Science+Business Media, LLC
All rights reserved. This work may not be translated or copied in whole or in part without the written permission of the publisher (Springer Science+Business Media, LLC, 233 Spring Street, New York, NY 10013, USA), except for brief excerpts in connection with reviews or scholarly analysis. Use in connection with any form of information storage and retrieval, electronic adaptation, computer software, or by similar or dissimilar methodology now known or hereafter developed is forbidden.
The use in this publication of trade names, trademarks, service marks, and similar terms, even if they are not identified as such, is not to be taken as an expression of opinion as to whether or not they are subject to proprietary rights.
Printed on acid-free paper
9 8 7 6 5 4 3 2 1
Preface
Bacterial contamination of food and water resources, as well as the increasing incidence of nosocomial infections, has us on our toes, looking for ways of recognizing these elements. In addition, the recent and growing threats to personal and territorial securities make this task even more urgent. Therefore, accurate assessment of the state of current technologies is a prerequisite for undertaking any course of action towards future improvements. In particular, development of new detection and identification technologies for the plethora of bacterial agents has become increasingly important to scientists and to regulatory agencies. In recent years, there has been much progress in the field of bacterial agents detection, resulting in the development of more accurate, fast, analyte-specific, robust, and cost effective techniques by incorporating emerging technologies from various disciplines.
Principles of Bacterial Detection: Biosensors, Recognition Receptors and Microsystems presents a significant and up-to-date review of various integrated approaches for bacterial detection by distinguished engineers and scientists. This work is a comprehensive approach to bacterial detection, presenting a thorough knowledge of the subject and an effective integration of disciplines in order to appropriately convey the state-of the-art fundamentals and applications of the most innovative approaches.
The book consists of four parts. The first part (Chapters 1–4) is an introduction to pathogenic bacteria and sampling techniques and provides an overview of the rapid microbio-logical methods. The second part (Chapters 5–20) describes the different transducers used for bacterial detection. It covers the theory behind each technique and delivers a detailed state-of-the-art review for all the new technologies used. The third part (Chapters 21–29) covers the different recognition receptors used in the latest methods for the detection of bacteria. It describes in detail the use of immunoassays, nucleic acids, oligonucleotide microarrays, carbohydrates, aptamers, protein microarrays, bacteriophage, phage display, and molecular imprinted polymers as recognition elements. The fourth part (Chapters 30–36) covers the different microsystems used for detection/identification and bacterial manipulations, mainly bacteria lysis in microfluidics, PCR in microfluidics, dielectrophoresis, ultrasonic manipulation techniques, and mass spectrometry.
We anticipate that the book will be helpful to academicians, practitioners, and professionals working in various fields, including biomedical sciences, physical sciences, microsystems engineering, nanotechnology, veterinary science and medicine, food QA, bioter-rorism and security as well as allied health, healthcare and surveillance. Since the fundamentals are also reviewed, we believe that the book will appeal to advanced undergraduate and graduate students who study in areas related to bacterial detection.
We gratefully acknowledge all authors for their participation and contributions, which made this book a reality. We give many thanks to Olivier Laczka and Joseph Piliero for the book cover design.
Mohammed Zourob Souna Elwary Anthony Turner June 2008
Contents
Part I
Introduction
1. Introduction to Pathogenic Bacteria
Tracey Elizabeth Love and Barbara Jones
1. Pathogenic Microorganisms... 3
1.1. Toxins ... 4
1.2. Adherence ... 4
1.3. Invasion... 7
1.4. Evasion of the Host Immune Response ... 7
1.5. Iron Acquisition ... 8
1.6. Regulation of Virulence Factors ... 8
2. Sources and Routes of Infection... 9
2.1. Natural Infection... 9
2.2. Food and Water ... 9
2.3. Hospital Acquired Infections... 10
2.4. Intentional Infection—Biological Warfare... 10
3. Detection of Pathogenic Microorganisms... 11
4. Conclusions ... 12
References... 12
2. Sample Preparation: An Essential Prerequisite for High-Quality Bacteria Detection Jan W. Kretzer, Manfred Biebl and Stefan Miller 1. Introduction ... 15
2. The Sample... 16
3. Sampling... 17
3.1. Sample drawing ... 17
4. Microbiological Examination of Foods ... 17
5. Microbiological Examination of Surfaces ... 17
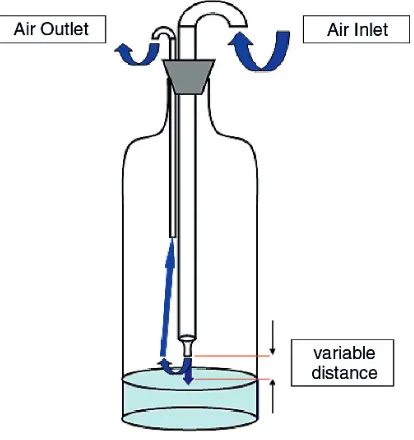
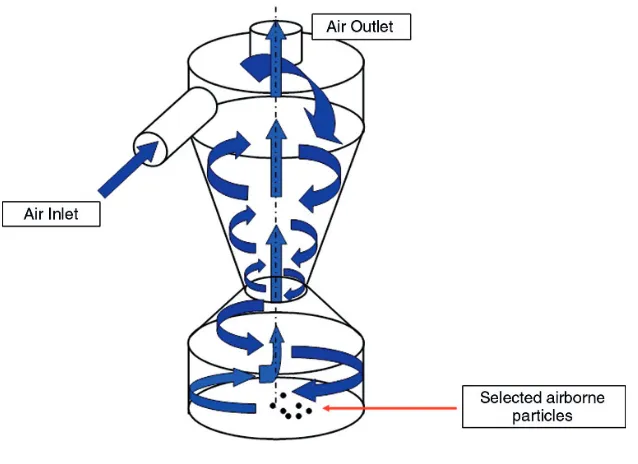
6. Microbiological Examination of Air... 18
7. Sample Handling ... 20
8. Sample Preparation ... 21
9. Sample Preparation for Detection of Intact Bacterial Cells... 21
10. Sample Preparation for Detection of Bacterial Nucleic Acids ... 23
11. Conclusions and Future Perspectives ... 27
References... 28
3. Detection of Bacterial Pathogens in Different Matrices: Current Practices and Challenges Ahmed E. Yousef 1. Introduction ... 31
2. Analytical Tools and Methods: A Historical Perspective ... 32
3. Defining the Terms ... 32
4. Matrix Complexity and Pathogen Detection ... 32
5. Techniques Currently Used in Pathogen Detection Methods ... 33
5.1. Culture Techniques ... 33
5.2. Enzyme-Linked Immunoassay... 35
5.3. Polymerase Chain Reaction (PCR) ... 36
6. Basics of Pathogen Detection ... 36
6.1. Sampling ... 37
6.1.1. Air Sampling... 37
6.1.2. Surfaces Sampling ... 37
6.1.3. Bulk Sampling ... 39
6.2. Sample Preparation ... 39
6.3. Pathogen Amplification ... 39
6.4. Selection and Screening... 40
6.5. Identification ... 40
6.5.1. Morphological Characteristics... 41
6.5.2. Biochemical and Physiological Traits... 41
6.5.3. Serological Properties... 42
6.5.4. Genetic Characteristics ... 42
6.6. Pathogenicity Testing... 43
6.6.1. Koch’s Postulates... 43
6.6.2. Mammalian Cell Culture (Tissue Culture)... 43
6.6.3. Virulence Genes and Gene Expression Products... 44
6.7. Testing for Specific Traits ... 44
7. Challenges to Current Detection Methods... 44
7.1. Pathogen Quantification Problems ... 44
7.2. Can a Small Bacterial Population be Detected Rapidly and Reliably?... 44
7.3. Which Traits to Analyze, and How Many Tests are Needed for Identifying a Bacterial Pathogen? ... 45
7.4. Real-Time Detection... 46
References... 46
4. Overview of Rapid Microbiological Methods Jeanne Moldenhauer 1. Introduction ... 49
2. A History of Rapid Microbiological Methods: Industry Reluctance to Accept These Methods ... 50
3. Types of Microbial Testing Performed ... 50
4. Types of Rapid Microbiological Methods... 50
4.1. Growth-Based Technologies... 50
4.2. Viability-Based Technologies... 50
4.3. Cellular Component or Artifact-Based Technologies... 51
4.4. Nucleic Acid-Based Technologies ... 51
4.5. Automated Methods... 51
4.6. Combination Methods... 51
5. Overview of Rapid Technologies and How They Work ... 51
5.1. Adenosine Tri-Phosphate (ATP) Bioluminescence ... 51
5.2. Adenylate Kinase... 52
5.3. Autofluorescence ... 52
5.4. Biochemical Assays and Physiological Reactions... 52
5.5. Biosensors and Immunosensors ... 53
5.6. Carbon Dioxide Detection... 53
5.8. Colorimetric Detection of Carbon Dioxide Production... 53
5.9. Concentric Arcs of Photovoltaic Detectors with Laser Scanning... 54
5.10. Direct Epifluorescent Filter Technique (DEFT)... 54
5.11. DNA Sequencing... 54
5.12. Endospore Detection ... 55
5.13. Enzyme Linked Immunosorbent Assay (ELISA)... 55
5.14. Flow Cytometry... 55
5.15. Fluorescent Probe Detection ... 55
5.16. Fatty Acid Profiles (Fatty Acid Methyl Esters, FAMEs)... 56
5.17. Fourier Transformed Infrared Spectroscopy (FTIR) ... 56
5.18. Gram Stains (Rapid Method) ... 56
5.19. Impedance... 57
5.20. Immunological Methods... 57
5.21. Lab-on-a-Chip (LOC), Arrays, Microarrays and Microchips ... 57
5.22. Limulus Amebocyte Lysate (LAL) Endotoxin Testing... 58
5.23. Mass Spectrometry (Matrix-Assisted Laser Desorption-Time of Flight (MALTI-TOF))... 58
5.24. Microcalorimetry ... 58
5.25. Micro-Electro-Mechanical Systems (MEMS) ... 59
5.26. Nanotechnology... 59
5.27. Near Infrared Spectroscopy (NIRS)... 59
5.28. Nucleic Acid Probes... 59
5.29. Optical Particle Detection ... 59
5.30. Polymerase Chain Reaction (PCR) ... 60
5.31. Rep-PCR ... 60
5.32. Raman Spectroscopy ... 61
5.33. Ribotyping/Molecular Typing ... 61
5.34. Solid Phase Laser Scanning Cytometry... 61
5.35. Southern Blotting/Restriction Fragment Length Polymorphism ... 62
5.36. Spiral Plating ... 62
5.37. Turbidimetry ... 62
6. Potential Areas of Application of Rapid Microbiological Methods ... 62
7. Disclaimer... 75
8. Conclusions ... 75
References... 75
Part II
Biosensors
5. Surface Plasmon Resonance (SPR) Sensors for the Detection of Bacterial Pathogens Allen D. Taylor, Jon Ladd, Jiˇrí Homola and Shaoyi Jiang 1. Introduction ... 832. Fundamentals of Surface Plasmon Resonance Biosensing ... 83
3. SPR Sensor Instrumentation ... 85
4. Surface Chemistries and Molecular Recognition Elements ... 88
5. Detection Formats ... 90
6. Quantification of Bacteria Cells ... 91
6.1. Challenges for the Detection of Whole Bacteria by SPR... 91
6.2. Effect of Bacteria Sample Treatment ... 92
6.3. Examples of Bacteria Detection ... 92
6.3.1. Escherichia coli... 93
6.3.2. Salmonellaspp. ... 97
6.3.4. Other Bacteria ... 98
6.3.5. Detection of Multiple Bacteria ... 99
7. Genetic Markers ... 101
8. Antibody Biomarkers ... 103
9. Conclusions and Future Perspectives ... 103
References... 104
6. Bacterial Detection Using Evanescent Wave-Based Fluorescent Biosensors Kim E. Sapsford and Lisa C. Shriver-Lake 1. Introduction ... 109
2. Current State of Bacterial Fluorescent TIRF Biosensors... 112
2.1. Non-Planar Substrates... 112
2.1.1. Fiber Optics ... 112
2.1.2. Capillaries... 112
2.2. Planar Substrates... 112
2.2.1. NRL Array Biosensor ... 113
2.2.2. Other Optical Waveguides ... 115
2.2.3. TIRF-Microscopy ... 116
3. Future Aspects of Bacterial Fluorescent TIRF Biosensors ... 117
4. Conclusions ... 119
References... 120
7. Fiber Optic Biosensors for Bacterial Detection Ryan B. Hayman 1. Fiber Optic Biosensors... 125
1.1. Whole-Cell Detection ... 126
1.1.1. Evanescent-Field Sensing ... 126
1.1.2. Sandwich Immunoassays ... 127
1.2. Bead-Based Arrays ... 128
1.3. Nucleic Acid Sandwich Assays... 129
1.4. Nucleic Acid Direct Hybridization... 131
1.5. Extension Reactions... 134
2. Conclusions and Future Perspectives ... 134
References... 135
8. Integrated Deep-Probe Optical Waveguides for Label Free Bacterial Detection Mohammed Zourob, Nina Skivesen, Robert Horvath, Stephan Mohr, Martin B. McDonnell and Nicholas J. Goddard 1. Introduction ... 139
1.1. Planar Optical Waveguides... 141
1.2. Total Internal Reflection and Evanescent Waves ... 141
1.3. Waveguide Modes ... 143
1.4. Frustrated Total Internal Reflection, Leaky Modes ... 144
1.5. Literature on Waveguides for Bacterial Detection ... 144
2. Deep-Probe Optical Waveguide Sensors with Tunable Evanescent Field ... 145
2.1. Waveguide Modes, Light Coupling and Sensing Depths of Evanescent Waves... 146
2.2. Waveguide Designs Based on Low-Index Substrates... 150
2.2.1. Bacteria Detection Using Reverse Symmetry Waveguides ... 151
2.3. Waveguide Designs Based on Metal- and Dye-Clad Substrates—Leaky Modes... 152
2.3.1. Results ... 156
3. Integrated Deep-Probe Optical Waveguides Systems ... 160
3.1. Integration with Electric Field... 161
3.2. Integration with Ultrasound Standing Waves (USW)... 163
4. Conclusions and Future Perspectives ... 166
References... 166
9. Interferometric Biosensors Daniel P. Campbell 1. Principles of Optical Interferometry ... 169
1.1. Optical Waveguides ... 171
1.2. Planar Waveguide Operation... 172
1.3. Types of Waveguides ... 175
2. Light Coupling Methods ... 178
2.1. Interferometers ... 180
2.2. Collinear or Single Channel Interferometers ... 183
2.3. Two-Channel Interferometers... 186
3. Interferometric Array Sensors... 192
4. Surface Plasmon Interferometry ... 195
5. Other Interferometric Methods and Designs ... 196
6. Surface Functionalization... 197
7. Sample Collection Systems... 198
8. Interferometric Applications for Whole-Cell Detection... 199
9. Advantages and Limitations... 206
10. Potential for Improving Current Performance... 206
References... 208
10. Luminescence Techniques for the Detection of Bacterial Pathogens Leigh Farris, Mussie Y. Habteselassie, Lynda Perry, S. Yanyun Chen, Ronald Turco, Brad Reuhs and Bruce Applegate 1. Beyond Robert Boyle’s Chicken... 214
2. The Bacterial (lux) Luminescent System for Direct Pathogen Detection... 215
3. The Firefly (luc) Luminescent System for Direct Pathogen Detection... 219
4. The Use of Alternative Luciferases in Pathogen Detection ... 222
5. Luminescent-Based Immunoassays... 222
6. Chemiluminescence Detection Methods ... 222
7. Conclusions and Future Perspectives ... 225
References... 226
11. Porous and Planar Silicon Sensors Charles R. Mace and Benjamin L. Miller 1. Introduction... 231
1.1. Porous Silicon: A Three-Dimensional Matrix for Biosensing ... 232
1.2. Effect of PSi Immobilization on Probe Viability: Experiments with GST ... 233
1.3. Toward Larger Targets: The First Macroporous Microcavity Structures... 235
1.4. Porous Silicon Bandgap Sensors in Novel Formats: “Smart Bandages” and “Smart Dust” ... 235
2. Arrayed Imaging Reflectometry—A Planar Silicon Biosensor... 236
2.1.1. Physical Rationale... 236
2.1.2. Substrate Design ... 237
2.1.3. Mathematical Model ... 238
2.1.4. Monitoring the Null Reflectance Condition... 240
2.2. Applications of AIR Biosensing ... 242
2.2.1. Limitations ... 242
2.2.2. Probe Immobilization ... 244
2.2.3. Pathogen Detection ... 246
3. Conclusions and Future Perspectives ... 250
References... 251
12. Acoustic Wave (TSM) Biosensors: Weighing Bacteria Eric Olsen, Arnold Vainrub and Vitaly Vodyanoy 1. Introduction... 255
2. Historical Perspective, Theory and Background... 256
2.1. Piezoelectricity and Acoustic Waves... 256
2.2. Acoustic Wave Devices ... 256
3. TSM Biosensors... 259
3.1. Detection of Microorganisms... 261
3.2. Measurement in Liquid ... 263
3.3. TSM Biosensor Characteristics... 264
3.4. Commercial TSM Microbalances ... 267
3.5. Immobilization of Probes onto Sensor Surface ... 269
3.5.1. Physical Adsorption... 271
3.5.2. Other Coupling Methods ... 272
3.5.3. Combined Langmuir-Blodgett/Molecular Assembling Method ... 272
3.5.4. Solvent-Free Purified Monolayers... 275
3.5.5. Immobilization of Monolayers of Phage Coat Proteins ... 276
3.5.6. Immobilization of Molecular Probes onto Porous Substrates ... 281
4. Problem of “Negative Mass”... 282
5. Coupled Oscillators Model... 286
6. Conclusions... 290
References... 291
13. Amperometric Biosensors for Pathogenic Bacteria Detection Ilaria Palchetti and Marco Mascini 1. Introduction... 299
2. Amperometric Biosensors... 300
2.1. Microbial Metabolism-Based Biosensors ... 302
2.2. Immunosensors ... 303
2.3. DNA-Based Biosensors... 306
3. Conclusion and Future Perspectives... 310
References... 310
14. Microbial Genetic Analysis Based on Field Effect Transistors Yuji Miyahara, Toshiya Sakata and Akira Matsumoto 1. Introduction... 313
2. Fundamental Principles of Field Effect Devices ... 314
2.1. Metal-Insulator-Semiconductor (MIS) Capacitor ... 314
3. Fundamentals of Genetic Analysis... 317
3.1. DNA... 317
3.2. Genetic Analysis... 317
3.3. DNA Chip / DNA Microarray ... 318
4. Immobilization of DNA Molecules on the Surfaces of Solid Substrates ... 318
4.1. Silanization ... 318
4.2. Thiol-Gold Bonding ... 320
4.3. Avidin, Streptavidin and Biotin ... 320
4.4. Others... 321
5. Genetic Analysis Based on Field Effect Devices ... 322
5.1. Fundamental Characteristics of Genetic Field Effect Devices... 322
5.1.1. Detection of DNA Molecular Recognition Events ... 322
5.1.2. Immobilization Density of Oligonucleotide Probes... 326
5.2. Single Nucleotide Polymorphisms (SNPs) Analysis ... 327
5.2.1. Controlling Hybridization Temperature for SNPs Analysis... 328
5.2.2. SNPs Analysis Based on Primer Extension ... 329
5.3. DNA Sequencing... 331
6. Conclusions and Future Perspectives ... 335
References... 336
15. Impedance-Based Biosensors for Pathogen Detection Xavier Muñoz-Berbel, Neus Godino, Olivier Laczka, Eva Baldrich, Francesc Xavier Muñoz and Fco. Javier Del Campo 1. Introduction... 341
2. Fundamentals of Electrochemical Impedance Spectroscopy... 342
2.1. Data Analysis: Plotting... 344
2.2. Data Analysis: Interpretation ... 344
2.2.1. Non-Faradaic Parameters... 345
2.2.2. Faradaic Parameters... 347
2.3. Measuring at Impedimetric Biosensors... 350
2.3.1. Measurement Modes... 350
2.4. Bacterial Parasitizing Effect on Electrode Surface... 353
3. Development of an Immunosensor... 354
3.1. Biological Recognition Elements in Biosensors for Pathogen Detection... 354
3.1.1. Antibodies ... 355
3.1.2. Nucleic Acids... 355
3.1.3. Aptamers ... 356
3.1.4. Other Recognition Strategies... 356
3.2. Surface Modification Methods... 357
3.2.1. Adsorption... 357
3.2.2. Self-assembled Monolayers... 358
3.2.3. Silanisation... 359
3.2.4. Protein A and Protein G ... 360
3.2.5. The Biotin-(Strept)Avidin System... 360
3.2.6. Chemical Conjugation ... 361
3.2.7. Entrapment ... 362
3.2.8. Microencapsulation... 362
3.3. Blocking... 362
3.4. Signal Amplification ... 363
3.5. The Need for Negative Controls... 364
3.6. Development of Novel Strategies: Assessing Performance Using ELISA and Microscopy ... 365
4. Current EIS Biosensors for Pathogen Detection... 365
4.2. Biosensors Based on Charge-Transfer Resistance Changes... 367
4.3. Biosensors Based on Conductivity Changes ... 369
4.4. Other Approaches... 370
5. Conclusions and Future Perspectives ... 370
References... 371
16. Label-Free Microbial Biosensors Using Molecular Nanowire Transducers Evangelyn Alocilja and Zarini Muhammad-Tahir 1. Introduction... 377
1.1. Rationale for Rapid Tests... 377
1.2. Target Microorganisms and Matrices ... 378
1.2.1.Escherichia coli... 378
1.2.2.Salmonella... 379
1.2.3. Bovine Viral Diarrhea Virus ... 380
1.3. Food Safety Applications ... 381
2. Biosensor Formats ... 382
2.1. Definition... 382
2.2. Antibodies as Biological Sensing Element... 382
2.3. DNA as Biological Sensing Element... 384
2.4. DNA-Based Biosensors... 385
2.5. Antibody-Based Biosensors ... 387
2.6. Biosensor Transducing Element: Conducting Polymer... 388
2.6.1. Polyaniline ... 390
2.6.2. Self-doped Polyaniline... 391
2.6.3. Carbon Nanotubes... 391
2.7. Conducting Polymer-Based Biosensor for Microbial/Viral Detection... 392
3. Illustration: Biosensor Using Self-doped and Non-self-doped Pani... 392
3.1. Pani Preparation... 392
3.2. Pani Characterization... 392
3.2.1. Conductivity Measurement... 392
3.2.2. Biosensor Fabrication ... 393
3.2.3. Indium Tin Oxide/Pani Biosensor... 393
3.2.4. Lateral Flow Conductometric Biosensor... 393
3.2.5. Signal Measurement ... 393
3.3. Properties of Pani ... 394
3.4. Detection Concept of the Biosensor ... 398
3.5. Biosensor Properties... 399
3.5.1. ITO-Pani Biosensor ... 399
3.6. Lateral Flow Conductometric Biosensor ... 403
3.7. Biosensor Performance... 404
3.7.1. ITO/Pani Biosensor... 404
3.8. Conductometric Biosensor ... 404
4. Conclusions and Future Perspectives ... 406
References... 406
17. Magnetic Techniques for Rapid Detection of Pathogens Yousef Haik, Reyad Sawafta, Irina Ciubotaru, Ahmad Qablan, Ee Lim Tan and Keat Ghee Ong 1. Introduction... 415
2. Synthesis of Magnetic Particles ... 417
2.2. Synthesis Techniques ... 423
2.3. Encapsulation of Magnetic Particles... 423
2.3.1. Methods of Preparing Polymer/Protein Coatings ... 424
2.3.2. Examples of Polymer/Protein Encapsulated Particles ... 426
3. Immobilization Strategies ... 426
3.1. Modification of Particle Surface with a Ligand ... 430
4. Biological Targets... 430
5. Magnetic Immunoassays... 430
5.1. Direct Immunoassay Detection Using Magnetic Beads ... 430
5.1.1. Superconducting Quantum Interference Devices... 431
5.1.2. ABICAP Column... 432
5.2. Indirect Immunoassay Detection Using Magnetic Beads... 433
5.2.1. ELISA ... 433
6. Handling Techniques ... 438
7. Magnetic Separation ... 439
7.1. Magnetic Force... 439
7.2. High-Field Electromagnets... 440
7.3. Permanent Magnets ... 441
7.4. Numerical Analysis for Permanent Magnet Arrangements... 442
8. Giant Magnetoresistive (GMR) Devices for Bacterial Detection... 446
9. Bacteria Detection with Magnetic Relaxation Signal... 448
10. Magnetoelastic Sensors for Bacterial Detection ... 449
10.1.E. coliDetection... 450
11. Conclusions and Future Perspectives ... 453
References... 454
18. Cantilever Sensors for Pathogen Detection Raj Mutharasan 1. Introduction... 459
2. Millimeter-Sized Cantilever Sensors... 460
3. Reported Work on Detecting Cells Using Cantilever Sensors... 461
4. Physics of Cantilever Sensors ... 463
5. Resonance Modes ... 466
6. Characterization of PEMC Sensors... 468
7. Mass Change Sensitivity ... 468
8. Antibody Immobilization Methods ... 469
9. Detection in Batch and Stagnant Samples ... 470
10. Detection in Flowing Samples ... 473
11. Selectivity of Detection ... 475
12. Conclusions... 477
References... 478
19. Detection and Viability Assessment of Endospore-Forming Pathogens Adrian Ponce, Stephanie A. Connon and Pun To Yung 1. Introduction... 481
1.1. Historical Perspective... 481
1.2. Endospore Dormancy, Resistance and Longevity ... 482
1.3. Endospores as Biodosimeters for Evaluating Sterilization Regimes ... 484
1.4. Endospore-Forming Pathogens ... 485
2. Detection of Endospore-Forming Pathogens and their Endospores ... 489
2.1. Phenotypic Identification... 489
2.1.1. Phenotypic Identification ofBacillus anthracis... 490
2.1.2. Phenotypic Identification ofClostridium perfringens... 490
2.2. Parameters of a Sensor... 491
2.3. Rapid Immunoassays... 492
2.3.1. Enzyme-Linked Immunosorbent Assays... 492
2.3.2. Lateral-Flow Immunoassays... 493
2.3.3. Immunomagnetic Electrochemiluminescence ... 493
2.3.4. Flow Cytometry ... 494
2.3.5. Vegetative Cells... 494
2.4. Rapid Nucleic Acid Assays... 495
2.4.1. PCR Sample Preparation and Endospore Lysis ... 495
2.4.2. The PCR Reaction ... 496
2.4.3. Specificity of PCR Primers forBacillus anthracisDetection... 496
2.4.4. Rapid PCR Detection Methods High Throughput and real-time PCR ... 497
2.4.5. Field Implementation of Rapid PCR for Analysis of Environmental Samples ... 498
2.4.6. Monitoring the Air forBacillus anthracisEndospores by PCR ... 500
2.5. Rapid Detection of Endospores via Dipicolinic Acid Biomarker... 501
2.5.1. Terbium Dipicolinic Acid Luminescence Assay ... 501
2.5.2. Anthrax Smoke Detector ... 503
3. Validation of Sterilization by Rapid Endospore Viability Assessment ... 505
3.1. Measuring Endospore Viability and Inactivation ... 505
3.2. Measuring Endospore Inactivation using Germinability Assays ... 508
3.2.1. Rapid Germinability Assays... 508
3.2.2. Nucleic Acid-Based Amplification Methods for Detecting Germinable, Viable Bacillus anthracisSpores... 508
3.2.3. Germination Observed via Loss of Phase Brightness... 509
3.2.4. Germination Observed via DPA release ... 510
3.3. Measuring Endospore Inactivation Using Metabolic Activity Assays ... 512
4. Conclusions and Future Perspectives ... 513
References... 514
20. Label-Free Fingerprinting of Pathogens by Raman Spectroscopy Techniques Ann E. Grow 1. Introduction... 525
2. Raman Microscopy for Whole-Organism Fingerprinting... 527
3. Surface-Enhanced Raman Scattering (SERS) for Whole-Organism Fingerprinting... 531
4. MicroSERS for the Detection and Identification of Pathogens and Toxins ... 534
4.1. MicroSERS Detection of Bacteria ... 535
4.1.1. SERS Fingerprinting of Bacteria... 535
4.1.2. Impact of Growth Conditions on Bacterial Fingerprints ... 536
4.1.3. Viable vs. Nonviable Bacteria... 539
4.1.4. Integrated MicroSERS Detection and Identification of Bacteria ... 542
4.1.5. Impact of Growth Conditions on Biomolecule Capture ... 544
4.1.6. Analysis of Bacteria in Complex Samples... 544
4.2. MicroSERS Detection of Spores... 545
4.2.1. SERS Fingerprinting of Spores ... 545
4.2.2. Impact of Growth Conditions on Spore Fingerprints ... 547
4.2.3. Viable vs. Nonviable Spores ... 550
4.3. MicroSERS Detection of Bacterial Toxins... 554
4.3.1. SERS Fingerprinting of Toxins... 555
4.3.2. Analysis of Toxins in Complex Samples... 558
5. Conclusion and Future Perspectives... 559
References... 560
Part III
Recognition Receptors
21. Antibodies and Immunoassays for Detection of Bacterial Pathogens Padmapriya P. Banada and Arun. K. Bhunia 1. Introduction... 5672. Antibodies ... 568
2.1. Polyclonal Antibody... 570
2.2. Monoclonal Antibody... 570
2.3. Use of Synthetic Peptides for Antibody Production ... 571
2.4. Recombinant DNA Technology... 573
2.4.1. Phage Display ... 573
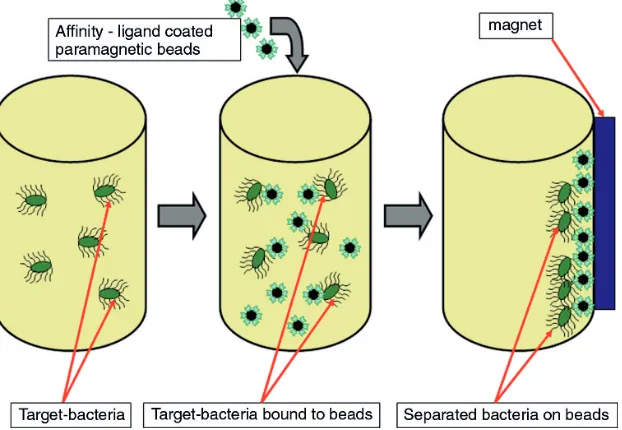
3. Capture and Concentration of Cells by Immunomagnetic Separation ... 575
3.1. Automated IMS Systems... 577
4. Immunoassays for Pathogen Detection ... 577
4.1. Radioimmunoassay... 577
4.2. Enzyme Immunoassays ... 577
4.2.1.Escherichia coli ... 580
4.2.2.Listeria monocytogenes ... 583
4.2.3.Salmonella... 583
4.2.4. Staphylococcal Enterotoxins... 583
4.2.5.Clostridium botulinumToxins... 584
4.3. Lateral Flow Immunoassay ... 584
4.4. Other Immunoassays ... 585
4.4.1. Latex Agglutination (LA) and Reverse Passive Latex Agglutination (RPLA) Tests.... 585
4.4.2. Enzyme-Linked Fluorescent Assay ... 585
4.4.3. Time-Resolved Fluorescence Immunoassay ... 585
4.4.4. Chemiluminescent Immunoassay ... 586
4.4.5. Capillary Microbead (Spheres) Immunoassay ... 586
4.4.6. Electrochemical-Immunoassay ... 586
4.5. Optical Biosensors... 587
4.5.1. Surface Plasmon Resonance... 587
4.5.2. Fiber-Optic Biosensors ... 587
4.5.3. Antibody-Based Microfluidic Sensors ... 588
4.5.4. Serodiagnosis ... 589
5. Recent Developments in Immunoassays... 590
5.1. Protein/Antibody Microarrays... 590
5.2. Mass Spectrometric Immunodetection... 591
5.3.SERS Biochip Technology ... 591
6. Limitations and Challenges ... 591
6.1. Specificity and Sensitivity... 591
6.2. Effect of Physical and Chemical Stresses on the Expression Profile of Antigens in Bacteria 592 6.2.1. Effect of Media Composition on the Expression of Proteins in Bacteria... 592
6.2.2. Effect of Stress on the Expression of Proteins in Bacteria ... 593
7. Conclusions and Future Perspectives ... 594
22. Rapid Nucleic Acid-Based Diagnostics Methods for the Detection of Bacterial Pathogens
Barry Glynn
1. Introduction... 603
1.1. Detection of Pathogenic Bacteria from Clinical Samples ... 604
1.2. NAD Assays for the Detection of Respiratory Infection, Sepsis and Sexually Transmitted Infection ... 604
1.3. Profiling of Multi-drug Resistance ... 606
1.4. Bioterrorism... 606
2. Detection of Bacterial Food-Borne Pathogens... 606
2.1. Recent Outbreaks... 606
2.2. Benefits and Limitations of Conventional Methods... 607
2.3. Development of Rapid Diagnostics Methods ... 607
3. Rapid Nucleic Acid Diagnostics for Bacterial Food-Borne Pathogens... 607
3.1. In Vitro Nucleic Acid Amplification-Based Detection of Food-Borne Pathogens ... 607
3.2. Requirements for a NAD-Based Food Assay... 608
3.3. Polymerase Chain Reaction (PCR) ... 608
3.4. Application of PCR-Based Tests to Pathogen Detection in Food Samples ... 609
3.5. Use of RNA as an Alternative Nucleic Acid Diagnostic Target ... 610
3.6. Sample Preparation for NAD from Clinical Sample Types... 611
3.7. Limitations of NAD in Clinical Settings ... 611
4. Formats of NAD Assays for Food Pathogen Detection ... 612
4.1. Nucleic Acid-Based Diagnostics Based on In Vitro Amplification Technologies... 612
4.2. PCR-ELISA and PCR-DNA Probe Membrane Based Assays forCampylobacterandSalmonella... 612
4.3. Specific Examples of Nucleic Acid Diagnostics Assays for the Detection of Bacterial Food-Borne Pathogens... 613
4.3.1. Commercially Available Conventional NAD Assays for Food-Borne Bacterial Pathogens... 614
4.3.2. Alternative In Vitro Amplification Technologies ... 615
4.4. Standardisation of In Vitro Amplification-Based NAD Assays and Inter-Laboratory Validation Studies ... 616
4.5. Real-Time In Vitro Amplification-Based Nucleic Acid Diagnostics ... 617
4.5.1. Specific Examples of Real-Time PCR Assays for the Detection of Bacterial Food-Borne Pathogens ... 617
4.5.2. Alternative Real-Time In Vitro Amplification-Based Diagnostics Technologies ... 618
4.6. Limitations and Other Considerations for In Vitro Amplification NAD Tests... 619
4.7. Non-Amplified Direct DNA Probe-Based Nucleic Acid Diagnostics ... 620
4.8. DNA-Probe Based Detection Methods ... 620
5. Conclusions and Future Perspectives ... 621
5.1. Emerging Nucleic Acid Diagnostic Technologies for Food-Borne Pathogen Detection ... 621
5.1.1. Biosensors ... 621
5.1.2. Microarrays ... 622
References... 623
23. Oligonucleotide and DNA Microarrays: Versatile Tools for Rapid Bacterial Diagnostics Tanja Kostic, Patrice Francois, Levente Bodrossy and Jacques Schrenzel 1. Introduction... 629
2. Microarray Technology ... 630
3. Technical Aspects of Microarray Technology... 632
3.1. Probes... 632
3.1.2. PCR Products... 632
3.1.3. Oligonucleotide Probes... 632
3.2. Substrates for Printing... 634
3.2.1. Slides with Poly-L-Lysine Coating ... 634
3.2.2. Slides with Amino Silane Coating ... 635
3.2.3. Slides with Aldehyde Coating... 635
3.2.4. Slides with Epoxy Coating ... 635
3.2.5. Proprietary Surface Chemistries... 636
3.2.6. Probe Spacers... 636
3.3. Targets for Microarray Analysis ... 637
3.3.1. Target Amplifications and Sensitivity Issues... 637
3.3.2. Labeling of the Targets... 638
3.3.3. Hybridization and Wash Conditions ... 638
3.4. Classical Commercially-Available Microarray Formats ... 639
3.4.1. Spotting Approaches... 639
3.4.2. In Situ Synthesis ... 639
3.5. Alternative Methods for Improving Microarray-Based Detection Sensitivity... 641
3.5.1. Resonance-Light Scattering (RLS)... 641
3.5.2. Planar-Waveguide Technology (PWT) ... 641
3.5.3. Liquid Arrays... 641
3.5.4. Three-Dimensional Microarray Formats ... 642
3.6. Marker Genes Used on Microbial Diagnostic Microarrays (MDMs)... 643
4. Analysis and QC Aspects ... 643
5. Applications of Microarray Technology in Microbial Diagnostics... 644
5.1. Gene Expression Studies... 644
5.2. Comparative Genome Hybridizations (CGH)... 645
5.3. Generic or Universal Microarrays... 646
5.4. Microarrays for Sequence Analysis ... 647
5.5. Microbial Diagnostic Microarrays ... 648
6. Conclusions... 649
References... 649
24. Pathogenic Bacterial Sensors Based on Carbohydrates as Sensing Elements Haiying Liu 1. Introduction... 660
2. Bacterial Surface Lectins... 661
3. Surface Carbohydrate Structures of Pathogenic Bacteria... 664
4. Carbohydrate Microarrays for Detection of Bacteria ... 668
5. Lectin Microarrays for Detection of Bacteria... 670
6. Conjugated Fluorescent Glycopolymers for Detection of Bacteria... 672
7. Glyconanoparticles for Detection of Bacteria... 676
8. Carbohydrate-Functionalized Carbon Nanotubes for Detection of Bacteria... 678
9. Conclusions and Future Perspectives ... 680
References... 681
25. Aptamers and Their Potential as Recognition Elements for the Detection of Bacteria Casey C. Fowler, Naveen K. Navani, Eric D. Brown and Yingfu Li 1. Functional Nucleic Acids ... 689
1.1. Properties of Nucleic Acids ... 690
1.2.1. DNA Polymerase and Polymerase Chain Reaction ... 692
1.2.2. RNA Polymerase and In Vitro Transcription ... 692
1.2.3. Reverse Transcription ... 693
1.2.4. Other Modifications... 693
2. Isolation of Functional Nucleic Acids... 694
2.1. Introduction to SELEX... 694
2.2. Selection Methods ... 694
2.2.1. Bead and Column Based Selections... 696
2.2.2. Polyacrylamide Gel Electrophoresis (PAGE) Based Selections ... 696
2.2.3. Capillary Electrophoresis (CE) Based Selections ... 697
2.3. Optimizing Functional Nucleic Acids... 697
3. Aptamers: Properties and Targets ... 697
3.1. The Growing Aptamer Catalogue ... 698
3.2. Aptamer Specificity... 698
3.3. Aptamer–Ligand Interactions... 700
3.4. Aptamers vs. Other Recognition Elements... 700
4. Applications of Aptamers... 701
4.1. Aptamers for Purification... 701
4.2. Aptamers with Therapeutic Potential... 702
4.3. Aptamers as Sensing Elements ... 702
4.3.1. Conformation-Dependent Fluorescent Sensors ... 703
4.3.2. Quantum Dot Sensors... 703
4.3.3. Target Detection by Fluorescence Anisotropy... 704
4.3.4. Enzyme Linked Aptamer Assays ... 705
4.3.5. Acoustic Sensors... 705
4.3.6. Electrochemical Sensors ... 706
5. Aptamers for Detection of Pathogenic Bacteria ... 706
5.1. Categories of Microbial Agents to be Detected ... 707
5.1.1. Gram-Positive Bacteria... 707
5.1.2. Gram-Negative Bacteria ... 708
5.2. Traditional Pathogen Detection Methods ... 708
5.3. Aptamers in Pathogen Detection... 709
6. Conclusions... 710
References... 710
26. Protein Microarray Technologies for Detection and Identification of Bacterial and Protein Analytes Christer Wingren and Carl AK Borrebaeck 1. Introduction... 715
1.1. Definition and Classification of Protein Microarrays... 716
1.2. Functional Protein Microarrays... 716
1.3. Affinity Protein Microarrays... 719
1.4. Alternative Microarray Setups ... 720
2. Detection of Bacteria and Bacterial Protein Analytes ... 721
2.1. Serotyping of Bacteria... 721
2.2. Detection of Pathogenic Organisms... 721
2.3. Detection of Multiple Toxins... 722
2.4. Simultaneous Detection and Identification of Bacterial Proteins and Bacteria... 723
3. Detection of Diagnostic Markers, Toxin Regulators and Associated Protein Expression Profiles. 724 3.1. Identification of Potential Diagnostic Markers and/or Vaccine Candidates... 724
3.2. Disease State Differentiation and Identification of Diagnostic Markers ... 724
3.3. Identification of Potential Toxin Modulators/Regulators... 725
4. Conclusions and Future Perspectives ... 726
References... 726
27. Bacteriophage: Powerful Tools for the Detection of Bacterial Pathogens Mathias Schmelcher and Martin J. Loessner 1. Introduction... 731
2. Detection by Phage Amplification ... 732
3. Detection Through Phage-Mediated Cell Lysis... 734
3.1. Measurement of ATP Release... 735
3.2. Detection of Other Cytoplasmic Markers... 736
3.3. Measurement of Impedance ... 737
4. Detection Through Cell Wall Recognition, Phage Adsorption and DNA Injection... 738
4.1. Immobilized Phage... 738
4.2. Detection Through Phage-Encoded Affinity Molecules ... 738
4.3. Fluorescently Labeled Phage ... 740
5. Detection by Reporter Phage... 741
5.1. Luciferase Reporter Phage (LRP) ... 743
5.2. Fluorescent Protein Reporter Phage... 745
5.3. Other Reporter Phages ... 746
6. Other Detection Methods Using Phage... 747
6.1. Phage Display for Production of Highly Specific Binding Molecules... 747
6.2. Dual Phage Technology ... 749
7. Conclusions and Future Perspectives ... 750
References... 750
28. Phage Display Methods for Detection of Bacterial Pathogens Paul A. Gulig, Julio L. Martin, Harald G. Messer, Beverly L. Deffense and Crystal J. Harpley 1. Introduction... 756
1.1. Why Detect Bacteria and What Tools Are Available? ... 756
1.2. Immunological Tools... 756
1.3. Nucleic Acid-Based Tools ... 758
2. What Types of Antigen Detection Methods Are Being Developed?... 758
3. Phage Display ... 759
3.1. Phage M13... 760
3.2. Principles of Phage Display ... 760
3.3. Phages Versus Phagemids... 762
3.4. Phage Display Formats... 764
3.4.1. Random Peptides ... 764
3.4.2. Antibody Fragments ... 764
3.5. The Phages Themselves Are Not the Ultimate Tool... 767
3.6. Using Phage Display ... 767
4. Review of Literature on Phage Display Against Bacterial Pathogens... 769
4.1. Random Peptide Phage Display... 770
4.2. scFv Libraries ... 772
4.3. Single Domain Antibodies (sdAbs) and Domain Antibodies (dAbs) ... 775
5. Summary of Our Results Using and Developing Phage Display scFv and Peptides ... 775
5.1. Panning Methods ... 776
5.2. Screening Methods ... 777
5.3. Genetic Modification of Phagemid Clones... 777
6. New Directions ... 778
6.1. Proteins Based on Phage Display ... 778
6.1.1. Affibodies... 778
6.1.2. Anticalins ... 778
6.1.3. Ankyrins... 778
6.1.4. Trinectins... 779
6.2. Alternatives to Phage Display... 779
6.2.1. Aptamers ... 779
6.2.2. Ribosome Display... 779
6.2.3. mRNA Display ... 780
7. Conclusions... 780
References... 780
29. Molecular Imprinted Polymers for Biorecognition of Bioagents Keith Warriner, Edward P.C. Lai, Azadeh Namvar, Daniel M. Hawkins and Subrayal M. Reddy 1. Introduction... 785
2. Principles of Molecular Imprinting ... 786
2.1. Imprinting Considerations ... 787
2.1.1. Versatility... 787
2.1.2. Template Molecule ... 788
2.1.3. Functional Monomer... 788
2.1.4. Cross-Linking... 789
2.1.5. Polymerization ... 790
2.1.6. Solvent ... 790
2.2. Aqueous Phase MIP ... 791
2.2.1. Hydrogels ... 792
2.2.2. MIP Within Hydrogels ... 793
2.2.3. Polyacrylamide Gels—HydroMIPs ... 793
3. Solid Phase Extraction Based on MIPs for Concentrating Bioagents... 795
3.1. Antibiotics... 795
3.2. Mycotoxins ... 798
3.3. Nano-Sized Structures ... 799
3.4. Peptides and Proteins ... 800
3.5. Viruses ... 801
3.6. Bacterial Cells and Endospores... 802
4. Biosensors Based on MIPs ... 803
4.1. MIP-based Sensors for Detection of Amino Acids ... 804
4.2. Molecular-Imprinted Films for Toxins ... 805
4.3. Microbial Imprinted Polymers ... 806
5. Conclusions and Future Perspectives ... 808
References... 809
Part IV
Microsystems
30. Microfluidics-Based Lysis of Bacteria and Spores for Detection and Analysis Ning Bao and Chang Lu 1. Introduction... 8172. Bench Scale Methods for Bacteria/Spore Lysis... 818
3.1. Mechanical Lysis... 820 3.2. Chemical Lysis ... 821 3.3. Thermal Lysis... 823 3.4. Laser-Based Lysis ... 826 3.5. Electrical Lysis ... 827 4. Conclusions and Future Perspectives ... 829 References... 829
31. Detection of Pathogens by On-Chip PCR
Pierre-Alain Auroux
1. Introduction... 833 2. Microfluidics... 834 2.1. History of Miniaturized Total Analysis System (TAS)... 834 2.2. Advantages of Miniaturized Analysis Systems ... 834 3. DNA Amplification ... 835 3.1. A Brief History of DNA ... 835 3.2. PCR Characteristics and Applications ... 836 3.3. Components to Perform PCR... 837 3.4. PCR Process ... 838 3.5. Conventional PCR ... 839 3.6. Real-Time PCR: Apparatus and Detection Techniques ... 840 3.7. On-Chip PCR... 841 3.7.1. Capillary-Based Thermocyclers ... 842 3.7.2. Microdevice-Based Thermocyclers ... 843 3.7.3. Static-Sample Systems... 843 3.7.4. Dynamic-Sample Systems ... 844 4. Minireview ... 846 5. Conclusions... 848 References... 849
32. Micro- and Nanopatterning for Bacteria- and Virus-Based Biosensing Applications
David Morrison, Kahp Y. Suh and Ali Khademhosseini
33. Microfabricated Flow Cytometers for Bacterial Detection Sung-Yi Yang and Gwo-Bin Lee
1. Introduction... 869 1.1. Bio-MEMS ... 871 1.2. Review of Microfabrication Techniques... 872 1.2.1. Bulk Micromachining Technique... 872 1.2.2. Surface Micromachining Technique ... 872 1.2.3. LIGA ... 872 1.2.4. Polymer-Based Micromachining Techniques for Microfluidic Devices ... 873 2. Operation Principles ... 874 2.1. Cell Transportation and Focusing ... 875 2.1.1. Hydrodynamic Approach... 875 2.1.2. Pneumatic Approach... 878 2.1.3. Electrokinetic Approach ... 879 2.2. Cell Detection... 880 2.2.1. Optical Waveguide Approach ... 881 2.2.2. Buried Optical Fiber Approach ... 882 2.2.3. Large-Scale Optical System Approach ... 882 2.3. Cell Sorting... 883 2.3.1. Hydrodynamic Sorting... 883 2.3.2. Pneumatic Sorting... 884 2.3.3. Electrokinetic Sorting ... 885 2.3.4. Magnetic Sorting... 885 3. Applications ... 885 3.1. Environmental Monitoring ... 886 3.2. Rapid Assessment of Bacterial Viability ... 888 3.3. Rapid Analysis of Bacteria Levels in Food... 888 3.4. Antibiotic Susceptibility Testing... 889 3.5. Bacterial Detection in Blood and Urine ... 889 4. Conclusions and Future Perspectives ... 889 References... 890
34. Bacterial Concentration, Separation and Analysis by Dielectrophoresis
Michael Pycraft Hughes and Kai Friedrich Hoettges
1. Introduction... 895 2. Theory ... 897 3. Applications of Electrokinetics to Bacteria... 901 4. Toward an Integrated Detection System ... 904 5. Conclusions and Future Perspectives ... 905 References... 906
35. Ultrasonic Microsystems for Bacterial Cell Manipulation Martyn Hill and Nicholas R. Harris
2.3. Acoustic Streaming ... 913 2.4. Cavitation... 914 3. Applications of Ultrasonic Particle Manipulation... 914 3.1. Practical Considerations ... 914 3.1.1. Transduction... 914 3.1.2. Mechanical Effects ... 915 3.1.3. Construction ... 916 3.2. Filtration and Fractionation of Cells... 917 3.2.1. Filtration and Concentration... 917 3.2.2. Fractionation of Cells ... 920 3.2.3. Trapping of Cells ... 921 3.3. Biosensor Enhancement by Forcing Cells to a Surface ... 922 4. Conclusions and Future Perspectives ... 924 References... 924
36. Recent Advances in Real-Time Mass Spectrometry Detection of Bacteria
Arjan L. van Wuijckhuijse and Ben L.M. van Baar
1. Introduction... 929 1.1. General... 929 1.2. Scope... 930 1.3. MS in the Whole Cell Analysis of Bacteria... 930 1.3.1. The Definition of ‘Identity’ of Bacteria... 930 1.3.2. Mass Spectrometry of Bacteria ... 931 1.4. Aerosol MS... 936 1.4.1. MS of Deposited Aerosols ... 936 1.4.2. Direct MS of aerosols... 938 2. Current State of the Technology ... 939 2.1. Considerations on Aerosol MS of Bacteria ... 939 2.2. Deposition and PyMS Based Technology ... 940 2.3. Deposition and MALDI MS Based Technology ... 941 2.4. Single Particle LDI MS Technology ... 941 2.5. Single Particle MALDI MS Technology ... 943 3. Conclusions and Future Perspectives ... 946 References... 947
Contributors
National Institute for Standards and Technology EEEL, Semiconductor Electronics Division
Stephanie A. Connon
Department of Molecular Genetics and Microbiology University of Florida College of Medicine
Gainesville, Florida
School of Chemical Engineering and Analytical Science (CEAS)
Department of Molecular Genetics and Microbiology University of Florida College of Medicine
Gainesville, Florida
University of Florida College of Medicine Gainesville, Florida
USA
Nicholas R. Harris
School of Electronics and Computer Science The University of Southampton
Southampton UK
Daniel M. Hawkins University of Surrey
Robert Horvath
National Institute of Standards and Technology Chemical Sciences and Technology Laboratory
Austrian Research Centres GmbH - ARC Seibersdorf, Austria
Institute for Food Science and Nutrition Zurich, Switzerland
Akira Matsumoto
University of Florida College of Medicine Gainesville, Florida
School of Chemical Engineering and Analytical Science (CEAS)
Ahmad Qablan The Hashemite University Zarqa, Jordan
Subrayal M. Reddy
School of Biomedical and Molecular Sciences University of Surrey
Institute for Food Science and Nutrition Zurich, Switzerland
Ahmed E. Yousef
Professor of Food Microbiology
Department of Food Science and Technology and
Department of Microbiology Parker Food Science Building Ohio State University Columbus, Ohio USA
Pun To Yung
California Institute of Technology Pasadena, California
USA
I
1
Introduction to Pathogenic Bacteria
Tracey Elizabeth Love and Barbara Jones
Abstract
This chapter is a brief introduction to pathogenic microorganisms and also discusses virulence factors. An understanding of virulence factors is important, as they represent potential targets for the detection of microbial pathogens. Sources and routes of infection are also briefly discussed with reference to specific examples. There are a number of ways in which infection could be acquired, including via contaminated food and water; hospital acquired infection; “naturally acquired” infection; and intentional infection, for example, through the use of biological warfare agents. The focus of the review is predominantly on human pathogens. However, there are a range of other microbial pathogens of particular importance in other areas; for example, animal and plant pathogens, which will not be discussed. Finally, a brief overview of the detection of pathogenic bacteria is presented.
1. Pathogenic Microorganisms
Over many years there has been considerable debate as to the exact definitions of pathogenicity and virulence. These two words are often used interchangeably, but pathogenicity has been defined as the ability of an organism to cause disease and virulence as the relative severity of the disease caused by the organism (Watson and Brandly 1949). It has become increasingly apparent that virulence is highly complex and is dependent on the interaction between the host and the microorganism (Casadevall and Pirofski 2001). Taking into account the problems associated with defining virulence, virulence factors have also been difficult to characterise. Two defini-tions that have been put forward are that a virulence factor is (1) a “component of a pathogen that when deleted specifically impairs virulence but not viability” (Wood and Davis 1980); or (2) a “microbial product that permits a pathogen to cause disease” (Smith 1977). However, these often do not apply to infections caused by commensal or opportunistic pathogens, where often classic virulence determinants do not exist. Furthermore, the definitions may not account for host tissue damage that has been caused by the induction of a particular part of the host’s immune response, such as cytokine synthesis (Henderson et al. 1996). Therefore an under-standing of virulence factors is important, as these can often be used to specifically detect pathogenic microorganisms. Classical virulence factors include factors that aid in a number of stages of infection:
1) host cell attachment; 2) entry to the host cell;
Tracey Elizabeth Love • Defence Science and Technology Laboratory, Porton Down, Wiltshire, UK.
Barbara Jones • National Institute of Standards and Technology, Chemical Sciences and Technology Laboratory,
Biochemical Science Division, Gaithersburg, Maryland, USA.
M. Zourob et al. (eds.),Principles of Bacterial Detection: Biosensors, Recognition Receptors and Microsystems, © Springer Science+Business Media, LLC 2008
3) evasion of detection by the hosts immune system;
4) intracellular or extracellular replication and inhibition of phagocytosis.
Virulence factors can be either requisite, where the gene products discriminate between pathogenic and nonpathogenic species; or contributory factors, that alter the severity of the disease. Again, the ability to cause disease and its severity will also be dependent on the immune status of the host. Contributory virulence factors do not fulfil the definition of virulence factors; nor do they separate pathogenic from nonpathogenic species, as they may be found in a wide range of microorganisms but still have a role in damage to host cells. A general consensus of opinion has been that regardless of the function of a gene product, if its expression leads to damage of the host cell then it is a virulence factor. Therefore Casadevall and Pirofski (1999) suggest that virulence factors should be defined as “attributes that mediate host damage.” Bacterial pathogens usually possess a number of virulence factors that are essential in estab-lishing infection and causing disease. Classical virulence factors include toxins, as well as molecules that are involved in adherence, invasion of the host, evasion of the host’s immune response, and iron acquisition.
1.1. Toxins
Some microorganisms (e.g.,Bacillus anthracis) produce toxins that are the major cause of clinical symptoms observed in patients. Toxins can be integral parts of the bacterium, such as lipopolysaccharide (endotoxins), or secreted molecules (exotoxins). Toxins often perform other functions, such as the making of adhesins (Tuomanen and Weiss 1985). Toxin secretion may also be regulated as part of an orchestrated response by the bacterium. The lipopolysaccharide (LPS) content of pathogenic Gram-negative cell walls is contained within a microorganism and usually released when the cell dies or is broken down (by autolysis or by the host’s immune response).
Unlike exotoxins, endotoxins are believed not to have any direct enzymatic action; and it is the lipid A portion, usually embedded within the bacterial membrane, that is believed to be the toxic component. As LPS is released from the bacterial cell, a number of host molecules involved in the inflammatory response are released (e.g., cytokines). One of the most important cytokines released is tumour necrosis factor-(TNF-). This molecule usually prevents the
spread of a localised infection. However, the rapid stimulation of high levels of TNF-within
the bloodstream results in fever, damage to host tissue, an alteration of metabolism, and the production of further cytokines (IL-6, IL-8, IL-1, and PAF, platelet activating factor). These cytokines produce further damage to host cells and tissue resulting in a dramatic decrease in blood pressure and reduced blood flow to major organs leading to multiple organ failure (Tracey and Cerami 1993, Rink and Kirchner 1996). Exotoxins can be divided into a number of broad categories summarised below.
1.2. Adherence
Another important factor in establishing an infection is the ability of a microorganism to attach to a host cell or to an extracellular matrix. The macromolecules and structures involved in specific attachments to host cell receptors are often referred to as adhesins. Proteinaceous adhesins can be classified into two groups: afimbrial adhesins (sometimes termed nonpilus adhesins) and fimbriae (or pili). However, not all adhesins are essential for microbial virulence (Krogfelt 1991).
that pili were homopolymeric, composed of a number of repeating pilin (or fimbrin) subunits. However, it has become apparent that for many pili (e.g., Type I and Pap pili) these structures are heteropolymeric. Furthermore, the protein subunits located either at the tip or the base of these structures are important for a particular function. For example, the minor protein subunits located at the distal end of the pilus (the tip fibrillum) are frequently involved in attachment to the host cell receptor (Hultgren et al. 1993). Pili have the capacity to attach to a number of different receptors (Table 1.1) and genetic variation in the tip adhesin confers differences in binding affinity to host cell receptors, which allows for differences in the tissue tropism. Bacterial DNA may encode multiple operons for fimbrial expression; for example,Salmonella. typhimurium encodes four fimbrial operons (fim, lpf, pef, and agf). Deletion of individual fimbrial operons resulted in a minor reduction in virulence, but a quadruple mutant demonstrated significant attenuation compared to the wild type strain (Van der Velde et al. 1998). This suggests that deletion of a single component of virulence can be compensated by the presence of related virulence factors in the case of some microorganisms.
Curli are another form of pilus type adhesins found in some strains ofEscherichia coliand Salmonella entereditisspp. They are highly stable, thin, irregular surface structures that facilitate binding to host proteins such as plasminogen and fibronectin. All pili are assembled in a highly ordered manner. and although the assembly mechanism may vary, common characteristics are often observed (Soto and Hultgren 1999). Nonpilus adhesins can be found in Gram-negative, Gram-positive, and mycobacterial pathogens. Examples of nonfimbrial adhesins are summarised in Table 1.2.
Table 1.1. Examples of bacterial toxins and modes of action (compiled from Merrit and Hol 1995, Schiavo et al. 1992, Welch 1991, Savarino et al. 1993, Falzano et al. 1993, and Schmitt et al. 1999).
Type of Toxin Mode of Action Example Organisms
A-B Toxins (Type III toxins)
The A subunit has enzymatic activity that mediates toxicity. The B-subunit binds to the host cell receptor and allows for delivery of the A-subunit.
Proteolytic cleavage of host cell molecules. Clostridum tetani(tetanus toxin), Clostridium botulinum (botulinum toxin) Pore-forming Toxins
(Type II Toxins)
Creation of a pore within the plasma membrane of the host cell that leads to cell lysis.
E. coli(hemolysin),Bordetella pertussis(adenylate cyclase) Interference with
Host Cell Function
One group affects the host cell cytoskeleton by modification of Rho family (small GTP binding proteins) resulting in various detrimental effects on actin polymerisation (toxin dependent).
Bacteria that secret these toxins have their own secretion systems encoded within the bacterial DNA. The mode of action of many of these toxins has yet to be elucidated.
Neisseria meningitidis, Haemophilus influenzae
Heat Stable Toxins Heat stable toxins – binding of the toxin to its receptor stimulates activation of guanylate cyclase increasing intracellular GMP in turn causing dramatic ion flux changes.
EnterotoxinogenicE. coli, Yersinia enterocolitica, Vibrio cholerae.
Superantigens (Type I Toxins)
Immunostimulatory toxins that bind to MHC class II molecules stimulating the production of T-cells and triggering the release of cytokines involved in the inflammatory response. This causes fever, shock, and erythematous rash.
Table 1.2. Examples of Gram negative pilin adhesins (modified from Wizemann et al. 1999).
Adhesin Strain Host Receptor
PapG Klebsiella pneumoniae Gal(1–4)Gal
FimH Escherichia Mannose oligosaccharides
MrkD Escherichia coli Type V collagen
HifE Haemophilus influenzae Sialylyganglioside-GM1
Bacteria can also produce other molecules that are involved in host cell attachment, such as intimins. These allow for close associations between the pathogens and result in rearrangement of the cytoskeleton, interference with host cell signalling, and possibly in bacterial internalisation. A more novel mechanism of host cell attachment is a pathogen secreted receptor, which is endocytosed by the host cell and subsequently presented on the host cell surface in a phosphorylated form. This then functions as a receptor for the bacterial cell, for example in enteropathogenicE. coli(Kenny et al. 1997).
Other surface structures may also be involved in specific and nonspecific adherence to a host cell, such as a slime layer, capsule, LPS, techoic acid and lipotechoic acid. For example, the capsular glucose and mannan polysaccharides ofMycobacteria species adhere to complement receptor 3 and the mannose receptors of host cells (Daffe and Etienne 1999). The techoic acids of StaphylococcusandStreptococcusspp can also function as adhesins (Walker 1998). Slime layers and capsules are usually composed of polysaccharides, but can be made of polypeptides. If the surrounding material is unorganised and loosely attached to the cell wall it is referred to as a slime layer, whereas an organised layer that is firmly attached to the bacterial cell is termed a capsule. Both may mediate specific or nonspecific attachment, but do not necessarily have a role in pathogenicity or virulence. Many adhesins have also demonstrated important roles in evasion of the host immune system (e.g., capsules, LPS, and techoic and liptotechoic acids).
Both Gram-negative and Gram-positive pathogens often express and utilise a large reper-toire of adhesins (Tables 1.1 and 1.2). Adhesion can occur as a protein or protein-carbohydrate interaction, and a vast array of host molecules are used as adhesin targets (Table 1.3). For example, surface immunoglobulin, glyocproteins, glycolipids, and extracellular matrix proteins such as fibronectin, collagen, or laminin have been shown to interact with adhesins (Finlay and Falkow 1997).
Table 1.3. Examples of afrimbrial adhesins in Gram-negative and Gram-positive bacteria (Brubaker 1995).
Adhesin Strain Host Cell or Receptor
Pertactin Bordetella pertussis Integrin
HMW1/HMW2 Haemophilus influenzae Human epithelial cells Envelope antigen F1 Yersinia pestis Not known
Lebbinding adhesion Helicobacter pylori Fucosylated Leb histocompatibility blood group antigens CpbA/SpsA/PbcA/PspC Streptococcus pneumoniae Cytokine activated
1.3. Invasion
As discussed previously, intimins often mediate a close association with the host cell, which can lead to internalisation of the pathogenic microorganism. Classes of adhesin that facilitate entry into the host cell are termed invasins and are the most common mode of entry of a bacterial pathogen into both phagocytic and nonphagocytic cells (Finlay and Falkow 1997). In phagocytic cells, pseudopod formation occurs as cell signals mediate cytoskeletal rearrange-ments, resulting in polymerisation and depolymerisation of actin. Internalisation of a bacterial pathogen by cells other than professional phagocytes is mediated by invasin-activated actin rearrangements. This has the same effect as in a phagocytic cell, resulting in forced phago-cytosis of the pathogen (Bliska et al. 1993, Rosenshine and Finlay 1993). Some pathogenic species also utilise host microtubules (polymerised tubulin) to enter nonphagoctyic cells e.g., N. gonorrrhoaeorK. pneumoniae. The exact mechanism is undefined, but they do not appear to utilise this mechanism as an essential virulence factor. Some pathogens target phagocytes and may use phagocytic pathways for internalisation. Once the pathogen is internalised, it resides initially within a membrane vesicle. The pathogen can either remain within or escape from the vesicle. Many pathogenic species remain within the vesicle and have evolved mechanisms to evade the cellular response of the host. For example, capsules and LPS can serve as a protective barrier for internalised pathogens. Other factors, such as the secretion of enzymes that neutralise oxygen radicals and proteolytic enzymes that can degrade host cell lysosyme, are also important for intracellular survival. Exploitation of host cell signals may also occur (e.g., acidic pH) to activate replication and the initiation of the expression of other virulence factors or cascades required for intracellular survival. Pathogenic bacteria may also have the ability to replicate within the host cell and spread to other host cells.
1.4. Evasion of the Host Immune Response
Many surface elements of bacterial pathogens serve to aid in the evasion of the host’s immune response. Capsules consisting of a mix of polysaccharide, protein, and glycoprotein prevent complement activation by inhibition of the assembly of C3 convertase on the bacterial cell surface using a variety of mechanisms. Prevention of C3 convertase assembly on the bacterial surface inhibits the efficiency of phagocytosis, as opsonisation of the pathogen is less likely to occur. C3 convertase may assemble beneath the capsule, and the C3b molecule may also be able to diffuse through; however, the capsular network blocks subsequent contact with phagocytic receptors (Taylor and Roberts 2005). Lack of C3 convertase on the surface also reduces the probability of the formation of a membrane attack complex (MAC) on the underlying bacterial surface. The capsule itself may provoke an immune response. One way by which a number of pathogenic species have overcome this is to produce a nonimmunogenic capsule composed of polysaccharides similar to host polysaccharides—for example, sialic or hyaluronic acid. LPS also aids in the evasion of complement activation and phagocytosis. The LPS O-antigen blocks C3 convertase assembly through the binding of sialic acid. Variation in the length of the LPS O-antigen side chain prevents the assembly of an effective MAC conferring serum resistance, which is important for establishing systemic infections. Another example of evasion of complement activation and phagocytosis is the production of enzymes and toxins to prevent the migration of phagocytes to the infected site (for example, through the enzymatic degradation of a the chemoattractant C5a) (Taylor and Roberts 2005).
1) prevention of phagosome-lysosome fusion;
2) release from the phagosome prior to lysosome fusion;
3) secretion of molecules that reduce the toxic effects of the components of the lysosome into the phagolysosome (e.g., the expression of enzymes such as superoxide dismutase that detoxify oxygen radicals).
Other mechanisms include the production of cell walls that are resistant to degradation by lysosyme, and interference with signalling pathways through the production of enzymes that are homologous to host cell enzymes.
Pathogens can also evade the host’s immune response by inhibiting the production of antibodies by utilising a range of strategies including phase variation (constant switching of the expression of surface antigens). Use of this strategy allows the microorganism to avoid antibodies, as those produced will only be effective against the forms previously expressed (Meyer 1991). Strain variation of immunodominant and highly expressed proteins may confer a selective advantage to pathogenic species. Furthermore, surface components such as LPS, capsules, S-layers, flagella, and outer membrane proteins may demonstrate antigenic variation (Brunham et al. 1993). As mentioned previously, nonimmunogenic structures or layers such as capsular polysaccharides or carbohydrates may be produced that resemble those of the host and even mimic the function of host cell proteins (Stebbins and Galán 2001). Bacteria may also be coated in host cell proteins, such as fibronectin and collagen. The coating of bacteria with host antibodies can prevent opsonisation, possibly through prevention of recognition by specific antibodies to surface located antigens or the inhibition of complement assembly. One example of this is protein A ofS. aureusand protein G ofS. pyogenes, which bind to the Fc portion of immunoglobulins, thus covering the pathogen in antibody. Some pathogenic species also express specific iron binding receptors. Although the primary function of these receptors is iron acquisition, it has also been suggested that they may have a role in masking surface antigens.
1.5. Iron Acquisition
Iron is required for bacterial replication; however, low iron concentrations are often found within a host, particularly within humans. Therefore for survival and growth, some form of iron acquisition mechanism is required. Siderophores are high affinity iron binding low molecular weight compounds, secreted by the bacteria to chelate iron (Neilands 1995). The iron-siderophore complex is then bound by siderophore receptors located on the bacterial surface. It has been demonstrated that this type of iron acquisition mechanism may contribute to bacterial virulence, but this has not always been found to be the case. In humans most iron is bound to proteins such as ferratin, lactoferratin, hemin, or transferrin. It has been shown that some pathogens are able to utilise these as an iron source through binding to receptors on the bacterial surface, although the exact mechanism of the removal of the iron has not been clearly defined. Another potential method of iron acquisition is the production of other virulence factors such as exotoxins, invasins, adhesins, and outer membrane proteins where expression is activated by low iron concentrations (Litwin and Calderwood 1993). It has been suggested that exotoxins kill host cells, thus releasing iron stores which can then be utilised by the pathogen. Many bacteria have more than one method of iron acquisition, therefore deletion may be compensated by another system.
1.6. Regulation of Virulence Factors
response to a number of biochemical and physical parameters such as temperature, pH, ion concentration, growth phase, osmolarity, oxygen, calcium, and iron levels (Gross 1993). Many virulence factors will be required only when an organism is within a host, others may also be essential for survival outside the host. Once a pathogen is within a host, environmental signals will induce the switching on and off of various virulence genes, dependent on the stage of infection. It is common for a single regulatory element to control the expression of numerous virulence factors that may not be related; these are sometimes termed global regulators. It has also become apparent that virulence factor expression is a complex interdependent process relying on various cues from the host and the pathogen. The expression of a number of virulence factors may be coordinated simultaneously by several different regulatory elements; alternatively, a single virulence factor can come under the control of several regulatory elements.
2. Sources and Routes of Infection
2.1. Natural Infection
Pathogenic bacteria are widely distributed and can be found in the soil, other animals or humans, food, or water, depending on bacterial species. All these represent potential sources of infection. Infection can be via a number of routes, such as inhalation, ingestion, abrasion to the skin, contaminated blood, or the bite of an insect vector. The way in which infection is established will again depend on the microorganism of concern and may also have an effect on the predicted outcome of the disease. For example, an organism such asB. anthracis, the aetiological agent of anthrax, has a number of different forms. In the case of humans, there are three main forms of the anthrax disease: cutaneous, inhalational, and gastrointestinal. Any of these types of infection can result in systemic anthrax, which is nearly always fatal (Mock and Fouet 2001). The severity of disease is usually greatest with inhalational anthrax, and if untreated this form of the disease has a mortality rate approaching 100% (Turnbull 1991, Webb 2003). Cutaneous anthrax is often self-limiting with or without the appropriate treatment (Hambleton and Turnbull 1990). There are a number of diseases that are of importance throughout the world; however, there are three areas for which the rapid sensitive detection of pathogenic microorganisms has been addressed in this review: food- and water-borne pathogens, hospital acquired infections, and the intentional use of pathogenic bacteria for biological warfare or bioterrorism. These are discussed mainly in the context of human infections, but obviously there is a range of pathogenic microorganisms that can infect animals and plants and are of importance, but that will not be discussed in detail within this review.
2.2. Food and Water