with Neuropathologic Evidence of Increased Vascular
or Alzheimer-Type Pathology
John O’Brien, Alan Thomas, Clive Ballard, Andrew Brown, Nicol Ferrier,
Evelyn Jaros, and Robert Perry
Background:Cognitive impairment is common in depres-sion, but underlying mechanisms remain unknown. We examined whether increases in Alzheimer-type or vascular pathology are associated with cognitive impairments in elderly depressed subjects.
Methods: Eleven subjects who had died during a well-documented episode of DSM-IV major depression were included. Neuropathologic assessments, blind to group membership, included standardized assessment of neuritic plaques, neurofibrillary tangles, and Lewy Bodies in frontal, temporal, parietal, and occipital cortices. Braak staging of Alzheimer pathology was also performed. Ce-rebral microvascular disease was scored according to a previously validated scale, and a score for cerebral and systemic atheroma of large and medium sized arteries was obtained.
Results:No subject had Lewy bodies. Plaque and tangle counts for all subjects were well within published norms for age-matched control subjects. There were no signifi-cant differences in plaque or tangle counts between subjects who were cognitively impaired (n55) and those who were nonimpaired (n 5 6) during their depressive illness. Similarly, neither total microvascular pathology nor deep frontal microvascular pathology differed be-tween the two groups.
Conclusions: Our results indicate that the liability for some patients to develop cognitive impairment during a depressive episode is not related to an increase in Alzhe-imer-type or vascular neuropathologic change. This indi-cates that other mechanisms must underlie both the cognitive impairment associated with depression and the observation that depression is a risk factor for dementia. Biol Psychiatry 2001;49:130 –136 ©2001 Society of Bi-ological Psychiatry
Key Words:Depression, affective disorder, neuropathol-ogy, cognitive impairment, postmortem, elderly
Introduction
I
t is well known that depression, particularly in elderly subjects, is associated with a number of deficits in cognitive function (Abas et al 1990; Austin et al 1999; Beats et al 1996). These deficits, when severe, can resemble those seen in an organic dementia such as Alzheimer’s disease, giving rise to the clinical syndrome of “pseudo dementia” and the emphasis placed on trying to obtain an accurate diagnosis in such patients (Bulbena and Berrios 1986; McAllister 1985). It is now clear that the relationship between cognitive impairments occurring as part of a depressive disorder and those seen in organic conditions is complex. Cognitive impairments occurring during depression do not always remit on recovery from illness. For example, Abas et al (1990) found that whereas 70% of depressed subjects were impaired on a learning task during illness, 30% remained impaired despite clini-cal recovery. The cause of such enduring cognitive deficits is unknown, though they may carry an adverse prognosis with regard to long-term outcome. Kral and Emery (1989) followed up 44 elderly patients who had previously suffered from what was thought to be “depressive pseudodementia” on the grounds that cognitive function reverted to premorbid levels following treatment. After an average follow-up of 8 years, 89% were felt to have developed Alzheimer’s disease. Epidemiologic studies have shown that depression is an independent risk factor for dementia (Jorm et al 1991). These lines of evidence suggest that cognitive impairment occurring during a depressive illness may indicate an increased liability to subsequently develop dementia and, in particular, Alzhei-mer’s disease. If so, neuropathologic study of such cases might be expected to show a greater burden of Alzheimer type pathology in those depressed subjects who develop significant cognitive dysfunction compared to those who do not.From the Institute for the Health of the Elderly (JO, AT, CB, AB, EJ, RP) and the Department of Psychiatry (NF), University of Newcastle upon Tyne, Newcastle upon Tyne, United Kingdom.
Address reprint requests to John O’Brien, D.M., Newcastle General Hospital, Wolfson Research Centre, Institute for the Health of the Elderly, Westgate Road, Newcastle upon Tyne NE4 6BE, United Kingdom.
Received January 3, 2000; revised May 19, 2000; accepted May 26, 2000.
There are, however, other reasons why elderly de-pressed patients may develop cognitive impairments. It is now clear that neuropsychological deficits affect most domains tested and include problems with attention, learn-ing and memory, frontal and executive tasks, as well as motivation (Abas et al 1990; Austin et al 1999; Beats et al 1996; Elliot 1998; Salloway et al 1996). It has also been suggested that some impairments, particularly those in learning and memory, may be associated with hippocam-pal damage due to the raised levels of corticosteroids known to be associated with depressive illness, particu-larly in the elderly (O’Brien 1997). The recent demonstra-tion of reduced hippocampal volume in depressed subjects that correlated with lifetime duration of depression (and therefore presumably exposure to corticosteroids) supports this view (Sheline et al 1999).
Another possible explanation for depressed subjects developing cognitive impairment is the presence of cere-brovascular pathology. One of the most consistently ob-served abnormalities in elderly depressed subjects is of an increased frequency of hyperintensities in the white matter and subcortical grey matter visualized on magnetic reso-nance imaging (Coffey et al 1990; Figiel et al 1991; Greenwald et al 1996, 1998; O’Brien et al 1996). These lesions are more common in depressed subjects with onset in late life (Figiel et al 1991; O’Brien et al 1996; Salloway et al 1996) and have been associated with cognitive impairments both during the depressive episode (Salloway et al 1996) and at follow-up (Hickie et al 1997). Partly on the basis of these lesions and the link between depression and cardiovascular disorder, the notion of “vascular de-pression” has been proposed (Alexopoulos et al 1997; Hickie and Scott 1998; Krishnan and McDonald 1995). Because microvascular change is a cause of white matter hyperintensities on magnetic resonance imaging (MRI) (Chimowitz et al 1992) and such lesions are associated with cognitive impairments in depression, it is possible that cognitive impairments occurring during depressive episode may be related to an increase in microvascular pathology.
There have been no previous studies of the neuropatho-logic basis of the cognitive impairments that occur during depression. We wished to test the hypothesis that such impairments would be associated with an increased burden of Alzheimer-type and/or microvascular pathology in cortical areas.
Methods and Materials
Subjects
Cases were obtained from records of the Neuropathology De-partment and the Newcastle Brain Tissue Bank. All depressed cases were from referrals to a secondary care unit, the Brighton
Clinic. All were inpatients except one who was attending the day hospital. Full ethical approval has been granted for this postmor-tem study and consent for postmorpostmor-tem examination obtained from the next of kin.
Clinical Assessment
Subjects were included if they were 60 years or over at death and had died during a well-documented episode of DSM-IV Major Depression (American Psychiatric Association 1994). They had all undergone extensive assessments including a full history, mental state examination, physical examination, screening blood tests and, in some cases, computed tomography (CT) or MRI scans. Subjects were excluded if they had committed suicide by violent means or had ever met DSM-IV criteria for dementia, schizophrenia, or other psychotic disorders, manic or hypomanic episode or a mood disorder due to a general medical condition. Subjects were divided into a cognitively impaired group who were significantly impaired when depressed, and a nonimpaired group who showed no evidence of impairment even when depressed.
Neuropathologic Assessment
All subjects received a full postmortem examination (except one whose body was taken by the undertakers after the brain was removed) and the delay to postmortem was recorded. The right hemisphere was fixed in 10% formalin and the brains were dissected in a standard manner (Perry et al 1990a). Blocks were taken from the frontal, temporal, parietal, and occipital cortices, the hippocampus, the cingulate, the basal ganglia, the cerebel-lum, the upper and lower midbrain, the pons and the medulla. Fivemm or 20mm sections were cut, and sections were prepared on glass slides. These were stained with haematoxylin and eosin (H&E), luxol fast blue (LFB) and/or Loyez (myelin), cresyl fast violet (CFV) or Nissl, methanamine silver and/or von Braunmuhl (plaques), Palmgren and/or tau-2 (tangles) and ubiquitin (Lewy bodies). These were then examined by experienced neuropath-ologists (RP and EJ) Subjects were excluded if they met neuropathologic criteria for any neurodegenerative disease such as Alzheimer’s disease or dementia with Lewy bodies.
Assessment of Alzheimer and Lewy Body Pathology
selected at random on 5 occasions for plaque and tangle counts. Co-efficients of variation (SD/mean) were 0% for tangle counts and 5.3% for plaque counts.
Assessment of Large and Medium-Sized Arteries
The extent of atheromatous degeneration in the coronary arteries, cerebral carotid and vertebrobasilar systems, and aorta was rated on a semiquantitative 0 –3 scale (RP and AT). 05none; 15 mild: atheroma only mild and patchy; 2 5 moderate: more extensive atheroma with less than 50% luminal stenosis; 3 5 severe: atheroma widespread with greater than 50% luminal stenosis in at least one vessel. An overall atheroma score was obtained for each subject by adding together the scores from each site.
Assessment of Small Arteries and Arterioles
For assessing microvascular disease the sections stained with H&E, LFB, and/or Loyez and Nissl from the frontal, temporal, parietal, and occipital cortices were used. In addition, large full-face coronal blocks were taken from the frontal and occipital cortices to include all the deep white matter in these two areas, coronal levels 4/5 and 27 (Perry 1993). Ten-mm or 20-mm sections were prepared on large (30 320) slides and stained with H&E, LFB, and/or Loyez (myelin) and Nissl. Microvascular disease was then scored blind to diagnosis by two raters (RP and AT) in each neocortical area in accordance with a scale previ-ously used in vascular dementia (Esiri et al 1997). Briefly, this is a 0 –3 scale in which 05normal; 15dilatation of perivascular spaces or hyaline thickening or a few perivascular macrophages; 25dilatation of perivascular spaces or hyaline thickening plus mild to moderate perivascular pallor of myelin staining or loosening of the nerve fibres or nerve cells with gliosis; 3 5 Binswanger’s disease (2 plus more widespread myelin pallor, nerve fibre or nerve cell disruption and gliosis). An overall microvascular score was calculated for each subject by adding together the scores for each neocortical area. A separate score was obtained by assessment of the frontal deep white matter.
Statistical Analysis
The two groups were compared statistically using standard software. Minitab (version 12; Minitab, State College, PA) was used to carry out Mann–Whitney tests for the quantitative data (age, post-mortem interval, plaques, tangles, Lewy bodies) and StatXact (version 4, Cytel Software, Cambridge, MA) was used to carry out Fisher–Freeman–Halton test (more appropriate than x2 because of the low cell frequency) for the categorical data (sex, Braak, atheroma, and microvascular scores).
Results
Eleven subjects were studied. Eight suffered from recur-rent unipolar depression, and all but one were depressed at death. All except one had received past treatment with
electroconvulsive therapy. Five subjects were cognitively impaired when depressed. Of these, two had such marked psychomotor retardation that meaningful standardized cognitive testing was not possible, though both were clearly clinically very impaired, as for a time genuine doubt existed as to whether or not they had an organic dementia or a “depressive pseudodementia.” Testing of the other three showed scores in the impaired range on either the Mini-Mental State Examination (MMSE, maximum score 30; Folstein et al 1975) or the Mental Test Score (MTS, maximum score 37; Blessed et al 1968). One subject had an MMSE score of 22; the others had MTS scores of 22 and 25. Six subjects showed no clinical evidence of cognitive impairment. Two were recorded in casenotes as having undergone cognitive testing and being completely oriented, with normal concentration and mem-ory, although neither the MMSE nor MTS was completed. Tests on the other four revealed MMSE scores of 28 and 29 and MTS scores of 36 and 37. Cause of death for the cognitively impaired patients was carcinoma (n5 2) and bronchopneumonia (n 5 3). Cause of death for the nonimpaired patients was cardiac arrest (n5 3), suicide (n 5 1), pulmonary embolus (n 5 1) and congestive cardiac failure (n 5 1). Four of the five cognitively impaired patients had failed to respond to adequate courses of two different antidepressants. Of unimpaired subjects, two had failed to respond to adequate courses of two different antidepressants, two had shown some re-sponse but were still depressed, and two died without apparent response to an antidepressant.
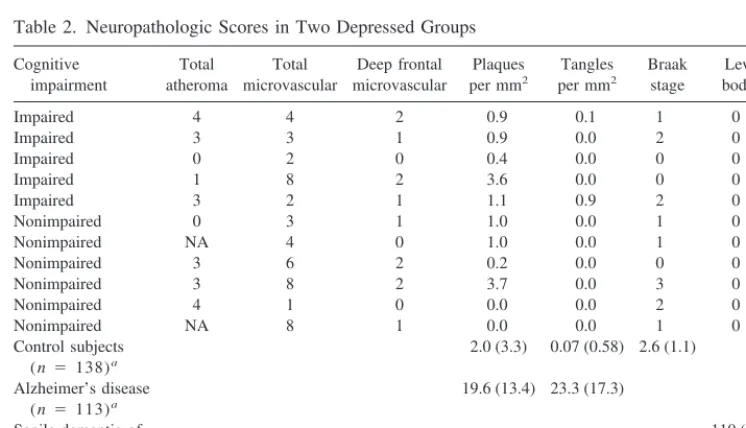
Subject characteristics and results are shown in Table 1, whereas Table 2 contains a detailed breakdown of neuro-pathologic scores for each subject. For comparison, scores are also presented in Table 2 from published data on control and demented subjects of very similar age obtained using the same methodology (Perry et al 1996). There were no significant differences in age (t5 36, p5 .31) or postmortem delay (t 5 22, p 5 .31), but there was a trend toward a difference in gender (Fisher statistic 4.8, p5.06), because all five cognitively impaired depressed subjects were female. No statistical differences were found between the groups on Alzheimer-type pathology (p .
.41) or Lewy body pathology (p 5 1.00, no Lewy
bodies were seen in any case). The total atheroma and total microvascular scores were very similar (Fisher statistic5
Discussion
This is the first study to investigate neuropathologic changes in depressed subjects with and without cognitive impairment. We found no evidence of increased neuro-pathologic abnormalities in the form of Alzheimer-type pathology or microvascular change in depressed subjects who developed cognitive impairment during depression compared to those who did not. A relationship with Alzheimer pathology might have been expected because of the similarity of some aspects of cognitive dysfunction in depression to those seen in Alzheimer’s disease (Abas et al 1990), the high proportion of cognitively impaired depressed patients who subsequently develop the disorder (Kral and Emery 1989), and the fact that depression is a risk factor for Alzheimer’s disease (Jorm et al 1991). The
original study of Blessed et al (1968) which found a correlation, now replicated by several groups, between plaque count and cognitive test score in dementia included some cases with functional illness, seven of whom were depressed; however, our more detailed study (which does not include any of the same cases used by Blessed et al) shows that this well-recognized relationship in dementia does not apply to cognitive impairment in depression.
Minimal Alzheimer-type pathology was seen in the depressed cases, of the same order as would be expected in age-matched control subjects (Perry et al 1990b). Micro-vascular pathology was also not extensive using currently available methods of assessment (Esiri et al 1997). Al-though the link between vascular disease and late life depression is well recognised (Hickie and Scott 1998), such pathology did not explain the occurrence of
signifi-Table 1. Comparison of Cognitively Impaired and Nonimpaired Groups
Impaired Nonimpaired Test statistic Significance
Age (years)a 79.0 (4.69) 73.0 (8.81) 36 .31
Gender (male/female)b 0/5 4/2 4.8 .06
Postmortem delay (hours)a 47.5 (28.62) 27.8 (6.34) 22 .31
Total atheroma scoreb 3 3 1.42 1.00
Total microvascular scoreb 3 5 4.31 .84
Deep white matter frontal microvascularb
0 2 0.55 1.00
Neocortical plaques (per mm2)a 1.4 (1.27) 0.98 (1.41) 34 .52
Neocortical tangles (per mm2)a 0.2 (0.39) 0 (0) ,34 ..41
Braak stageb 1 1 2.59 .65
Lewy bodies (per cm2)a 0 (0) 0 (0) Not applicable 1.00
aValues are mean (SD) and comparisons are made using the Mann–Whitney test. bValues are medians and comparisons are made using the Fisher–Freeman–Halton test.
Table 2. Neuropathologic Scores in Two Depressed Groups
Cognitive impairment
Total atheroma
Total microvascular
Deep frontal microvascular
Plaques per mm2
Tangles per mm2
Braak stage
Lewy bodies
Impaired 4 4 2 0.9 0.1 1 0
Impaired 3 3 1 0.9 0.0 2 0
Impaired 0 2 0 0.4 0.0 0 0
Impaired 1 8 2 3.6 0.0 0 0
Impaired 3 2 1 1.1 0.9 2 0
Nonimpaired 0 3 1 1.0 0.0 1 0
Nonimpaired NA 4 0 1.0 0.0 1 0
Nonimpaired 3 6 2 0.2 0.0 0 0
Nonimpaired 3 8 2 3.7 0.0 3 0
Nonimpaired 4 1 0 0.0 0.0 2 0
Nonimpaired NA 8 1 0.0 0.0 1 0
Control subjects (n 5 138)a
2.0 (3.3) 0.07 (0.58) 2.6 (1.1)
Alzheimer’s disease (n 5 113)a
19.6 (13.4) 23.3 (17.3)
Senile dementia of Lewy body type (n 5 61)a
110 (134)
NA, not available.
cant cognitive dysfunction in our patients during their depression. Consistent with this, we found no difference between impaired and nonimpaired groups for atheroma-tous degeneration score that reflected cerebral and sys-temic large and medium vessel pathology.
Although many studies have examined neurotransmitter and receptor changes during depression, there has been only very limited focus on neuropathologic changes. This is partly because affective illness has been viewed as a state-dependent, reversible condition. This is, particularly in the elderly, now known to be flawed, as elderly depressed subjects have both enduring cognitive impair-ments (Abas et al 1990) and demonstrable neurobiological changes, which suggest structural abnormalities. For ex-ample, depressed subjects have increased cortical atrophy and ventricular enlargement when compared to control subjects (Elkis et al 1995; Jacoby and Levy 1980; Rabins et al 1991). In an MRI study, Rabins et al (1991) was unable to differentiate those with depression and Alzhei-mer’s disease on the basis of either cortical atrophy or the presence of white matter change. Our findings show that the causes of cognitive impairment and structural brain changes in depression lie outside those of degenerative or microvascular pathology. Results are consistent with the notion that deficits in some areas, particularly in learning and memory, may be related to excess cortisol secretion as a result of hypothalmic–pituitary–adrenal axis activation during the illness (O’Brien 1997). Several lines of evi-dence support this view. First, in animal models prolonged raised corticosteroid secretion is associated with deficits in learning and memory and hippocampal cell loss (Sapolsky and Plotsky 1990; Sapolsky et al 1986). Second, the hippocampus is a major site of glucocorticoid receptors, and at a cellular level excess glucocorticoids increase calcium dependent influx, which is known to be neuro-toxic (Landfield and Eldridge 1994). Third, following exogenous steroid administration or raised endogenous levels as a result of stress, cognitive deficits can be demonstrated in healthy subjects (Kirschbaum et al 1996). Fourth, in Cushing’s syndrome excess cortisol production is associated with cognitive impairments and hippocampal change (Starkman et al 1992). Finally, depression has been shown to be associated with mild hippocampal atrophy (Sheline et al 1999), which correlates with the total duration of depression. To determine whether hippocam-pal cell loss is a cause of cognitive decline associated with depression, further neuropathologic study of cell mor-phometry in the hippocampus would need to be performed.
Cognitive impairments during depression have also been associated with abnormalities in other brain areas, particularly the medial prefrontal cortex (Dolan et al 1992). Changes in similar brain areas have also been
described on MRI (Drevets et al 1997), and a preliminary neuropathologic report suggests glial loss in the absence of neuronal loss in one part of the prefrontal cortex, at least in younger patients with depression (Drevets et al 1998). Changes in these or related areas may also underlie some of the neuropsychological impairments that occur during depression. Salloway et al (1996) found white matter lesions to be associated with impairments in attentional function. Although such lesions may be an important correlate of impairment, we could find no evidence that this was accompanied by microvascular change, at least in the cortical areas that we examined (which did include frontal cortex). The tests of cognition used in this study, however, the MMSE and the MTS, are global measures of cognitive function not particularly sensitive to executive function, processing speed and other tasks subserved by frontal–subcortical areas. Because these are the regions most affected in imaging studies (Coffey et al 1993; Greenwald et al 1998), it may be that there would be correlations between microvascular pathology in these regions and more specific frontal tests.
Some other limitations of our study should be consid-ered. Our cases were not prospectively assessed, and so information regarding their depressive illness and cogni-tive function came from case notes; however, case notes within the service were of a very high quality, with detailed typed summaries. All cases had detailed comment about cognitive performance, and the majority had re-ceived a standardized assessment of cognitive function. Numbers of depressed subjects were small and, although the cognitive impairment documented during the depres-sive illness was felt to be secondary to depression, we were not able to document reversibility on recovery from the depression and so cannot be certain that this impair-ment was not due to another cause. Although this may have been a possible confounding explanation if we had found an increase in microvascular or Alzheimer type pathology in the group with cognitive impairment, we do not feel this can explain our negative results. Although our subjects were impaired on clinical measures of cognition, scores were in the moderate range and it may be that by studying a group with more pronounced cognitive impair-ments during depression correlations with neurodegenera-tive pathology may exist.
cholinergic loss may be important (Perry et al 1978). To investigate this further, in vivo study of subjects who have undergone detailed neuropsychological testing, assess-ment of endocrine function, and brain imaging should be combined with clinico-pathological study that includes detailed assessment of hippocampal cell morphometry, investigation of glucocorticoid receptor changes, and de-tailed neurochemical analysis.
The authors thank the Medical Research Council and the Stanley Foundation for financial support.
References
Abas MA, Sahakian BJ, Levy R (1990): Neuropsychological deficits and CT scan changes in elderly depressives.Psychol Med20:507–520.
Alexopoulos GS, Meyers BS, Young RC, Campbell S, Silbers-weig D, Charlson M (1997): “Vascular depression” hypoth-esis.Arch Gen Psychiatry54:915–922.
American Psychiatric Association (1994):Diagnostic and Sta-tistical Manual of Mental Disorders,4th ed. Washington, DC: American Psychiatric Association.
Austin M-P, Mitchell P, Wilhelm K, Parker G, Hickie I, Brodaty H, et al (1999): Cognitive function in depression: A distinct pattern of frontal impairment?Psychol Med29:73– 85. Beats BC, Sahakian BJ, Levy R (1996): Cognitive performance
in tests sensitive to frontal lobe dysfunction in the elderly depressed.Psychol Med26:591– 603.
Blessed G, Tomlinson BE, Roth M (1968): The association between quantitative measures of dementia and of senile change in the cerebral grey matter of elderly subjects. Br J Psychiatry114:797– 811.
Braak H, Braak E (1991): Neuropathological stageing of Alzhe-imer-related changes.Acta Neuropathol82:239 –59. Bulbena A, Berrios GE (1986): Pseudodementia: Facts and
figures.Br J Psychiatry148:87–94.
Chimowitz MI, Estes ML, Furlan AJ, Awad IA (1992): Further observations on the pathology of subcortical lesions identified on magnetic resonance imaging.Arch Neurol49:747–752. Coffey CE, Figiel GS, Djang WT, Weiner RD (1990):
Subcor-tical hyperintensity on magnetic resonance imaging: a com-parison of normal and depressed elderly subjects. Am J Psychiatry147:187–189.
Coffey CE, Wilkinson WE, Weiner RD, Parashos IA, Djang WT, Webb MC, et al (1993): Quantitative cerebral anatomy in depression: A controlled magnetic resonance imaging study.
Arch Gen Psychiatry50:7–15.
Dolan RJ, Bench CJ, Brown RG, Scott LC, Friston KJ, Frack-owiak RS (1992): Regional cerebral blood flow abnormalities in depressed patients with cognitive impairment. J Neurol Neurosurg Psychiatry55:768 –773.
Drevets WC, Ongur D, Price JL (1998): Neuroimaging abnor-malities in the subgenual prefrontal cortex: Implications for the pathophysiology of familial mood disorders.Mol Psychi-atry3:220 –226.
Drevets WC, Price JL, Simpson JR Jr, Todd RD, Reich T, Vannier M, Raichle ME (1997): Subgenual prefrontal cortex abnormalities in mood disorders.Nature386:824 – 827. Elkis H, Friedman L, Wise A, Meltzer HY (1995): Meta-analyses
of studies of ventricular enlargement and cortical sulcal prominence in mood disorders. Comparisons with controls or patients with schizophrenia. Arch Gen Psychiatry 52:735– 746.
Elliot R (1998): The neuropsychological profile in unipolar depression.Trends Cogn Sci2:447– 454.
Esiri MM, Wilcock GK, Morris JH (1997): Neuropathological assessment of the lesions of significance in vascular demen-tia.J Neurol Neurosurg Psychiatry63:749 –753.
Figiel GS, Krishnan KR, Doraiswamy PM, Rao VP, Nemeroff CB, Boyko OB (1991): Subcortical hyperintensities on brain magnetic resonance imaging: A comparison between late age onset and early onset elderly depressed subjects. Neurobiol Aging12:245–247.
Folstein MF, Folstein SE, McHugh PR (1975): “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician.J Psychiatr Res12:189 –198. Greenwald BS, Kramer-Ginsberg E, Krishnan KRR, Ashtari M,
Auerbach C, Patel M (1998): Neuroanatomic localization of magnetic resonance imaging signal hyperintensities in geri-atric depression.Stroke29:613– 617.
Greenwald BS, Kramer-Ginsberg E, Krishnan KRR, Ashtari M, Aupperle PM, Patel M (1996): MRI signal hyperintensities in geriatric depression.Am J Psychiatry153:1212–1215. Hickie I, Scott E (1998): Late-onset depressive disorders: A
preventable variant of cerebrovascular disease?Psychol Med
28:1007–1013.
Hickie I, Scott E, Wilhelm K, Brodaty H (1997): Subcortical hyperintensities on magnetic resonance imaging in patients with severe depression—a longitudinal evaluation.Biol Psy-chiatry42:367–374.
Jacoby RJ, Levy R (1980): Computed tomography in the elderly. 3. Affective disorder.Br J Psychiatry136:270 –275. Jorm AF, Van Duijn CM, Chandra V, Fratiglioni L, Graves AB,
Heyman A, et al (1991): Psychiatric history and related exposures as risk factors for Alzheimer’s disease: A collab-orative re-analysis of case-control studies. EURODEM.Int J Epidemiol20:43– 47.
Kirschbaum C, Wolf OT, May M, Wippich W, Hellhammer DH (1996): Stress- and treatment-induced elevations of cortisol levels associated with impaired declarative memory in healthy adults.Life Sci58:1475–1483.
Kral VA, Emery OB (1989): Long-term follow-up of depressive pseudodementia of the aged.Can J Psychiatry34:445– 446. Krishnan KR, McDonald WM (1995): Arteriosclerotic
depres-sion.Med Hypotheses44:111–115.
Landfield PW, Eldridge JC (1994): Evolving aspects of the glucocorticoid hypothesis of brain aging: hormonal modula-tion of neuronal calcium homeostasis. Neurobiol Aging15: 579 –588.
McAllister TW (1985): Recognition of pseudodementia.Am Fam Physician32:175–181.
O’Brien JT (1997): The “glucocorticoid cascade” hypothesis in man.Br J Psychiatry170:199 –201.
Perry EK, Tomlinson BE, Blessed G, Bergmann K, Gibson PH, Perry RH (1978): Correlation of cholinergic abnormalities with senile plaques and mental test scores in senile dementia.
Br Med J2:1457–1459.
Perry R, Jaros E, Irving D, Scoones D, Brown A, McMeekin W, et al (1996): What is the neuropathological basis of dementia associated with Lewy bodies? In: RH Perry, IG McKeith, EK Perry editors.Dementia with Lewy Bodies,Vol 1. Cambridge, UK: Cambridge University Press, 212–223.
Perry RH (1993): Coronal Maps of Brodmann Areas in the Human Brain.London: Wolfe.
Perry RH, Irving D, Blessed G, Fairbairn A, Perry EK (1990a): Senile dementia of Lewy body type. A clinically and neuro-pathologically distinct form of Lewy body dementia in the elderly.J Neurol Sci95:119 –139.
Perry RH, Irving D, Tomlinson BE (1990b): Lewy body preva-lence in the aging brain: Relationship to neuropsychiatric disorders, Alzheimer-type pathology and catecholaminergic nuclei.J Neurol Sci100:223–33.
Rabins PV, Pearlson GD, Aylward E, Kumar AJ, Dowell K (1991): Cortical magnetic resonance imaging changes in elderly inpatients with major depression. Am J Psychiatry
148:617– 620.
Salloway S, Malloy P, Kohn R, Gillard E, Duffy J, Rogg J, et al (1996): MRI and neuropsychological differences in early- and late-life-onset geriatric depression.Neurology
46:1567–1574.
Sapolsky RM, Krey LC, McEwen BS (1986): The neuroendo-crinology of stress and aging: The glucocorticoid cascade hypothesis.Endocr Rev7:284 –301.
Sapolsky RM, Plotsky PM (1990): Hypercortisolism and its possible neural bases.Biol Psychiatry27:937–952.
Sheline YI, Sanghavi M, Mintun MA, Gado MH (1999): De-pression duration but not age predicts hippocampal volume loss in medically healthy women with recurrent major depres-sion.J Neurosci19:5034 –5043.