Mononuclear cell adhesion to collagen ex vivo is related to pulse
pressure in elderly subjects
J.C. Williams
a, M.D. Fotherby
b, L.A. Forster
a, S.P. Tull
a, G.A.A. Ferns
c,*
aDi
6ision of Chemical Pathology,Uni6ersity of Leicester,Glenfield General Hospital,Groby Rd,Leicester LE3 9QP, UK bDepartment of Medicine for the Elderly,Uni
6ersity of Leicester,Glenfield General Hospital,Groby Rd,Leicester LE3 9QP, UK cCentre for Clinical Science and Measurement,School of Biological Sciences,Uni
6ersity of Surrey,Guildford,Surrey GU2 5XH, UK Received 9 June 1999; received in revised form 22 September 1999; accepted 13 October 1999
Abstract
Mononuclear cells and platelets are intimately involved in the pathogenesis and complications of cardiovascular disease. Platelet activation has been reported in hypertension, though the activation-state of monocytes has received less attention. In this study the adhesiveness of monocytes and platelets was assessed and any relationship between the adhesive properties of these cellular elements and plasma levels of soluble adhesion molecules and blood pressure parameters determined. Fifty six elderly volunteers, of whom 32 were classified hypertensive (daytime SBP]135 mmHg), underwent 24 h blood pressure monitoring, assessment of monocyte and platelet adhesion and measurement of the plasma soluble adhesion molecules ICAM-1, L-selectin, E-selectin and vWF. In the elderly hypertensive subjects, monocyte adhesion to collagen coated (PB0.05) and tissue culture plastic microwells (PB0.05) was significantly elevated and circulating levels of soluble ICAM-1 (PB0.01) and soluble E-selectin (PB0.05) were significantly raised compared to their normotensive counterparts. A significant correlation was found to exist between monocyte adhesion to collagen and daytime pulse pressure (r=0.39,PB0.01) and also between plasma levels of soluble E-selectin and clinic DBP (r=0.40,PB0.001). The increased monocyte adhesion witnessed in hypertensive subjects and with increasing pulse pressure may contribute to the increased risk of cardiovascular disease in hypertension. Whether this increased adhesiveness is a property of the monocytes, or reflects endothelial cell activation, remains to be determined. © 2000 Elsevier Science Ireland Ltd. All rights reserved.
Keywords:Monocytes; Platelets; Adhesion; Pulse pressure; Hypertension
www.elsevier.com/locate/atherosclerosis
1. Introduction
Mononuclear cells and platelets are intimately in-volved in athero-thrombotic vascular disease [1] a con-dition whose prevalence increases with age and blood pressure. The relationship between platelet reactivity and blood pressure has previously been studied [2 – 4] and although reports have been conflicting, the consen-sus indicates that platelets are activated in subjects with hypertension [5,6]. In contrast the activation-state of mononuclear cells, which play a pivotal role in athero-genesis, has received less attention.
Adhesion of monocytes to the arterial wall, via spe-cific cell surface adhesion molecules, prior to
transendothelial migration is an important early event in the development of atherosclerotic lesions [7]. It has been suggested that various risk factors such as hyperc-holesterolaemia, hypertension, smoking and diabetes may lead to endothelial dysfunction and activation [8 – 10]. Upon activation one of the earliest events is the
upregulation of the expression of the adhesion
molecules of the selectin family, which results in the tethering and rolling of monocytes along the endothe-lium [11]. Some of these monocytes will disengage and re-enter the circulation, while others will become more firmly attached via integrin mediated adhesion [12]. Monocytes subsequently migrate across the endothe-lium under the influence of chemotactic factors. Once within the subendothelial space they differentiate into macrophages and take up modified low-density lipo-proteins to become the characteristic lipid laden foam cell of an atherosclerotic lesion.
* Corresponding author. Tel.: +44-1483-300800; fax: + 44-1483-300374.
E-mail address:[email protected] (G.A.A. Ferns)
The aim of the present study was to assess the adhesiveness of monocytes and platelets ex vivo in elderly hypertensive and normotensive subjects free of clinical vascular disease and to determine whether the adhesive properties of these cellular elements were re-lated to plasma levels of the soluble adhesion molecules L- or E-selectin, ICAM-1 and von Willebrand factor (vWF) or the blood pressure parameters.
2. Methods
2.1. Materials
RPMI 1640 was purchased from Gibco (Uxbridge, UK). All other reagents including apyrase and prosta-cyclin were purchased from Sigma Chemical (Dorset, UK).
2.2. Subjects
Healthy elderly (60 – 80 years) volunteers were screened to exclude those with a history of symptomatic vascular disease, established hypertension, diabetes mel-litus or subjects using medication including aspirin and vitamin supplements, and current smokers. This re-search has been carried out in accordance with the Declaration of Helsinki (1989) of the World Medical Association, and ethical approval was granted by the Local Ethics Committee prior to the start of the study. Informed consent was obtained from all subjects. Con-ventional blood pressure was recorded in triplicate on three occasions, taking the mean of three sitting blood pressures. Home 24-h blood pressure monitoring was undertaken with the SpaceLabs 90 207 ambulatory blood pressure device (Space Labs Inc, Redmond, Washington, USA) programmed to take readings every 15 min; daytime blood pressure was defined as the mean of readings from 07:00 to 22:00 h. During the final clinic visit blood samples were taken between 09:00 and 11:00 h for the measurement of plasma lipid levels, glucose, urea, electrolytes, soluble L- and E-se-lectin, soluble ICAM- and vWF and assessment of monocyte and platelet adhesion.
2.3. Mononuclear cell isolation
Whole blood anticoagulated with 1/10th volume of 0.013 mol/l trisodium citrate was centrifuged at 200×g
for 20 min and the upper platelet-rich layer removed without disturbing the ‘buffy-coat’. The blood was re-stored to its original volume with phosphate buffered saline (PBS), and 5-ml aliquots overlaid onto Ficoll – Paque. Blood mononuclear cells were prepared as pre-viously described [13]. Briefly, the tubes were centrifuged at 400×gfor 30 min; the mononuclear cell
layer was recovered and washed twice in five volumes of PBS. Immediately prior to use, the cells were resus-pended in serum-free RPMI 1640 medium at a concen-tration of 6×109/l. Prior to use, cell viability was
assessed by trypan blue exclusion, and on all occasions viability exceeded 95%. The content of contaminating platelets was low, with a ratio of platelets:mononuclear cells being B1.
2.4. Measurement of monocyte adhesion
Mononuclear cell (MNC) suspensions in serum-free RPMI 1640 were added to plastic and collagen coated (20 mg/ml, overnight at 4°C) 96 well microtitre plates. The plates were incubated at 37°C for 30 min, and non-adherent cells removed by washing twice with PBS. Monocyte specific adherence was determined by a mod-ification of the method described by Bath et al. [14]. This method relies on conversion of a colourless sub-strate tetramethyl benzidine (TMB) to a blue product by the action of myeloperoxidase, which is present in monocytes but not lymphocytes [14]. The cells con-tained in each well were lysed in 100 ml hexade-cyltrimethyl-ammonium bromide (5 mg/ml in PBS, pH 5.0) at 37°C for 60 min. A fresh solution of TMB [0.1 mg/ml in 0.05 mol/l phosphate citrate buffer (pH 6.0) containing 0.3 mg/ml sodium perborate] was added to each well and the plate incubated for 10 min at room temperature. The reaction was stopped by the addition of 2.5 mol/l sulphuric acid and the absorbance of each well was measured at 450 nm using an Anthos HTIII automatic microplate reader. A standard curve of cell number versus absorbance was constructed for each batch of MNC and absolute adhesion calculated by reference to this curve using Biolise software (Labtech International, East Sussex, UK).
2.5. Platelet isolation
Whole blood anticoagulated with 1/10th volume of 0.013 mol/l trisodium citrate was centrifuged at 200×g
for 20 min and the upper platelet-rich layer collected. Apyrase (final concentration 10mg/ml) and prostacyclin (final concentration 0.33 mg/ml) were added to the platelet-rich plasma to prevent premature platelet acti-vation, followed by centrifugation at 800×g for 15 min. The platelet pellet that resulted was resuspended in Ca2+-free Tyrodes buffer (10 mmol/l HEPES, 145
mmol/l NaCl, 2.7 mmol/l K Cl, 1.8 mmol/l MgCl2, 5.55
mmol/l glucose, 5.95 mmol/l NaHCO3and 0.42 mmol/l
NaHPO4, pH 7.4) containing bovine serum albumin (2
mg/ml) and the count adjusted to 1×1011
/l in Ca2+
2.6. Measurement of platelet adhesion
Platelet adhesion was measured according to the method of Bellavite et al. [15], which quantitates the number of platelets by measuring the activity of acid phosphatase, a platelet enzyme whose activity is stable independently of stimulation and is not released [15]. One hundredml of the washed platelet suspension was added to plastic and collagen coated (20 mg/ml, overnight at 4°C) wells of a 96 well microtitre plate and incubated at 37°C for 1 h. Nonadherent platelets were removed by washing the plates twice with PBS. Subse-quently 150 ml of 0.1 mol/l citrate buffer, pH 5.4, containing 0.1% (v/v) Triton-X-100 and 5 mmol/lp -ni-trophenol phosphate was rapidly added to each well and the plate incubated for 1 h at room temperature. The reaction was then terminated by the addition of 50
ml of 4 mol/l NaOH and the absorbance of each well was measured at 450 nm using an Anthos HTIII auto-matic microplate reader (Labtech International, East Sussex, UK). A standard curve of cell number versus absorbance was constructed for each batch of platelets and absolute adhesion calculated by reference to this curve using Biolise software (Labtech International, East Sussex, UK).
2.7. Measurement of plasma soluble adhesion molecules
Fasted blood samples were collected into lithium heparin tubes (1.5 international units/ml) and cen-trifuged at 1500×g for 10 min at 4°C. The plasma obtained was stored in the dark at −70°C. Plasma
levels of sE-selectin, sL-selectin and sICAM-1 were determined by the use of commercial monoclonal anti-body – based enzyme immunoabsorbent assay (ELISA) kits (R&D Systems, Abingdon, Oxfordshire, UK). Plasma levels of vWF were also determined using a commercial ELISA (Shield Diagnostics, Dundee, UK). All analyses were performed in duplicate.
2.8. Statistical analysis
Results are expressed as mean 9S.E.M. Differences between two means were compared using unpaired Student’s t-test. To evaluate correlations between vari-ables Pearson’s r correlation test was performed. All calculations were performed on MINITAB issue 10 (State College, PA, USA). PB0.05 was considered statistically significant.
3. Results
3.1. Baseline characteristics
Fifty six subjects (29 male) mean age 6995 years (range 64 – 80 years) underwent 24-h blood pressure monitoring, assessment of monocyte and platelet adhe-sion and measurement of plasma soluble ICAM-1, E-selectin, L-selectin and vWF. All subjects had blood glucose, urea and electrolytes within normal ranges. Selected clinical and biochemical characteristics of the hypertensive and normotensive subjects are summarised in Table 1. No significant differences were observed
Table 1
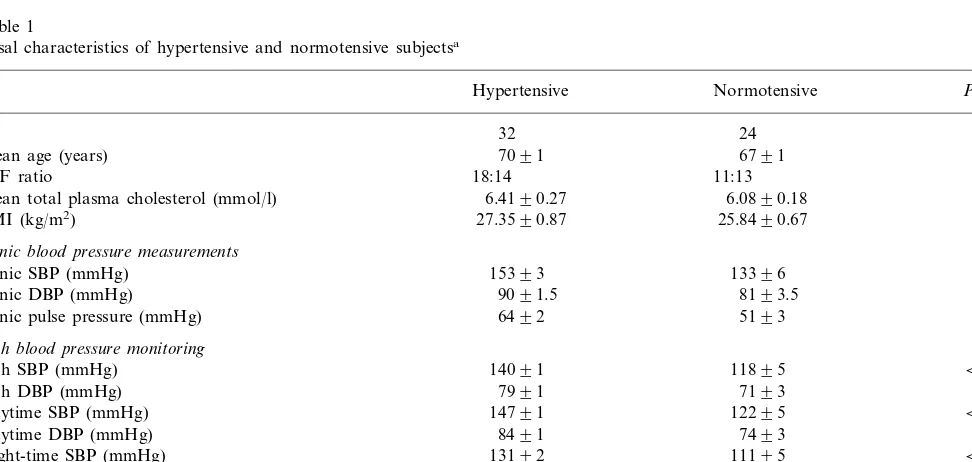
Basal characteristics of hypertensive and normotensive subjectsa
Hypertensive Normotensive Pvalue
N 32 24
Mean age (years) 7091 6791 0.263
0.590
M:F ratio 18:14 11:13
6.4190.27
Mean total plasma cholesterol (mmol/l) 6.0890.18 0.300
BMI (kg/m2) 27.3590.87 25.8490.67 0.199
Clinic blood pressure measurements
Clinic SBP (mmHg) 15393 13396 0.0014
0.0091
Clinic DBP (mmHg) 9091.5 8193.5
0.0024
Clinic pulse pressure (mmHg) 6492 5193
24-h blood pressure monitoring
24-h SBP (mmHg) 14091 11895 B0.0001
24-h DBP (mmHg) 7991 7193 0.0028
12295 B0.0001
14791 Daytime SBP (mmHg)
0.0010
Daytime DBP (mmHg) 8491 7493
13192
Night-time SBP (mmHg) 11195 B0.0001
Night-time DBP (mmHg) 7191 6393 0.0038
6291 4892 B0.0001
24-h pulse pressure (mmHg)
6391
Daytime pulse pressure (mmHg) 4992 B0.0001
6092 4892
Night-time pulse pressure (mmHg) 0.0002
Table 2
Correlation of monocyte adhesion to collagen coated microwells with pulse pressure measured on ambulatory monitoring and clinic read-ings
Night PP Clinic PP 24-h PP Day PP
r
r P P r P r P
0.38 0.005 0.25 0.076 0.29 0.051 0.34 0.012
between the two groups, except for systemic blood pressure (Table 1).
3.2. Monocyte adhesion
Univariate correlation coefficients of monocyte adhe-sion to collagen coated wells with 24 h, daytime, night time and clinic pulse pressure are shown in Table 2. Monocyte adhesion to collagen coated microwells corre-lated significantly with daytime pulse pressure (r=0.39,
P=0.005) (Fig. 1); on multiple linear regression analysis this relationship was independent of age, vitamin E, total, low density and high density cholesterol (R2=8%, P=0.016). In contrast monocyte adhesion to tissue culture plastic microwells was not significantly corre-lated with daytime or clinic pulse pressure (r=0.21,
P=0.13,r=0.24,P=0.11, respectively). Linear corre-lations of monocyte adhesion with blood pressure were not significant. However, subjects (n=32) with daytime SBP]135 mmHg, a level above which subjects can be defined as hypertensive [16,17] compared to those with lower SBP, had greater monocyte adhesion to collagen coated (7393.5 vs. 6193.7%, P=0.04) and tissue culture plastic microwells (7992.1 vs. 7093.5%, P= 0.04) (Fig. 2).
3.3. Platelet adhesion
Platelet adhesion to collagen coated or tissue culture microwells was not significantly correlated with any of the ambulatory blood pressure, clinic blood pressure or pulse pressure measurements. Basal platelet adherence to collagen coated (41.7191.33 vs. 46.3092.66%, P\
0.05) or tissue culture plastic microwells (34.1091.82 vs. 39.1492.88%, P\0.05) was not significantly different in hypertensive (daytime SBP]135 mmHg) compared to normotensive subjects.
3.4. Soluble adhesion molecules
Circulating levels of soluble ICAM-1 (389.49920.21 vs. 327.65910.47 ng/ml,PB0.01) (Fig. 3) and soluble
Fig. 1. Correlation between monocyte adhesion to collagen (%) and daytime pulse pressure (PP day) (mmHg);r=0.3796,P=0.0051.
Fig. 2. Monocyte adhesion to collagen coated and tissue culture plastic microwells (%) in hypertensive (HT) and normotensive (NT) subjects. *PB0.05.
Fig. 3. Levels of soluble ICAM-1 in hypertensive (HT) and nor-motensive (NT) subjects. Data are expressed as means, with S.E.M. shown by vertical error bars. **PB0.01.
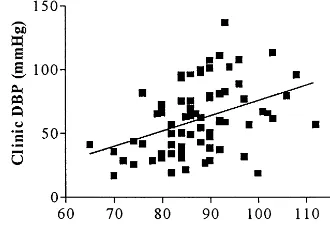
Fig. 5. Correlation between plasma soluble E-selectin levels (ng/ml) and clinic diastolic blood pressure (Clinic DBP) (mmHg);r=0.396, P=0.0005.
increasing age, hypertension is characterised by pre-dominant elevation of systolic rather than diastolic pressure leading to a wide pulse pressure. A wide pulse pressure may reflect the increased peripheral vas-cular resistance, whereby large arteries become increas-ingly stiff and less compliant [25,27]. We have found that there is a significant correlation between mono-cyte adhesion to collagen under static conditions, and pulse pressure. Although this association was weak, the increased adhesiveness of monocytes with increas-ing pulse pressure may be another way in which pulse pressure contributes to the increased risk of cardiovas-cular disease. It also probably indicates that mono-cytes from the hypertensive subjects express higher levels of adhesion molecules. Although circulating monocytes are unlikely to come into contact with col-lagen in the absence of endothelial damage, our data has possible implications for atherosclerotic lesion ex-pansion through enhanced monocyte binding to dis-rupted atherosclerotic plaques, or regions of denuded endothelium, for example following balloon angio-plasty.
Co-existing coronary risk factors may alter MNC adhesiveness and confound for effects of hypertension. For example, monocytes isolated from patients with hypercholesterolaemia adhered to cultured human um-bilical vein cells to a greater extent than those from subjects with normal cholesterol levels [28 – 30]. In the present study an attempt was made to control for possible confounding variables. Hypertensive and nor-motensive subjects were screened to exclude those with clinical evidence of vascular disease or diabetes melli-tus, and those who smoked. Despite entering other possible confounding variables, including blood lipids, into the regression equation, blood pressure remained significantly correlated with monocyte adhesion to col-lagen coated microwells.
In contrast to the results for mononuclear cell adhe-sion, no significant association was found between platelet adhesion and either blood pressure or pulse pressure. Increased platelet activation has been re-ported in hypertensive subjects [5,6]. Despite this and the observation of Andrioli et al. [2] that hypertensives exhibited increased platelet adhesion compared to healthy normotensive controls, we failed to observe any significant differences in basal platelet adhesion between our hypertensive and normotensive subjects or any correlations with blood pressure.
In our study significantly higher levels of soluble ICAM-1 and E-selectin were observed in hypertensive compared to normotensive subjects, this is in accor-dance with Gearing et al. [31] and Blann et al. [32], who have also observed that hypertensive subjects ex-hibit increased plasma levels of soluble ICAM-1 and E-selectin respectively. A significant correlation was found between soluble E-selectin and clinic DBP, simi-E-selectin (69.8297.14 vs. 47.9694.78 ng/ml, PB
0.05) (Fig. 4) were significantly raised in the
hyperten-sive (daytime SBP]135 mmHg) compared to
normotensive subjects, while basal levels of vWF and soluble L-selectin were not significantly different be-tween these two groups. A significant correlation ex-isted between plasma levels of soluble E-selectin and clinic DBP (r=0.396, PB0.001; Fig. 5). The regres-sion equation used to predict clinic DBP (r2=0.157, PB0.001) was
E-selectin (ng/ml)=79.6+0.131 clinic DBP (mmHg).
No significant correlation existed between any of parameters measured and soluble ICAM-1, soluble L-selectin and vWF.
4. Discussion
In the present study we found that mononuclear cell adhesion to collagen ex vivo was significantly greater in elderly hypertensive subjects compared to nor-motensive individuals. Whether this increased mono-cyte adhesion can be attributed to properties of the monocyte itself or is merely a reflection of increased activation of the endothelium remains to be conclu-sively determined. Increased monocyte adhesion in hy-pertension has been demonstrated in both in vitro studies [18,19] and in animal models [20,21]. It may be attributed to increased endothelial cell adhesion molecule expression including VCAM-1 and ICAM-1 [21,22]. Elevated monocyte chemotactic protein-1 (MCP-1) expression has also been demonstrated in the aortae of hypertensive rats [23] and in endothelial cells following cyclic strain [24]. Angiotensin II can also increase monocyte adhesion independent of endothelial adhesion molecule expression [19].
lar to that also observed by Blann et al. [32], who suggested that the increased levels of soluble E-selectin might indicate endothelial activation. A failure to demonstrate an increase in the levels of vWF the es-tablished marker of endothelial cell damage in hyper-tension, in our study and that of Blann et al. [32], may suggest that the endothelium is activated as opposed to damaged in hypertension, or that proposed activa-tion resulting in elevated E-selectin is brought about by a different mechanism to that which results in vWf release.
Soluble adhesion molecules in plasma probably re-sult from proteolytic cleavage from the cell surface. The mechanism by which levels of soluble adhesion molecules are increased is unknown, but their levels are increased in conditions in which expression on the cell membrane has been shown to be increased [33,34]. Therefore, it is possible that a raised blood pressure causes endothelial cell activation in vivo. This in turn results in increased adhesion molecule expression, which has been demonstrated in vivo in an animal model [21] and ex vivo upon cytokine stimulation [18,22].
It has been suggested that soluble adhesion
molecules may influence leucocyte adhesion [35,36]; however, in this study we failed to observe any corre-lation between any of the soluble adhesion molecules and monocyte adhesion. If increased levels of soluble ICAM-1 and E-selectin truly represent increased ex-pression of these molecules on the endothelium, this may result in increased monocyte adhesion in vivo. Adhesion and transmigration of monocytes do not necessarily correspond and once adhered to the en-dothelium, monocytes may become detached [12]. Al-though the detachment mechanism is unclear, perhaps this transient adhesion may result in activation of monocytes, which is reflected by increased monocyte adhesion ex vivo.
In summary, hypertension and especially increasing pulse pressure in elderly hypertensive subjects appears to have little effect on platelet adhesion but is associ-ated with marked mononuclear cell adhesion, an early step in the formation and extension of atherosclerotic lesions. This association of pulse pressure with mononuclear cell activation may contribute to the pro-gression of atherosclerotic lesions and increased atherothrombotic disease seen in older hypertensives.
Acknowledgements
We are grateful to C. Dawson and K. Lowe for their help with this study. We also thank all the volun-teers who generously participated in this study. This work was funded by the British Heart Foundation and The Medical Research Council.
References
[1] Ross R. The pathogenesis of atherosclerosis: a pathogenesis for the 1990s. Nature 1993;362:801.
[2] Andrioli G, Ortolani R, Fontana L, Gaino S, Bellavite P, Lechi C, Minuz P, Manzato F, Tridente G, Lechi A. Study of platelet adhesion in patients with uncomplicated hypertension. J Hyper-tens 1996;14:1215.
[3] Valtier D, Guicheney P, Bandouin-Legros M, Meyer P. Platelets in human essential hypertension: in vitro hyperreactivity to thrombin. J Hypertens 1986;4:551.
[4] Ashida T, Tanaka T, Yokouchi M, Kuramochi M, Deguchi F, Kimura G, Kojima S, Ito K, Ikeda M. Effect of dietary sodium on platelet alpha-2 adrenergic receptors in essential hyperten-sion. Hypertension 1985;7:972.
[5] Mehta J, Mehta P. Platelet function in hypertension and effect of therapy. Am J Cardiol 1981;47:331.
[6] Coccheri S, Fiorentine P. Platelet adhesiveness and aggregation in hypertensive patients. Acta Med Scand (Suppl) 1971;525:273. [7] Gerrity RG. The role of monocyte in atherogenesis. I. Transition of blood borne monocytes into foam cells in fatty lesions. Am J Pathol 1981;103:181.
[8] Simon BC, Haudenschild CC, Cohen RA, Palacino J. Preserva-tion of endothelium-dependent relaxaPreserva-tion in atherosclertic rabbit aorta by probucol. J Cardiovasc Pharmacol 1993;21:893. [9] Lockette W, Otsuka Y, Carretero O. The loss of endothelium
dependent vascular relaxation in hypertension. Hypertension 1986;8(Suppl II):II161.
[10] Ball K, Turner R. Smoking and the heart. The basis for action. Lancet 1974;2:822.
[11] Butcher EC. Leucocyte-endothelial cell recognition: three (or more) steps to specificity and diversity. Cell 1991;67:1033. [12] Takahashi M, Ikeda U, Masuyama J-I, Kitagawa S-I, Kasahara
T, Saito M, Kano S, Shimada K. Involvement of adhesion molecules in human monocyte adhesion to and transmigration through endothelial cells in vitro. Atherosclerosis 1994;108:73. [13] Bøyum A. Isolation of mononuclear cells and granulocytes from
human blood. J Clin Invest 1968;21:77.
[14] Bath PMW, Booth RFG, Hassall DG. Monocyte – lymphocyte discrimination in a new microtitre-based adhesion assay. J Im-munol Methods 1989;118:59 – 65.
[15] Bellavite P, Andrioli G, Guzzo P, Arigliano P, Chirumbolo S, Manzato F, Santonastaso C. A colorimetric method for the measurement of platelet adhesion in microtitre plates. Anal Biochem 1994;216:444.
[16] Mancia G, Sega R, Bravi C, De Vito G, Valagussa F, Cesana G, Zanchetti A. Ambulatory blood pressure normality: results from the PAMELA study. J Hypertens 1995;13:1377.
[17] Manning G, Rushton L, Millar-Craig MW. Twenty four hour ambulatory blood pressure: a sample from a normal British population. J Hum Hypertens 1998;12:123.
[18] McCarron RM, Wang L, Sire´n A-L, Spatz M, Hallenbeck JM. Monocyte adhesion to cerebromicrovascular endothelial cells derived from hypertensive and normotensive rats. Am J Physiol 1994;267:H2491.
[19] Kim JA, Berliner JA, Nadler JL. Angiotensin II increases mono-cyte binding to endothelial cells. Biochem Biophys Res Commun 1996;226:862.
[20] Liu Y, Liu T, McCarron RM, Spatz M, Feuerstein G, Hallen-beck JM, Sire´n A-L. Evidence for activation of endothelium and monocytes in hypertensive rats. Am J Physiol 1996;270:H2125. [21] Tropea BI, Huie P, Cooke JP, Tsao PS, Sibley RK, Zarins CK.
Hypertension-enhanced monocyte adhesion in experimental atherosclerosis. J Vasc Surg 1996;23:596.
[23] Caper IVQ, Alexander RW, Lou P, De Leon H, Wilcox JN, Ishizaka N, Howard AB, Taylor WR. Monocyte chemoattrac-tant protein-1 expression in aortic tissue of hypertensive rats. Hypertension 1997;30:1397.
[24] Wang DL, Wung B-S, Shyy Y-J, Lin C-F, Chao Y-J, Usami S, Chien S. Mechanical strain induces monocyte chemotactic protein-1 gene expression in endothelial cells: effects of mechan-ical strain on monocyte adhesion to endothelial cells. Circ Res 1995;77:294.
[25] Madhavan S, Ooi W, Cohen H, Alderman MH. Relation of pulse pressure and blood pressure reduction to the incidence of myocardial infarction. Hypertension 1994;23:395.
[26] Flack JM, Neaton J, Grimm R, et al. Blood pressure and mortality among men with prior myocardial infarction. Circula-tion 1995;92:2437.
[27] Bots ML, Witteman JCM, Hofman A, de Jong PTVM, Grobbee DE. Low diastolic blood pressure and atherosclerosis in elderly subjects. Arch Int Med 1996;156:843.
[28] Bath PM, Gladwin AM, Martin JF. Human monocyte charac-teristics are altered in hypercholesterolaemia. Atherosclerosis 1991;90:175.
[29] Lo¨sche W, Krause S, Pohl A, Pohl C, Liebrenz A, Schauer I, Ru¨hling K, Till U. Functional behaviour of mononuclear blood cells from patients with hypercholesterolemia. Thromb Res 1992;65:337.
[30] Stragliotto E, Camera M, Postiglione A, Sirtori M, Di Minno
G, Tremoli E. Functionally abnormal monocytes in hyperc-holesterolemia. Arterioscler Thromb 1993;13:944.
[31] Gearing AJH, Hemingway I, Pigott R, Hughes J, Rees AJ, Cashman SJ. Soluble forms of vascular adhesion molecules, E-selectin, ICAM-1 and VCAM-1: pathological significance. Ann N Y Acad Sci 1992;667:324.
[32] Blann AD, Wai T, Maxwell SJR, Waite MA. Increased levels of the soluble adhesion molecule E-selectin in essential hypertension. J Hypertens 1994;12:925.
[33] Adams DH, Mainolfi E, Elias E, Neuberger JM, Rothlein R. Detection of circulating intercellular adhesion molecule-1 after liver transplantation, evidence of local release within the liver during graft rejection. Transplantation 1993;55:83.
[34] Ballantyne CM, Mainolfi EA, Young JB, Windsor NT, Co-canougher B, Lawrence EC, Pollack MS, Entman ML, Rothlein R. Relationships of increased levels of circulating intercellular adhesion molecule-1 after heart transplantation to rejection: human leukocyte antigen mismatch and survival. J Heart Lung Trans 1994;13:597.
[35] Schleiffenbaum B, Olivier S, Tedder TF. Soluble L-selectin is present in human plasma at high levels and retains functional activity. J Cell Biol 1992;119:229.
[36] Lo SK, Lee S, Ramos RA, Lobb R, Rosa M, Chi-Rosso G, Wright SD. Endothelial-leukocyte adhesion molecule-1 stimulates the adhesive activity of leukocyte integrin CR3 (CD11b/CD18, Mac-1,a1b2) on human neutrophils. J Exp Med 1991;173:1493.