Utilization of Lampung Natural Zeolite as Immobilization Media
on Biogas Production from Palm Oil Mill Effluent (POME)
SRI Ismiyati Damayanti
1,a, SIMPARMIN Br.Ginting
2,b,
AMELIA Virgiyani Sofyan
2,c, ALIP Tania Putri
2,d, WIRATNI Budhijanto
1,e* 1Chemical Enginering Department, Engineering Faculty,Universitas Gadjah Mada, Grafika Street, Yogyakarta 55281, Indonesia
2Chemical Engineering, Engineering Faculty,
University of Lampung, Soemantri Brojonegoro Street, Bandar Lampung 35141, Indonesia
a[email protected], b[email protected], c[email protected], d[email protected], e[email protected]
Keywords: Biogas; Immobilization Media; Zeolite; AFBR; Methane Concentration
Abstract. Lampung is one of provinces with wide area of palm oil plantations and processing
industries in Indonesia. Each palm oil mill produces 0.7-1 m3 of POME per ton of fresh palm
bunches and pollutes the environment when untreated. Furthermore, Lampung is also a region with abundant Natural Zeolites (ZAL) availability. This research was performed to study the influences of modified ZAL as the microorganisms immobilization media (Biocarrier) in biogas production of POME. Hereafter, ZAL as Biocarrier will be applied on Anaerobic Fluidized Bed Reactor (AFBR). AFBR with biocarrier will have higher efficiency than conventional biogas reactor. Furthermore the vertical design of the AFBR does not require excessive land. Modification of ZAL was conducted to provide appropriate place for microorganisms, especially methanogen. This research was started by physical modification of ZAL (CV. Minatama, Lampung), followed by impregnation of ZAL by
Fe2+ and Mg2+. Each of the modified ZAL was analyzed by XRF, then characterized by XRD and
FTIR before used. Based on XRF analysis, SiO2/Al2O3 mol ratio of physical activated ZAL was
8.913, while ZAL-Fe2+, and ZAL-Mg2+ were 9.957 and 8.8, respectively. The anaerobic process
took place in a 2.8 L batch anaerobic digester by adding POME and an active digester effluent of cattle manure as microbial inoculum and 280 grams of each type of the modified ZALs to each digester. Result showed that that impregnated ZAL decreased the organic content of POME more rapidly than the physically treated ZAL. Besides, impregnated zeolite also resulted in higher methane concentration than the digester using physical activated.
Introduction
Indonesia is one of country known as the largest palm oil producer with 33.5 million tonnes of Crude Palm Oil (CPO) in 2016. According the data, it is estimated that Indonesia’s palm oil production will increase to 45 million tonnes of total CPO production in 2020. Indonesia’s crude palm oil production is spread in many provinces, one of them is Lampung province with 202,774 hectares of plantation area, 504 million tonnes of annual crude palm oil production, and will be still increasing further towards the national target in 2020 [1]. This estimation of fast growing production leads to more waste, especially liquid waste. Each palm plantation produces
0.7-1 m3 of Palm Oil Mill Effluent (POME) per ton of fresh palm bunches with acidic condition, pH
of 3.3-4.6 and methane emission to the atmosphere when untreated [2]. Direct discharge POME to environment will degrade water quality because of its organic content, which is as high as 15,103-65,100 mg/L of COD [3]. With the high organic content, however, actually POME can be converted into energy in the form of biogas (50-70% of methane) [4, 5].
Besides the richness from palm oil plantation, Lampung is also a region that has an easily
obtained and abundant Natural Zeolites (ZAL), which records a reserve of 2.14 million m3 of ZAL
[6]. Zeolite is a mineral of hydrated alumina silicate crystals containing alkali cations in three-dimensional framework [7]. Lampung Natural zeolite type is Clinoptilolite with SiO2/Al2O3 mole
ratio of 8.913 [8] and mean pore size of 3x7.6 Å [9].
In order to utilize the abundance of the POME waste and also ZAL, this research was performed to utilize POME as a raw in biogas production and to use zeolites as the immobilization media. Hereafter, ZAL as biocarrier of the anaerobic microbes will be applied on Anaerobic Fluidized Bed Reactor (AFBR). AFBR with microbial biocarrier will have higher efficiency than conventional biogas reactor. For stronger effect of ZAL to attract microbes, this study used modified ZAL by magnesium and iron impregnation as trace elements required in biogas production through anaerobic digestion of POME. THe trace elements are necessary to increase the performance of methanogens. There are several trace elements for methanogens, especially cobalt, iron, nickel, and magnesium [4, 12]. This research tested iron and magnesium as the impregnated cation into ZAL.
Methodology
This research was carried at the Laboratory of Applied Chemistry and Research, Chemical Engineering, University of Lampung. Raw materials in this study was POME collected from one of palm oil mill in Lampung, an active digester effluent (cattle manure) of Biogas Installation in Kediri village, Gading Rejo Pringsewu, Lampung as microbial inoculum, and ZAL of CV. Minatama
Lampung. The ZAL was activated physically by aquadest and dried in an oven at 110oC for 3 h.
The characteristics of each material used in this research were presented in Table 1, 2, and 3, respectively:
Table 1. Characteristics of Lampung Palm Oil Mill Effluent (POME)
Parameter Value Unit
pH 4 -
Chemical Oxygen Demand 11,995 [mg/L]
Protein 0.15 [%]
Potassium 1,459.86 [mg/L]
Sulphate 1,032.93 [mg/L]
Ammonia 125 [mg/L]
Source: [11]
Table 2. Characteristics of Active Digester Effluent
Parameter Value Unit
pH 7.5 -
Chemical Oxygen Demand 1,250 [mg/L]
Volatile Solid 34,968 [mg/L]
Volatile Fatty Acid 201,000 [mg acetic acid/L]
Source: Analysis Data, 2016
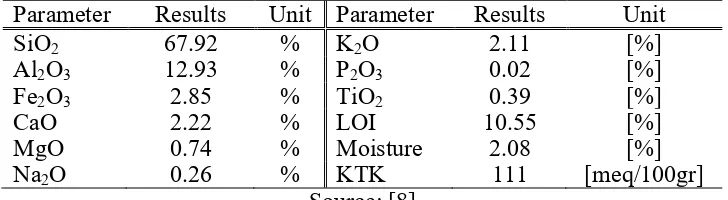
Table 3. Characteristics of Lampung Natural Zeolite (ZAL)
Parameter Results Unit Parameter Results Unit
Experiment Procedures
Modification of ZAL by Mg2+ and Fe2+ Impregnation. Twenty grams of physical activated
ZAL was added into 2 L of 400 ppm FeSO3.3H2O (Uni-Chem) for Fe impregnation and into 2 L of
400 ppm of MgSO4.7H2O (Uni-Chem) solution for Mg impregnation. The suspension was stirred
by magnetic stirrer for 30 minutes and then let be immersed for 24 hours. The ZAL was then
filtered and calcined at a temperature of 500oC for 1 hour. Those procedures were done until 280
gram modified ZAL was obtained [13].
Modified ZAL Characterization and Analysis. All modified ZAL were characterized by Fourier Transform Infrared Spectroscopy (FTIR) and X-ray Diffraction (XRD), and then analyzed by X-ray fluorescence (XRF).
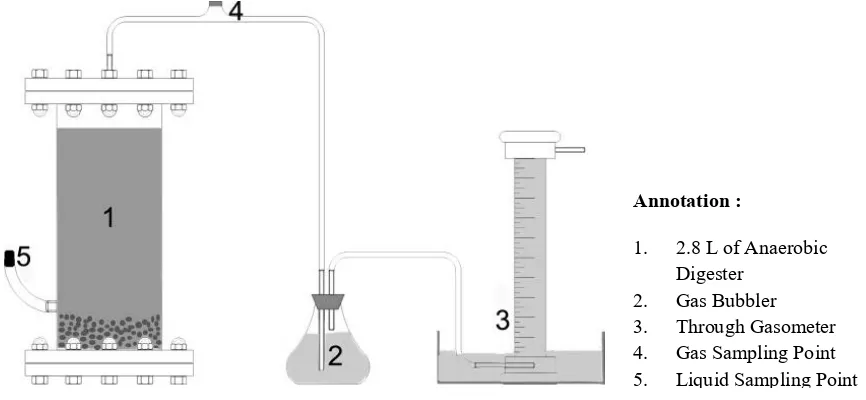
Anaerobic Digestion of Biogas Production. This study used batch digester to produce biogas in anaerobic digestion with 2.8 L of total volume and 2.1 of working volume. The reactor was connected to bubbler as gas visual indicator and through gasometer for gas quantitative measurement (Fig. 1). The gasometer was filled with acidified salt water to prevent biogas solubility [14]. The experiment was carried by added 280 grams of each modified ZAL (physical
activated, ZAL-Mg2+, and ZAL-Fe2+) and diluted POME (sCOD 5,000 mg/L) that mixed with
active digester effluent on ratio 3:1 (v/v) into every single digester. The nitrogen flushing was done for five minutes prior to the process starting time.
Figure 1. Experimental batch digester with ZAL equipped with bubbler and through gasometer for gas measurement
Biogas Analysis. Analysis of biogas included pH, sCOD, VFA, and methane concentration. The value of pH was analyzed to determine the conditions inside the digester using pH meter calibrated in 4 and 9 buffer solutions. Soluble Chemical Oxygen Demand (sCOD) analysis was conducted to determine the organic matter content of substrate, by taking liquid samples from each digester and
then analyzed them using HACH Colorimeter DR900. Volatile Fatty Acid (VFA analysis was
conducted by distillation method, measuring VFA concentration as acetic acid [15]. Methane concentration was measured by Shimadzu Gas Chromatography.
Annotation :
1. 2.8 L of Anaerobic Digester
Result and Discussion
Characterization and Analysis of Modified ZAL
Fourier Transform Infrared Spectroscopy (FTIR) and X-ray Diffraction (XRD) Characterization
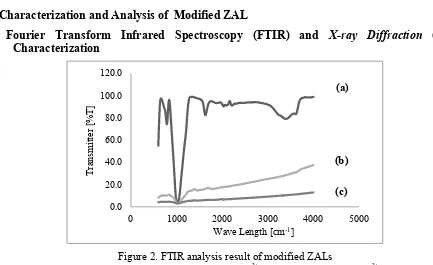
Figure 2.FTIR analysis result of modified ZALs
(a. Natural ZAL, b. ZAL Impragnated Fe2+, c. ZAL Impragnated Mg2+)
Table 4. Comparison of FTIR sample wave numbers of physical activated ZAL, ZAL impregnated with Fe2+ and ZAL impregnated with Mg2+
Information Wave Numbers [cm
-1
]
Natural ZAL Fe2+ Mg2+
O-H Stretch Vibration 3395,41 - -
O-H Buckling Vibration 1632,93 1763,52 1768,44
Asymmetric Stretch Vibration
of Si-O or Al-O on TO4
1017,96 1019,78 1026,66
Si-O Groups 787,78 - 779,27
T-O Buckling Vibration - 445,95 526,56
Fe-O Vibration - - -
Band absorption peaks on modified ZAL by Fe2+ and Mg2+ impregnation changed as shown in
Fig. 2 and Table 4/ The changes showed the presence of H atoms that were exchanged with Fe2+
and Mg2+ metals. Nonetheless, ZAL-Fe2+ and ZAL-Mg2+ had the same properties with Natural ZAL
as shown in Table 3, which showed the same groups.
a b
0 1000 2000 3000 4000 5000
Fig. 3 shows that ZAL-Fe2+ had peaks at 2θ = 11.066o; 17.2599o; 19.0123o; 24.980o; and 28.487o, while ZAL-Mg2+ had peaks at 2θ = 22.35o; 23.15o; 26.112o; 32.0401o; and 35.8101o.
X-ray fluorescence (XRF) Analysis
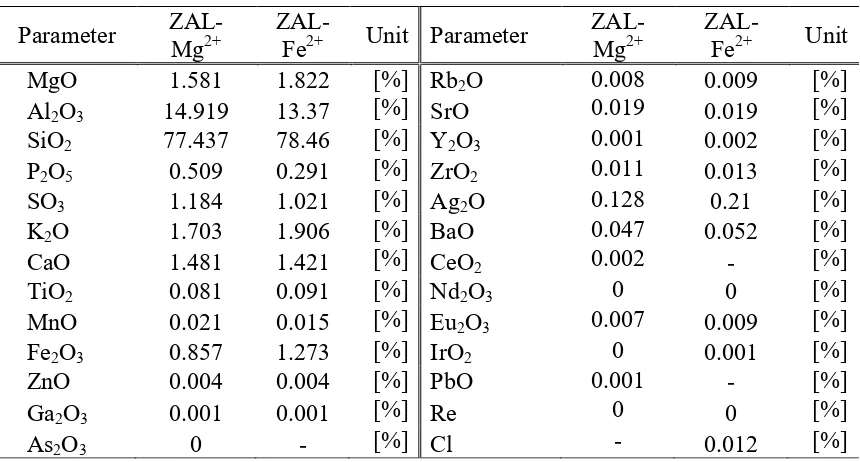
Table 5. XRF analysis result of modified ZAL-Mg2+ and ZAL-Fe2+
Parameter
ZAL-Table 5 shows the elements and their concentrations in the modified ZAL-Mg2+ and ZAL-Fe2+.
According to the data, ZAL Mg2+ had SiO2/Al2O3 mole ratio of 8.8 and ZAL- Fe2+ of 9.957, while
physical activated ZAL’s mole ratio was 8.913.
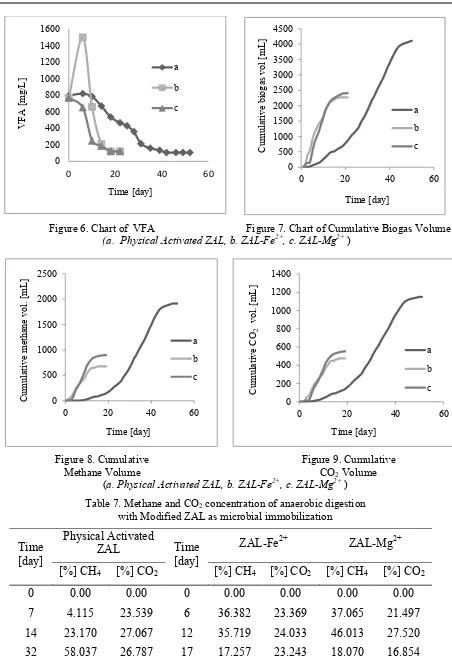
Biogas Analysis. The effect of modified ZAL addition in this research could be investigated by monitoring the pH of the digester, concentration of substrate, and the concentration of product in anaerobic digestion which was measured by sCOD and VFA as the product of acidogenic stage as well as substrate for methanogenic stage, and methane concentration. Initial condition data is shown in Table 6. Complete digesters experiment data of pH, sCOD, VFA, cumulative biogas volume, and methane concentration are shown in Fig. 4-9 and Table 7, respectively.
Table 6. Initial Condition Data
Modified ZAL pH SCOD
Figure 6. Chart of VFA Figure 7. Chart of Cumulative Biogas Volume
(a. Physical Activated ZAL, b. ZAL-Fe2+, c. ZAL-Mg2+ )
Table 7. Methane and CO2 concentration of anaerobic digestion
with Modified ZAL as microbial immobilization
Discussion
All modified ZALs were characterized and analyzed before being used in digester as microbial immobilization in the anaerobic digestion on biogas production from POME. Physically activated
ZAL properties was considered still the same as Natural ZAL properties. According to Fig.2, Fe2+
impregnation was not quite successful yet. The lack of Fe2+ is also shown by the reduction of Fe2O3
concentration in Table 5. This failure was occurred since FeSO4(3H2O) particle size was wider than
ZAL mean pore size of 3x7.6 Å [9]. However, Fe2+ as immobilization media trace element was
found on ZAL surface, which was shown by the color of orange. This deposited Fe2+ might cause
ZAL component release in acidic condition of pH 3 and increased SiO2/Al2O3 mole ratio to 9.957
(higher than 8.913 of natural ZAL). Table 5 shows the increasing MgO concentration on ZAL-Mg2+, 1.581% higher than 0.74% on natural ZAL and reducing of SiO2/Al2O3 mole ratio of 8.8
lower than 8.913 on natural ZAL. This study used different modified ZAL crystal type as immobilization media on each digester. According to research of Razzak [17] XRD results, ZAL
had three highest peaks on 2θ, they were 27.9583; 22.3963; 9.8631, however strongest peaks of ZAL-Fe2+ were 11.066o; 17.2599o; 19.0123o; 24.980o; and 28.487o, there was no similar peaks
between natural ZAL and ZAL-Fe2+. These strongest peaks conversion indicated that ZAL-Fe2+
crystal type was changed to Henlaundite-Na Monoclinic type with strongest peaks on 2θ of 11.066o; 17.2599 o; 19.0123 o ; 24.980 o; and 28.487 o on almost similar d-spacing. While ZAL-Mg2+ was still in Clinoptilolite because of its strongest peaks on 2θ : 22.35o.
According to Table 6, initial conditions of substrates that used in all digesters were in similar range, such as pH, sCOD, and VFA. These processes took place at optimum pH of methanogenic bacterial activity, that was 6.5-7.2 [5]. According to Fig.4, it can be seen that significant decreasing
of pH was obtained in digester with ZAL-Fe2+ and ZAL-Mg2+ until day-6. This condition was
caused by ZAL-Fe2+ and ZAL-Mg2+ that stimulated organic substrates (sCOD) degradation to
acidic VFA. After day-6 pH of ZAL-Fe2+ and ZAL-Mg2+ digester increased and keep constant on
range of pH 7 because of fast VFA degradation. However the highest pH increasing on digester
with ZAL-Mg2+ to 7.1 was caused by alkaline cations in ZAL-Mg2+ such as Ca, K, and more Mg
(1.822% in Table 5) that can be exchanged with H+ as pH neutralizer [18].
Based on research of Ersoy and Celik [19], zeolite is an adsorben, ion exchanger, and catalyst that increases organic matters degradation with zeolite structure pore roles. Fig.5 showed decreasing of organic molecule’s chemical oxygen requirement that measured by sCOD in organic molecules degradation (Hydrolysis). There was more significant sCOD reduction on immobilized
anaerobic digestion by ZAL-Fe2+ and ZAL-Mg2+ in 21 days, than immobilized digester by physical
activated ZAL’s sCOD reduction that was in 55 days. This condition caused by both of ZAL with Fe2+ and Mg2+ which were thermal impregnated in 500 oC, that resulted cleaner and wider pores than physical activated ZAL, so they absorbed more sCOD than physical activated ZAL.
Hydrolysis products were decomposed into VFA on acidogenesis stage [12] that showed in
Fig.6. According to it, ZAL with Fe2+ increased VFA significantly from 769.121 mg/L in day 0 to
1500 mg/L in day 6, while VFA of ZAL-Mg2+ decreased until the end of digestion period. This
condition indicated that methanogenic bacteria was in a great condition to degrade VFA into CO2
and CH4 [12]. VFA reduction was better in ZAL-Fe2+ and ZAL-Mg2+ digester, because zeolites
released Fe2+ and Mg2+ that could be micro carrier that improve metabolite transfer between
microorganisms [21].
The anaerobic processes were undertaken in different processing time of each physical activated
ZAL digester, ZAL-Fe2+, and ZAL-Mg2+, which were 55, 21, and 21 days, respectively. All
processes were stopped when there was no more change of sCOD, VFA, and biogas volume. Based on Fig.7, immobilized digesters by ZAL-Fe2+ and ZAL-Mg2+ required less processing time than physical activated ZAL to produce 2.27 , 2.4, and 4.1 L of biogas, respectively. Biogas volume that
resulted by ZAL-Fe2+ and ZAL-Mg2+ digester was lower than physically activated ZAL. It was
caused by the tendency of ZAL with Fe2+ and Mg2+ to adsorb more sCOD and VFA. Table 7
ZAL-Fe2+ and ZAL-Mg2+ stimulated hydrogenotrophic methanogenesis stage to convert acidogenesis
products into CH4 and CO2 [22]. The highest methane concentration was produced by each digester
of physical activated ZAL, ZAL-Fe2+, and ZAL-Mg2+ were 61.648% on day 41; 36.38% on day 6;
and 46.013% on day 12, respectively with methane volume result in Fig.8.
Conclusion
Based on the results and discussion of research can be concluded that :
a. Reduction of sCOD and VFA on biogas production from POME was not only occured by
anaerobic degradation. Some of sCOD and VFA contents also adsorbed into ZAL’s pore.
b. ZAL-Fe2+ and ZAL-Mg2+ adsorbed sCOD and VFA better than physical activated ZAL in
shorter processing time, caused by cleaner pores and cationics.
c. Modified ZAL with trace elements of Fe2+ and Mg2+ stimulated methanogenesis stage to degrade
VFA to methane more rapidly, that were 35% and 45% respectively, in 10 days.
Acknowledgment
This research was funded through the competitive research grant “Tim Pascasarjana”, from the Ministry of Research, Technology, and Higher Education (first year/2017).
References
[1] Directorate General of Estate, Buku Statistik Komoditas Kelapa Sawit Indonesia, Directorate General of Estate, Jakarta, 2014.
[2] Winrock International, Buku Panduan Konversi POME Menjadi Biogas Pengembangan Proyek di Indonesia. Winrock International, Jakarta, 2015.
[3] Indonesia Government Rule of Environment Number 3 about Wastewater Quality Standard, 2010.
[4] Gerardi, M. H., The Microbiology of Anaerobic Digesters, John Wiley & Sons, New York, 2003.
[5] Deublein, D., Steinhauser, A., Biogas From Waste and Renewable Resources An Introduction, Weinheim, Wiley-Vch Verlag GmbH & Co., 2008.
[6] Asriani, Profil Lampung Mencakup Data Penduduk Sumber Daya Alam dan Ekonomi Makro, 2008.
[7] Information on http://jurnal.pdii.lipi.go.id/admin/jurnal/
[8] CV Minatama., Certificate of Sampling and Analysis Zeolite, Lampung, 2013
[9] O. Korkuna., R. Leboda., J. Skubiszewska-Zieba., T. Vrublevs’ka., V.M. Gun’ko., J. Ryczkowski, Structural and physicochemical properties of natural zeolite : clinoptilolite and mordenite, Micropore Mesopor Mater. 87 (2006) 243-254.
[10] Damayanti, S. I., Pemanfaatan Stillage Menjadi Biogas Melalui Proses Co-digestion Stillage dan Kotoran Sapi, Thesis in Chemical Engineering Department, Engineering Faculty, Universitas Gadjah Mada, Yogyakarta, 2012.
[11] Halim, L., Peningkatan Produksi Biogas dari Stillage dengan Imobilisasi Bakteri Anaerobik pada Media Padatan Berpori, Thesis in Chemical Engineering Department, Engineering Faculty, Universitas Gadjah Mada, Yogyakarta, 2015.
[13] Rina, U., Modified Zeolite with Nanokitosan As Heavy Metal Ion Adsorbent and Study Kinetikanya Against Ion Pb (II), Faculty of Mathematics and Natural Sciences Graduate Program of Chemistry, Universitas Indonesia, Depok, 2012.
[14] Walker, M., Zhang, Y., Heaven, S., Banks, C., Potential errors in the quantitative evaluation of biogas production in anaerobic digestion processes, Bioresource Technology. 100 (2009) 6339-6346.
[15] APHA, Standard Methods for The Examination of Water and Wastewater, New York, 2005.
[16] Petcharoen, K., Sirivat, A., Materials Science and Engineering. 29 (2012) 249-261.
[17] Razzak, Mirzan T., Las, Thamzil., and Priyambodo., The characterization of Indonesian’s natural zeolite for water filtration system center for integrated laboratory, Valensi, 3 (2013) 129-137.
[18] Susanti.P.D and Panjaitan.S., Manfaat zeolit dan rock phosphat dalam pengomposan limbah
pasar, Standard Proceeding August 4th 2010 ISSN 2000-0188, Banjarmasin, 2010.
[19] Ersoy.B and Celik.M.S., Effect of hydrocarbon chain length on adsorption of cationic into clinoptilolite, Journal Clays and Clays Minerals, 51 (2003) 172-180.
[20] Darmansyah, Lilis Hermida, Arjun Fatahillah, M.Yuli Atrafatin., Sintesis dan aplikasi zeolit modifikasi surfaktan sebagai adsorben limbah cair tapioka (perbandingan dengan zeolit alam kalsinasi), Proceeding of National Seminar “Contribution of Academician in Sustainable Development”, Universitas Brawijaya, Malang, 2016.
[21] Cavaleiro, A.J., Sousa, D.Z., Alves, M.M., Methane production from oleate: assesing the bioaugmentation potential of syntrophomonas zehnderi, Water Research. 44 2010 4940-4947.